- PCSA Dashboard
- Financials
- Filings
-
Holdings
- Transcripts
- ETFs
- Insider
- Institutional
- Shorts
-
S-1 Filing
Processa Pharmaceuticals (PCSA) S-1IPO registration
Filed: 29 Dec 23, 12:00am
As filed with the U.S. Securities and Exchange Commission on December 29, 2023
Registration No. 333-
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
Processa Pharmaceuticals, Inc.
(Exact name of registrant as specified in its charter)
| Delaware | 2834 | 45-1539785 | ||
(State or other jurisdiction of incorporation or organization) | (Primary Standard Industrial Classification Code Number) | (I.R.S. Employer Identification Number) |
7380 Coca Cola Drive, Suite 106
Hanover, Maryland 21076
(443) 776-3133
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices)
George Ng
Chief Executive Officer
Processa Pharmaceuticals, Inc.
7380 Coca Cola Drive, Suite 106
Hanover, Maryland 21076
(443) 776-3133
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Copies to:
Michael B. Kirwan John J. Wolfel, Jr. Neda Sharifi Foley & Lardner LLP One Independent Drive, Suite 1300 Jacksonville, Florida 32202 (904) 359-2000 |
Approximate date of commencement of proposed sale to the public: As soon as practicable after the effective date of this registration statement.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933 check the following box. ☐
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration number of the earlier effective registration statement for the same offering. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer | ☐ | Accelerated filer | ☐ | |
| Non-accelerated filer | ☒ | Smaller reporting company | ☒ | |
| Emerging growth company | ☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided to Section 7(a)(2)(B) of the Securities Act. ☐
The registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933, as amended, or until this registration statement shall become effective on such date as the Commission, acting pursuant to said Section 8(a), may determine.
The information in this preliminary prospectus is not complete and may be changed. These securities may not be sold until the registration statement filed with the Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell nor does it seek an offer to buy these securities in any jurisdiction where the offer or sale is not permitted.
PRELIMINARY PROSPECTUS, SUBJECT TO COMPLETION, DATED DECEMBER 29, 2023
Up to ● Shares of Common Stock
Up to ● Pre-Funded Warrants to Purchase up to ● Shares of Common Stock
Up to ● Common Warrants to Purchase up to ● Shares of Common Stock
Up to ● Placement Agent Warrants to Purchase up to ● Shares of Common Stock
Up to ● Shares of Common Stock underlying such Pre-Funded Warrants, Common Warrants and Placement Agent Warrants
We are offering up to ● shares of our common stock and accompanying common warrants to purchase up to ● shares of our common stock (the “Common Warrants”), at an assumed combined public offering price of $● per share of common stock and accompanying Common Warrant (equal to the last sale price of our common stock as reported by The Nasdaq Capital Market on ●, 202●). The Common Warrants have an exercise price of $● per share (●% of the combined public offering price per share of the common stock and accompanying Common Warrant) and will be exercisable beginning on the effective date of stockholder approval of the issuance of the shares upon exercise of the warrants (“Warrant Stockholder Approval”), provided however, if the Pricing Conditions (as defined below) are met, the Common Warrant will be exercisable upon issuance (the “Initial Exercise Date”). The Common Warrants will expire on the five-year anniversary date of the Initial Exercise Date. As used herein “Pricing Conditions” means that the combined offering price per share and accompanying Common Warrant is such that the Warrant Stockholder Approval is not required under Nasdaq rules because either (i) the offering is an at-the-market offering under Nasdaq rules and such price equals or exceeds the sum of (a) the applicable “Minimum Price” per share under Nasdaq rule 5635(d) plus (b) $0.125 per whole share of common stock underlying the Common Warrant or (ii) the offering is a discounted offering where the pricing and discount (including attributing a value of $0.125 per whole share underlying the warrants) meet the pricing requirements under the Nasdaq rules.
We are also offering to each purchaser whose purchase of shares of common stock in this offering would otherwise result in the purchaser, together with its affiliates and certain related parties, beneficially owning more than 4.99% (or, at the election of the investor 9.99%) of our outstanding common stock immediately following the consummation of this offering, the opportunity to purchase, if the purchaser so chooses, pre-funded warrants (the “Pre-Funded Warrants”), in lieu of shares of common stock that would otherwise result in the purchaser’s beneficial ownership exceeding 4.99% of our outstanding common stock. Subject to limited exceptions, a holder of Pre-Funded Warrants will not have the right to exercise any portion of its Pre-Funded Warrants if the investor, together with its affiliates, would beneficially own in excess of 4.99% (or, at the election of the holder, 9.99%) of the number of shares of common stock outstanding immediately after giving effect to such exercise. Each Pre-Funded Warrant will be exercisable for one share of common stock at an exercise price of $0.0001 per share of common stock. The public offering price per Pre-Funded Warrant and accompanying Common Warrant, is equal to the public offering price per share of common stock and accompanying Common Warrant less $0.0001. Each Pre-Funded Warrant will be exercisable upon issuance and will expire when exercised in full. This prospectus also relates to the offering of the shares of common stock issuable upon exercise of the pre-funded warrants.
For each Pre-Funded Warrant we sell, the number of shares of common stock we are offering will be decreased on a one-for-one basis. Because a Common Warrant is being sold together in this offering with each share of common stock and, in the alternative, each Pre-Funded Warrant to purchase one share of common stock, the number of Common Warrants sold in this offering will not change as a result of a change in the mix of the shares of our common stock and Pre-Funded Warrants sold. The shares of common stock or Pre-Funded Warrants, as applicable, and the accompanying Common Warrants, can only be purchased together in this offering but will be issued separately and will be immediately separable upon issuance.
We are also registering shares of common stock that are issuable from time to time upon exercise of the Pre-Funded Warrants, Common Warrants and Placement Agent Warrants (as defined below).
This offering will terminate on ●, 202●, unless we decide to terminate the offering (which we may do at any time in our discretion) prior to that date. We will have one closing for all the securities purchased in this offering. The combined public offering price per share (or pre-funded warrant) and accompanying Common Warrants will be fixed for the duration of this offering.
We have engaged ●, or the placement agent, to act as our exclusive placement agent in connection with this offering. The placement agent has agreed to use its reasonable best efforts to arrange for the sale of the securities offered by this prospectus. The placement agent is not purchasing or selling any of the securities we are offering and the placement agent is not required to arrange the purchase or sale of any specific number or dollar amount of securities. We have agreed to pay to the placement agent the placement agent fees set forth in the table below, which assumes that we sell all of the securities offered by this prospectus. Since we will deliver the securities to be issued in this offering upon our receipt of investor funds, there is no arrangement for funds to be received in escrow, trust or similar arrangement. There is no minimum offering requirement as a condition of closing of this offering. Because there is no minimum offering amount required as a condition to closing this offering, we may sell fewer than all of the securities offered hereby, which may significantly reduce the amount of proceeds received by us, and investors in this offering will not receive a refund in the event that we do not sell an amount of securities sufficient to pursue our business goals described in this prospectus. In addition, because there is no escrow account and no minimum offering amount, investors could be in a position where they have invested in our company, but we are unable to fulfill all of our contemplated objectives due to a lack of interest in this offering. Further, any proceeds from the sale of securities offered by us will be available for our immediate use, despite uncertainty about whether we would be able to use such funds to effectively implement our business plan. See the section entitled “Risk Factors” for more information. We will bear all costs associated with the offering. See “Plan of Distribution” on page 35 of this prospectus for more information regarding these arrangements.
Our common stock is listed on The Nasdaq Capital Market under the symbol “PCSA.” The closing price of our common stock on ●, 202●, as reported by The Nasdaq Capital Market, was $● per share. There is no established public trading market for the Pre-Funded Warrants or the Common Warrants, and we do not expect a market to develop. We do not intend to apply for listing of the Pre-Funded Warrants or the Common Warrants on any securities exchange or nationally recognized trading system. Without an active trading market, the liquidity of the Pre-Funded Warrants and the Common Warrants will be limited.
The public offering price per share of common stock and accompanying Common Warrant and any Pre-Funded Warrant and accompanying Common Warrant will be determined by us at the time of pricing, may be at a discount to the current market price, and the recent market price used throughout this prospectus may not be indicative of the final public offering price.
Investing in our securities involves a high degree of risk. See “Risk Factors” beginning on page 10 of this prospectus before investing. You should also consider the risk factors described or referred to in any documents incorporated by reference in this prospectus, and in any applicable prospectus supplement, before investing in these securities.
We are a “smaller reporting company” as defined under federal securities law and we have elected to comply with certain reduced public company reporting requirements available to smaller reporting companies. See the section titled “Prospectus Summary — Implications of Being a Smaller Reporting Company.”
| Per share of Common Stock and accompanying Common Warrant | Per Pre-Funded Warrant and accompanying Common Warrant | Total | ||||||||||
| Public offering price | $ | $ | $ | |||||||||
| Placement agent fees (1) | $ | $ | $ | |||||||||
| Proceeds to us, before expenses (2) | $ | $ | $ | |||||||||
| (1) | We have agreed to pay the placement agent a cash fee equal to 7.0% of the gross proceeds raised in this offering (other than proceeds received from the Company’s current directors and officers). We have also agreed to reimburse the placement agent for certain of its offering related expenses, including reimbursement for non-accountable expenses in legal fees and expenses in the amount of up to $112,500, and for its clearing expenses in the amount of $15,950. In addition, we have agreed to issue the placement agent or its designees warrants (“Placement Agent Warrants”) to purchase a number of shares of common stock equal to 4.0% of the shares of common stock sold in this offering (including the shares of common stock issuable upon the exercise of the Pre-Funded Warrants, but not including shares of common stock sold to current directors and officers of the Company), at an exercise price of $● per share, which represents 125% of the public offering price per share and accompanying warrant. For a description of compensation to be received by the placement agent, see “Plan of Distribution” for more information |
| (2) | Because there is no minimum number of securities or amount of proceeds required as a condition to closing in this offering, the actual public offering amount, placement agent fees, and proceeds to us, if any, are not presently determinable and may be substantially less than the total maximum offering amounts set forth above. For more information, see “Plan of Distribution.” |
Neither the Securities and Exchange Commission nor any other state securities commission has approved or disapproved of these securities or passed upon the adequacy or accuracy of this prospectus. Any representation to the contrary is a criminal offense.
Delivery of the shares of common stock is expected to be made on or about ●, 202●.
Prospectus dated ●, 202●
TABLE OF CONTENTS
| i |
You should rely only on the information we have provided or incorporated by reference into this prospectus, any applicable prospectus supplement and any related free writing prospectus. We incorporate by reference important information into this prospectus. You may obtain the information incorporated by reference without charge by following the instructions under “Where You Can Find More Information.” You should carefully read this prospectus as well as additional information described under “Incorporation of Certain Information By Reference,” before deciding to invest in our securities.
We have not, and the placement agent and its affiliates have not, authorized anyone to provide you with any information or to make any representation not contained or incorporated by reference in this prospectus or any related free writing prospectus. We do not, and the placement agent and its affiliates do not, take any responsibility for, and can provide no assurance as to the reliability of, any information that others may provide to you. This prospectus is not an offer to sell or an offer to buy securities in any jurisdiction where offers and sales are not permitted. The information in this prospectus is accurate only as of its date, regardless of the time of delivery of this prospectus or any sale of securities. You should also read and consider the information in the documents to which we have referred you under the caption “Where You Can Find More Information” in the prospectus.
To the extent there is a conflict between the information contained in this prospectus, on the one hand, and the information contained in any document incorporated by reference filed with the Securities and Exchange Commission (“SEC”) before the date of this prospectus, on the other hand, you should rely on the information in this prospectus. If any statement in a document incorporated by reference is inconsistent with a statement in another document incorporated by reference having a later date, the statement in the document having the late date modifies or supersedes the earlier statement.
We further note that the representations, warranties and covenants made by us in any agreement that is incorporated by reference or filed as an exhibit to the registration statement of which this prospectus is a part were made solely for the benefit of the parties to such agreement, including, in some cases, for the purpose of allocating risk among the parties to such agreements, and should not be deemed to be a representation, warranty or covenant to you. Moreover, such representations, warranties or covenants were accurate only as of the date when made. Accordingly, such representations, warranties and covenants should not be relied on as accurately representing the current state of our affairs.
For investors outside the United States: neither we nor the placement agent have done anything that would permit this offering or possession or distribution of this prospectus or any free writing prospectus we may provide to you in connection with this offering in any jurisdiction where action for that purpose is required, other than in the United States of America. Persons outside the U.S. who come into possession of this prospectus must inform themselves about, and observe any restrictions relating to, the offering of the shares of our common stock and the distribution of this prospectus and any such free writing prospectus outside of the U.S.
Unless otherwise indicated, information contained in this prospectus concerning our industry and the markets in which we operate, including our general expectations, market position and market opportunity, is based on our management’s estimates and research, as well as industry and general publications and research, surveys and studies conducted by third parties. We believe that the information from these third-party publications, research, surveys and studies included in this prospectus is reliable. Management’s estimates are derived from publicly available information, their knowledge of our industry and their assumptions based on such information and knowledge, which we believe to be reasonable. These data involve a number of assumptions and limitations which are necessarily subject to a high degree of uncertainty and risk due to a variety of factors, including those described in “Risk Factors.” These and other factors could cause our future performance to differ materially from our assumptions and estimates.
This prospectus includes trademarks, service marks and trade names owned by us or other companies. All trademarks, service marks and trade names included in this prospectus are the property of their respective owners.
As used in this prospectus, unless the context indicates or otherwise requires, “the Company,” “our Company,” “we,” “us,” and “our” refer to Processa Pharmaceuticals, Inc., a Delaware corporation, and its consolidated subsidiary.
| 1 |
This summary highlights selected information contained elsewhere in this prospectus and does not contain all of the information that you should consider in making an investment decision. Before investing in our common stock, you should carefully read this entire prospectus, including the information set forth under the “Risk Factors” section of this prospectus and in the documents incorporated by reference into this prospectus for a discussion of the risks involved in investing in our securities.
Overview
We are a clinical-stage biopharmaceutical company focused on utilizing our “regulatory science” approach, including the principles associated with FDA’s Project Optimus Oncology initiative and the related FDA Draft Guidance, in the development of Next Generation Chemotherapy (“NGC”) oncology drug products. Our mission is to provide better treatment options than those that presently exist by extending a patient’s survival and/or improving a patient’s quality of life. This is achieved by improving upon FDA-approved, widely used oncology drugs or the cancer-killing metabolites of these drugs by altering how they are metabolized and/or distributed in the body, including how they are distributed to the actual cancer cells.
Regulatory science was conceived in the early 1990s when the founders of Processa and other faculty at the University of Maryland worked with the FDA to develop multiple FDA Guidances. Regulatory science is the science of developing new tools, standards, and approaches to assess the safety, efficacy, quality, and performance of all FDA-regulated products. Over the last 30 years, two of our founders, Dr. David Young and Dr. Sian Bigora, have expanded the original regulatory science concept by including the pre-clinical and clinical studies to justify the benefit-risk assessment required for FDA approval when designing the development programs of new drug products.
Our regulatory science approach defines the scientific information that the FDA requires to determine if the benefit outweighs the risk of a drug in a specific population of patients and at a specific dosage regimen for a specific drug product. The studies are designed to obtain the necessary scientific information to support the regulatory decision.
The FDA has more recently taken steps to define some of the regulatory science required for the FDA approval of oncology products. Through the FDA’s Project Optimus Oncology Initiative and the related Draft Guidance on determining the “optimal” dosage regimen for an oncology drug, the FDA has chosen to make the development of oncology drugs more science-based than in the past. Since the principles of the FDA’s Project Optimus and the related Draft Guidance have been used by our regulatory science approach in a number of non-oncology drugs, our experience with the principles of Project Optimus differentiates us from other biotechnology companies by focusing us not only on the clinical science, but also on the equally important regulatory process. We believe utilizing our regulatory science approach provides us with three distinct advantages:
| ● | greater efficiencies (e.g., the right trial design and trial readouts); | |
| ● | greater possibility of drug approval by the FDA or other regulatory authorities; and | |
| ● | greater ability to evaluate the benefit-risk of a drug compared to existing therapy, which allows prescribers to provide better treatment options for each patient. |
In January 2023, we announced our strategic prioritization to advance our pipeline of NGC proprietary small molecule oncology drugs. The NGC products are new chemical entities, but they work by changing the metabolism, distribution and/or elimination of already FDA-approved cancer drugs or their active metabolites while maintaining the mechanism of how the drug kills cancer cells. We believe our NGC treatments will provide improved safety-efficacy profiles when compared to their currently marketed counterparts – capecitabine, gemcitabine, and irinotecan. All future studies of these drugs are subject to availability of capital to conduct the trials.
The three NGC treatments in our pipeline are as follows:
| ● | NGC-Capecitabine (NGC-Cap) is a combination of PCS6422 and capecitabine, capecitabine being the oral prodrug of the cancer drug 5-fluorouracil (5-FU). PCS6422, without having any clinically meaningful biological effect itself, alters the metabolism of 5-FU, resulting in more 5-FU distribution to the cancer cells. In clinical trials, NGC-Cap has shown a safety profile different than capecitabine when administered alone. Side effects such as Hand-Foot Syndrome (HFS) and cardiotoxicity typically occur in up to 50-70% of patients treated with capecitabine and are caused by specific capecitabine metabolites. These types of toxicities frequently result in decreased doses, interrupted doses, or discontinuation of treatment with capecitabine. Since a much smaller amount of these metabolites are formed with NGC-Cap, these side effects appear in less patients and are less severe when they do occur. In addition, NGC-Cap has been found to be up to 50 times more potent than capecitabine based on the systemic exposure of the capecitabine metabolite 5-FU, which is metabolized to the cancer-killing metabolites. Like capecitabine, NGC-Cap could be used to treat patients with various cancers, such as metastatic colorectal, gastrointestinal, breast, and pancreatic. We estimate at least 200,000 patients in the United States were newly diagnosed in 2022 with metastatic colorectal, gastrointestinal, breast, and pancreatic cancers. On December 11, 2023, we had a successful meeting with the FDA regarding the next Phase 2 study supporting the advancement of NGC-Cap for cancer patients. The meeting with the FDA was supported by the interim results from the ongoing Phase 1B study that should complete enrollment in the first quarter of 2024. |
| 2 |
| ● | PCS3117, also referred to as NGC-Gemcitabine (NGC-Gem), is an oral analog of gemcitabine that is converted to its active metabolite by a different enzyme system than gemcitabine resulting in a positive response in gemcitabine patients as well as some gemcitabine treatment-resistant patients. Like gemcitabine, NGC-Gem could be used to treat patients with various cancers such as pancreatic, biliary tract, lung, ovarian, and breast. We estimate more than 275,000 patients in the United States were newly diagnosed in 2022 with pancreatic, biliary tract, lung, ovarian, and breast cancer. We plan to meet with the FDA to discuss potential study designs including implementation of the Project Optimus initiative as part of the design in 2024. | |
| ● | PCS11T, also referred to as NGC-Irinotecan (NGC-Iri), is a prodrug of the active metabolite of irinotecan (SN-38). The chemical structure of NGC-Iri influences the uptake of the drug into cancer cells, resulting in more NGC-Iri entering cancer cells than normal cells in mice. These levels were significantly greater than those seen with irinotecan, resulting in lower doses of NGC-Iri having greater efficacy than irinotecan and improved safety in animal models. Like irinotecan, NGC-Iri could be used to treat patients with various cancers such as lung, colorectal, gastrointestinal, and pancreatic cancer. We estimate at least 200,000 patients in the United States were newly diagnosed in 2022 with lung, colorectal, gastrointestinal, and pancreatic cancer. We plan to conduct IND-enabling and toxicology studies in 2024. |
We have completed our Phase 2A trial for PCS12852 in gastroparesis patients with positive results. Additionally, in February 2023, due primarily to the inability to identify and enroll patients in our rare disease Phase 2 trial for PCS499 in ulcerative Necrobiosis Lipoidica (uNL), we decided to cease further enrollment in the PCS499 trial and terminated the trial. We did not experience any safety concerns during the conduct of either the PCS12852 or PCS499 trial. We are currently evaluating options to monetize these non-core drug assets, which may include out-licensing or partnering these assets with one or more third parties.
Our shift in prioritization to NGC oncology drugs does not change our mission. We continue to be focused on drug products that improve the survival and/or quality of life for patients by improving the safety and/or efficacy of the drug in a targeted patient population, while providing a more efficient and probable path to FDA approval and differentiating our drugs from those on the market or are currently being developed.
Historically, much of oncology drug development has searched for novel or different ways to treat cancer. Our approach is to take three current FDA-approved cancer drugs and modify and improve how the human body metabolizes and/or distributes these NGC treatments compared to their presently approved counterpart drugs while maintaining the cancer-killing mechanism of action; thus, our reason for calling our drugs Next Generation Chemotherapy (or NGC) treatments. Part of the development includes determining the optimal dosage regimen based on the dose-response relationship as described in the FDA’s Project Optimus Initiative and Draft Optimal Dosage Regimen Oncology Guidance. To date, we have data that suggests our NGC treatments are likely to have a better safety-efficacy profile than the current widely used marketed counterpart drugs, not only potentially making the development and approval process more efficient, but also clearly differentiating our NGC treatments from the existing treatment. We believe our NGC treatments have the potential to extend the survival and/or quality of life for more patients diagnosed with cancer while decreasing the number of patients who are required to dose-adjust or discontinue treatment because of side effects or lack of response.
| 3 |
Our Strategy
Our strategy is to develop our pipeline of NGC proprietary small molecule oncology drugs using our regulatory science approach to determine the optimal dosage regimen of our oncology drugs.
By changing either the metabolism, distribution, and/or elimination of already FDA-approved cancer drugs (e.g., capecitabine, gemcitabine, and irinotecan) or their active metabolites, we believe that our three new oncology drugs represent the next generation of chemotherapy with an improved safety profile, improved efficacy profile and/or potentially benefiting more patients while maintaining the mechanism of how the drug kills cancer cells. By combining these modified approved cancer treatments with our regulatory science approach and our experience using the principles of FDA’s Project Optimus initiative, we anticipate that we will be able to increase the probability of FDA approval, improve the safety-efficacy profile over the existing counterparts of our NGC drugs, and more efficiently develop each drug.
Our pipeline of NGCs (i) already has data demonstrating the desired pharmacological activity in humans or appropriate animal models and is able to provide improved safety and/or efficacy by some modification in the formation and/or distribution of the active moieties associated with the drug and (ii) targets cancers for which a single positive pivotal trial demonstrating efficacy might provide enough evidence that the clinical benefits of the drug and its approval outweighs the risks associated with the drug.
Our Drug Pipeline
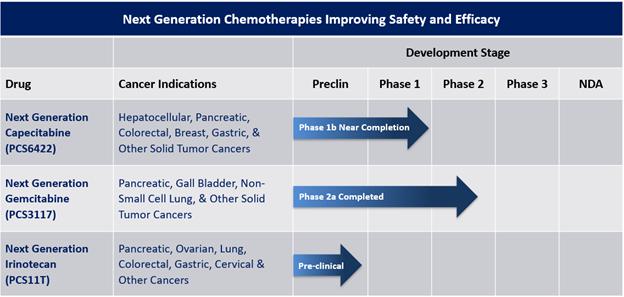
Our pipeline currently consists of NGC-Cap, NGC-Gem and NGC-Iri (also identified as PCS6422, PCS3117 and PCS11T, respectively) and two non-oncology drugs (PCS12852 and PCS499). The non-oncology drugs are not included in the pipeline chart above, as we are exploring our options for those drugs. A summary of each drug is provided below.
Next Generation Chemotherapy Pipeline
| ● | Next Generation Capecitabine (NGC-Cap) is a combination of PCS6422 and a lower dose of the FDA-approved cancer drug capecitabine. PCS6422 is an orally administered irreversible inhibitor of the enzyme dihydropyrimidine dehydrogenase (DPD). DPD metabolizes 5-Fluorouracil (5-FU), the major metabolite of capecitabine and widely used itself as an intravenous chemotherapeutic agent in many types of cancer, to multiple metabolites classified as catabolites. These catabolites do not have any cancer-killing properties but frequently cause dose-limiting side effects that may require dose adjustments or discontinuation of therapy. |
| 4 |
Capecitabine, as presently prescribed and FDA-approved, forms the cancer drug 5-FU which is then further metabolized to anabolites (which kill both cancer cells and normal duplicating cells) and catabolites (which cause side effects and have no cancer killing properties). When capecitabine is given in combination with PCS6422 in NGC-Cap, PCS6422 significantly changes the metabolism of 5-FU, which results in a change in the distribution of 5-FU within the body. Due to this change in metabolism and the overall metabolite profile of anabolites and catabolites, the side effect and efficacy profile of NGC-Cap has been found to be different from capecitabine given without PCS6422. Since the potency of NGC-Cap is also greater than FDA-approved capecitabine based on the 5-FU systemic exposure per mg of capecitabine administered, the amount of capecitabine anabolites formed from 1 mg of capecitabine administered in NGC-Cap will, therefore, be much greater than formed from the administration of 1 mg of existing capecitabine.
On August 2, 2021, we enrolled the first patient in our Phase 1B dose-escalation maximum tolerated dose trial in patients with advanced refractory gastrointestinal (GI) tract tumors. Our interim analysis of Cohorts 1 and 2A of the ongoing clinical trial found no dose-limiting toxicities (DLTs), no drug-related adverse events greater than Grade 1, and no adverse events associated with the catabolites of 5-FU such as HFS. In this Phase 1B trial, it was demonstrated that the irreversible inhibition of DPD by PCS6422 could alter the metabolism, distribution and elimination of 5-FU, making NGC-Cap significantly (up to 50 times) more potent than capecitabine alone and potentially leading to higher levels of anabolites which can kill replicating cancer and normal cells. By administering NGC-Cap to cancer patients, the balance between anabolites and catabolites changes depending on the dosage regimens of PCS6422 and capecitabine used, making the efficacy-safety profile of NGC-Cap different than that of FDA-approved capecitabine and requiring further evaluation of the PCS6422 and capecitabine regimens to determine the optimal NGC-Cap regimens for patients.
In order for NGC-Cap to provide a safer and more efficacious profile for cancer patients compared to existing chemotherapy, understanding how the different regimens of PCS6422 and capecitabine may affect the systemic and tumor exposure to the anabolites, as well as the systemic exposure to the catabolites, is required. This can be achieved by following the timeline of DPD irreversible inhibition and the formation of new DPD using the plasma concentrations of 5-FU and its catabolites.
In an effort to better estimate the timeline of DPD inhibition and formation of new DPD, we modified the protocol for the Phase 1B trial and began enrolling patients in the amended Phase 1B trial in April 2022. On November 1, 2022, we announced that data from the Phase 1B trial identified multiple dosage regimens with potentially better safety and efficacy profiles than currently existing chemotherapy regimens. Since 5-FU exposure is dependent on both the PCS6422 regimen and the capecitabine regimen, safe regimens were identified as well as regimens that cause DLTs. One of the regimens in the Phase 1B trial did cause DLTs in two patients, one of whom died. The Phase 1B trial is continuing to enroll patients and is expected to complete enrollment in early 2024. The next trial will be a Phase 2 trial to determine which regimens provide an improved efficacy-safety profile over present therapy using the principles of the FDA’s Project Optimus initiative to help guide the design of the trial. This FDA initiative requires us to consider NGC regimens that are not at the maximum tolerated dose or exposure level.
Discussions with the FDA in April 2023 have clarified that the major goal for the next Phase 2 trial will be to evaluate and understand the dose- and exposure-response relationship for anti-tumor activity and safety. The specific dosage regimens for the trial will be defined following the determination of the MTD from our ongoing Phase 1B trial. Cohort 3 in the Phase 1B trial, which dosed patients with PCS6422 in combination with capecitabine at 150 mg BID (twice a day), completed with no dose-limiting toxicities. Enrollment in Cohort 4 was expanded to include six patients to further evaluate the safety at this dose. Enrollment in this cohort is now complete and to date, no DLTs have been observed in this cohort, but safety evaluation for this cohort is still ongoing. Once the cohort and the safety evaluation is complete, the need for any additional cohorts will be further evaluated. Based on the data from the Phase 1B trial and ongoing discussions with the FDA from a successful FDA meeting on December 11, 2023, we have begun Phase 2 trial preparation tasks and will collaborate with the FDA to further define the final design prior to the trial. The trial is planned for 2024, but we will need to obtain additional funding before we can conduct this trial.
| 5 |
Our license agreement with Elion Oncology, Inc. (“Elion”) for NGC-Cap requires us to use commercially reasonable efforts, at our sole cost and expense, to research, develop and commercialize products in one or more countries, including meeting specific diligence milestones that include dosing a first patient with a product in a Phase 2 or 3 clinical trial on or before October 2, 2024. We are currently conducting pre-trial activities and planning to dose the first patient in our Phase 2 trial in the second or third quarter of 2024.
| ● | PCS3117 is a cytidine analog similar to gemcitabine (Gemzar®), but different enough in chemical structure that some patients are more likely to respond to PCS3117 than gemcitabine. The difference in response occurs because NGC-Gem is metabolized to its active metabolite through a different enzyme system than gemcitabine. We continue to evaluate the potential use of NGC-Gem in patients with pancreatic and other potential cancers and to evaluate ways to identify patients who are more likely to respond to NGC-Gem than gemcitabine. We plan to meet with the FDA in 2024 to discuss potential trial designs including implementation of the Project Optimus initiative as part of the design. Similar to NGC-Cap, we will need to obtain additional funding before we can begin the Phase 2 trial for NGC-Gem. |
Our license agreement with Ocuphire Pharma, Inc. (“Ocuphire”) for NGC-Gem requires us to use commercially reasonable efforts, at our sole cost and expense to oversee such commercialization efforts, to research, develop and commercialize products in one or more countries, including meeting specific diligence milestones that consist of: (i) dosing a patient in a clinical trial prior to June 16, 2024; and (ii) dosing a patient in a pivotal clinical trial or in a clinical trial for a second indication of the drug prior to June 16, 2026. We are currently negotiating with Ocuphire to extend these deadlines.
| ● | PCS11T is an analog of SN38 (SN38 is the active metabolite of irinotecan) and should have an improved safety/efficacy profile in every type of cancer that irinotecan is presently used. The manufacturing process and sites for drug substance and drug product are presently being evaluated and IND-enabling toxicology studies will then be initiated. In addition, we are defining the potential paths to approval, which include defining the targeted patient population and the type of cancer. We plan to conduct IND enabling and toxicology studies in 2024, subject to available funding. |
Non-Oncology Pipeline for Out-licensing or Partnership
| ● | PCS12852 is a highly specific and potent 5HT4 agonist that has already been evaluated in clinical studies in South Korea for gastric emptying and gastrointestinal motility in healthy volunteers and volunteers with a history of constipation. In October 2021, the FDA cleared our IND application to proceed with a Phase 2A trial for the treatment of gastroparesis. We enrolled our first patient on April 5, 2022 and completed enrollment of the trial on September 2, 2022. Results from this Phase 2A trial, which included 25 patients with moderate to severe gastroparesis, demonstrated improvements in gastric emptying in patients receiving 0.5 mg of PCS12852 as compared to placebo. The results indicated that for the patients in the PCS12852 group, the mean time for 50% of the gastric contents to empty (t50) compared to their baseline value (±SD) decreased by -31.90 min (±50.53) (compared to the change seen in the placebo group of only -9.36 min (±42.43). Significant gastric emptying differences were not observed between the placebo and the 0.1 mg dose. Adverse events associated with the administration of PCS12852 were generally mild to moderate as expected, limited in duration, and quickly resolved without any sequelae. There were no cardiovascular safety events or serious adverse events reported during the trial. Additionally, the 0.5 mg of PCS12852 showed a greater improvement than placebo in the gastroparesis symptomology scales used in the trial, including both total scores in the scales, as well as sub-scores such as nausea, vomiting and abdominal pain. With the trial now complete, we have the data necessary to finalize the development plan for the treatment of diabetic gastroparesis patients. We are exploring options for PCS12852, which may include licensing, partnering and/or collaborating opportunities. |
| ● | PCS499 is an oral tablet of the deuterated analog of one of the major metabolites of pentoxifylline (PTX or Trental®). PCS499 is a drug that can be used to treat unmet medical need conditions caused by multiple pathophysiological changes. We completed a Phase 2A trial for PCS499 in patients with ulcerative and non-ulcerative necrobiosis lipoidica (uNL and NL, respectively) in late 2020, and in May 2021, we enrolled the first patient in our Phase 2B trial for the treatment of uNL. Although we initiated several recruitment programs to enroll patients in this trial, we were only able to recruit four patients. We experienced extremely slow enrollment in the trial given the extreme rarity of the condition (rarer than reported in the literature), the impact of COVID-19, and the reluctance of patients to be in a clinical trial. We completed the Phase 2B uNL trial for those currently enrolled but halted further efforts to enroll new patients in the trial and have terminated the trial. There were no safety concerns during the conduct of the trial. Although we believe that PCS499 can be effective in treating uNL, preliminary data indicated that the placebo response was likely to be much greater than the literature and clinical experts believe; thus, a much larger sample size would be required in a pivotal trial for an indication where it was extremely difficult to enroll even four patients. We are exploring options for PCS499, which may include licensing, partnering and/or collaborating opportunities. |
| 6 |
Going Concern
This offering is being made on a best-efforts basis and we may sell fewer than all of the securities offered hereby and may receive significantly less in net proceeds from this offering. Assuming that we receive a minimum of $● of proceeds from this offering, we believe that the net proceeds from this offering, together with our cash on hand, will satisfy our capital needs until ● under our current business plan. In late 2024 or early 2025, we will need to raise additional capital to fund our operations and continue our planned development of our NGC drugs.
Compliance With the Nasdaq Capital Market Listing Requirements
Our common stock is currently listed for trading on Nasdaq Capital Market (the “Nasdaq”). On March 16, 2023 we received notice from Nasdaq indicating that we are not in compliance with the requirement to maintain a minimum bid price of $1.00 per share for continued listing on Nasdaq. We were provided a compliance period of 180 calendar days from the date of the notice, or until September 16, 2023, to regain compliance with the minimum closing bid requirement, pursuant to Nasdaq Listing Rule 5810(c)(3)(A). On September 19, 2023, Nasdaq notified us that it granted an extension until March 18, 2024 to regain compliance with the minimum closing bid requirement. If we fail to evidence compliance by March 18, 2024, we may be subject to delisting.
We will continue to monitor the closing bid price of our common stock and may, if appropriate, consider available options, including implementation of a reverse stock split of our common stock, to regain compliance with the minimum closing bid requirement. If we seek to implement a reverse stock split in order to remain listed on Nasdaq, the announcement or implementation of such a reverse stock split could negatively affect the price of our common stock and/or warrants.
We must satisfy Nasdaq’s continued listing requirements or risk delisting, which could have a material adverse effect on our business. If our common stock is delisted from Nasdaq, it could materially reduce the liquidity of our common stock and result in a corresponding material reduction in the price of our common stock as a result of the loss of market efficiencies associated with Nasdaq and the loss of federal preemption of state securities laws. In addition, delisting could harm our ability to raise capital through alternative financing sources on terms acceptable to us, or at all, and may result in the potential loss of confidence by investors, suppliers, customers and employees and fewer business development opportunities. If our common stock is delisted, it could be more difficult to buy or sell our common stock or to obtain accurate quotations, and the price of our common stock could suffer a material decline. Delisting could also impair our ability to raise capital on acceptable terms, if at all.
Implications of Being a Smaller Reporting Company
We are a “smaller reporting company” as defined by Rule 12b-2 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”). We may take advantage of certain of the scaled disclosures available to smaller reporting companies such as including: (i) not being required to comply with the auditor attestation requirements of Section 404(b) of the Sarbanes Oxley Act of 2002, as amended; (ii) scaled executive compensation disclosures; and (iii) the requirement to provide only two years of audited financial statements, instead of three years.
Corporate Information
We were incorporated under the laws of the state of Delaware on March 29, 2011. Our principal executive offices are located at 7380 Coca Cola Drive, Suite 106, Hanover, Maryland 21076, and our telephone number is (443) 776-3133. Our website address is www.processapharmaceuticals.com. The information contained in, or accessible through, our website is not incorporated by reference into this prospectus, and you should not consider any information contained in, or that can be accessed through, our website as part of this prospectus or in deciding whether to purchase our common stock.
Additional Information
For additional information related to our business and operations, please refer to the reports incorporated herein by reference, including our Annual Report on Form 10-K for the year ended December 31, 2022 and our subsequently filed reports on Form 10-Q, as described in the section entitled “Incorporation of Certain Documents by Reference” in this prospectus.
| 7 |
| Issuer | Processa Pharmaceuticals, Inc. | |
| Shares of common stock being offered by us | Up to ● shares of common stock at an assumed combined public offering price of $● per share which is the last reported sales price of our common stock on The Nasdaq Capital Market on ●, 202● and assuming no sale of any Pre-Funded Warrants. | |
| Pre-Funded Warrants offered by us | We are also offering to each purchaser whose purchase of shares of common stock in this offering would otherwise result in the purchaser, together with its affiliates and certain related parties, beneficially owning more than 4.99% (or, at the election of the investor, 9.99%) of our outstanding common stock immediately following the consummation of this offering, the opportunity to purchase, if the purchaser so chooses, Pre-Funded Warrants, in lieu of shares of common stock that would otherwise result in the purchaser’s beneficial ownership exceeding 4.99% (or, at the election of the investor, 9.99%) of our outstanding common stock. Subject to limited exceptions, a holder of Pre-Funded Warrants will not have the right to exercise any portion of its Pre-Funded Warrants if the holder, together with its affiliates, would beneficially own in excess of 4.99% (or, at the election of the holder, 9.99%) of the number of shares of common stock outstanding immediately after giving effect to such exercise. Each Pre-Funded Warrant will be exercisable upon issuance for one share of our common stock and will expire when exercised in full. The purchase price of each Pre-Funded Warrant will equal the public offering price per share of common stock and accompanying Common Warrant less $0.0001, and the exercise price of each Pre-Funded Warrant will be $0.0001 per share. This offering also relates to the shares of common stock issuable upon exercise of any Pre-Funded Warrants sold in this offering. The exercise price and number of shares of common stock issuable upon exercise will be subject to certain further adjustments as described herein. See “Description of Securities” on page 25 of this prospectus.
For each Pre-Funded Warrant we sell, the number of shares of common stock we are offering will be decreased on a one-for-one basis. Because a Common Warrant to purchase one share of our common stock is being sold together in this offering with each share of common stock and, in the alternative, each Pre-Funded Warrant to purchase one share of common stock, the number of Common Warrants sold in this offering will not change as a result of a change in the mix of the shares of our common stock and Pre-Funded Warrant sold. | |
| Common Warrants offered by us | Each share of common stock or pre-funded warrant is being offered together with one Common Warrant to purchase one share of common stock. The Common Warrants will have an exercise price of $● per share (●% of the combined public offering price per share of common stock and accompanying warrants) and will be exercisable beginning on the effective date of the Warrant Stockholder Approval, provided however, if the Pricing Conditions are met, the Common Warrants will be exercisable upon the Initial Exercise Date. The Common Warrants will expire on the five-year anniversary of the Initial Exercise Date. See “Description of Securities We Are Offering” for additional information. | |
| Placement Agent Warrants | We have agreed to issue to the placement agent or its designees, warrants, or the Placement Agent Warrants, to purchase up to 4.0% of the aggregate number of shares of common stock sold in this offering (including the shares of common stock issuable upon the exercise of the Pre-Funded Warrants, but not including shares of common stock sold to the Company’s current directors and officers) at an exercise price equal to 125% of the public offering price per share and accompanying warrants to be sold in this offering. The Placement Agent Warrants will be exercisable upon issuance and will expire three (3) years from the commencement of sales under this offering. See “Plan of Distribution” for additional information |
| 8 |
| Common Stock Outstanding prior to this Offering (1) | 24,631,474 shares | |
| Common Stock to be Outstanding After this Offering (1) | ● shares assuming we sell only shares of common stock and no Pre-Funded Warrants. | |
| Use of Proceeds | We estimate that the net proceeds of this offering assuming no exercise of the warrants, after deducting placement agent fees and estimated offering expenses, will be approximately $●, assuming we sell only shares of common stock and no pre-funded warrants and assuming no exercise of the warrants. We intend to use the net proceeds from the offering to begin a Phase 2 clinical trial of NGC-Cap and for working capital and other general corporate purposes. We may also use a portion of the net proceeds, together with our existing cash and cash equivalents, to in-license, acquire, or invest in complementary businesses, technologies, products or assets; however, we have no current commitments or obligations to do so. Assuming that we receive a minimum of $● of proceeds from this offering, we believe that the net proceeds from this offering, together with our cash on hand, will satisfy our capital needs until ● under our current business plan. In late 2024 or early 2025, we will need to raise additional capital to fund our operations and continue our planned development of our NGC drugs. See the section titled “Use of Proceeds” for more information. | |
| Risk Factors | Investing in our securities involves a high degree of risk. For a discussion of factors to consider before deciding to invest in our securities, you should carefully review and consider the “Risk Factors” section of this prospectus, as well as the risk factors described or referred to in any documents incorporated by reference in this prospectus, and in any applicable prospectus supplement. | |
| Market Symbol and trading | Our common stock is listed on The Nasdaq Capital Market under the symbol “PCSA.” There is no established trading market for any of the warrants being issued and we do not expect a market to develop. In addition, we do not intend to apply for the listing of the Common Warrants or Pre-Funded Warrants on any national securities exchange or other trading market. Without an active trading market, the liquidity of the Common Warrants and Pre-Funded Warrants will be limited. |
| (1) | The number of shares of our common stock to be outstanding immediately after this offering as shown above is based on 24,631,474 shares of common stock outstanding as of September 30, 2023. The number of shares outstanding used throughout this prospectus, unless otherwise indicated, excludes: |
| ● | 141,611 shares of our common stock issuable upon exercise of outstanding options, which have a weighted average exercise price of $18.22 per share; | |
| ● | 4,554,867 shares of common stock issuable for restricted stock units (RSUs) (of which 2,291,923 are vested) issuable upon meeting distribution restrictions; | |
| ● | 3,366,480 shares of common stock issuable upon exercise of outstanding vested common warrants at a weighted-average exercise price of $1.61 per share; | |
| ● | 467,735 shares of common stock reserved for issuance and available for future grant under our 2019 Omnibus Incentive Plan; and |
Unless otherwise indicated or the context requires otherwise, all information in this prospectus assumes (i) we issue no Pre-Funded Warrants and (ii) no exercise of the Common Warrants offered hereby.
| 9 |
An investment in our securities involves a high degree of risk. Before deciding whether to purchase our securities, including the shares of common stock offered by this prospectus, you should carefully consider the risks and uncertainties described under “Risk Factors” in our Annual Report on Form 10-K for the fiscal year ended December 31, 2022, any subsequent Quarterly Report on Form 10-Q and our other filings with the SEC, all of which are incorporated by reference herein. If any of these risks actually occur, our business, financial condition and results of operations could be materially and adversely affected and we may not be able to achieve our goals, the value of our securities could decline and you could lose some or all of your investment. Additional risks not presently known to us or that we currently believe are immaterial may also significantly impair our business operations. If any of these risks occur, our business, results of operations or financial condition and prospects could be harmed. In that event, the market price of our common stock and the value of the warrants could decline, and you could lose all or part of your investment.
Risks Related to Our Financial Position and Need for Capital
We need to raise additional capital to fund our operations.
We have incurred recurring losses since inception and had an accumulated deficit of approximately $73.0 million as of September 30, 2023. At November 30, 2023, we had cash and cash equivalents totaling $5.4 million and a prepaid expense with the clinical research organization of our Phase 1B trial of $660,000. Assuming that we receive a minimum of $● of proceeds from this offering, we believe that the net proceeds from this offering, together with our cash on hand, will allow us to begin our Phase 2 trial of NGC-Cap and satisfy our capital needs until ● under our current business plan. In late 2024 or early 2025, we will need to raise additional capital to fund our operations and continue our planned development of our NGC drugs.
Following this offering, we will need to raise additional capital to complete the development efforts for NGC-Cap, NGC-Gem and/or NGC-Iri. If we are unable to raise capital when needed, we could be forced to delay, reduce or terminate certain of our development programs or other operations.
Following this offering, we will need to raise additional capital to fund our operations and continue to support our planned development of our next generation chemotherapy drugs. Our estimates of the amount of cash necessary to fund our activities may prove to be wrong and we could spend our available financial resources much faster than we currently expect. Our future funding requirements will depend on many factors, including, but not limited to:
| ● | the timing, rate of progress and cost of any clinical trials and other manufacturing/product development activities for our current and any future product candidates that we develop, in-license or acquire; | |
| ● | the results of the clinical trials for our product candidates; | |
| ● | the timing of, and the costs involved in, FDA approval and any foreign regulatory approval of our product candidates, if at all; | |
| ● | the number and characteristics of any additional future product candidates we develop or acquire; | |
| ● | our ability to establish and maintain strategic collaborations, licensing, co-promotion or other arrangements and the terms and timing of such arrangements; | |
| ● | the degree and rate of market acceptance of any approved products; | |
| ● | costs under our third-party manufacturing and supply arrangements for our current and any future product candidates and any products we commercialize; | |
| ● | costs and timing of completion of any additional outsourced commercial manufacturing or supply arrangements that we may establish; | |
| ● | costs of preparing, filing, prosecuting, maintaining, defending and enforcing any patent claims and other intellectual property rights associated with our product candidates; | |
| ● | costs associated with prosecuting or defending any litigation that we are or may become involved in and any damages payable by us that result from such litigation; | |
| ● | costs of operating as a public company; | |
| ● | the emergence, approval, availability, perceived advantages, relative cost, relative safety and relative efficacy of alternative and competing products or treatments; | |
| ● | costs associated with any acquisition or in-license of products and product candidates, technologies or businesses; and | |
| ● | personnel, facilities and equipment requirements. |
| 10 |
We cannot be certain that additional funding will be available on acceptable terms, or at all. In addition, future debt financing into which we may enter may impose upon us covenants that restrict our operations, including limitations on our ability to incur liens or additional debt, pay dividends, redeem our stock, make certain investments and engage in certain merger, consolidation or asset sale transactions.
If we are unable to raise additional capital when required or on acceptable terms, we may be required to significantly delay, scale back or discontinue the development of our product candidates, restrict our operations or obtain funds by entering into agreements on unattractive terms, which would likely have a material adverse effect on our business, stock price and our relationships with third parties with whom we have business relationships, at least until additional funding is obtained. If we do not have sufficient funds to continue operations, we could be required to seek bankruptcy protection or other alternatives that would likely result in our security holders losing some or all of their investment in us. In addition, our ability to achieve profitability or to respond to competitive pressures would be significantly limited.
In addition, if we are unable to secure sufficient capital to fund our operations, we may have to enter into strategic collaborations that could require us to share license rights with third parties in ways that we currently do not intend or on terms that may not be favorable to us or our security holders.
We have incurred a history of operating losses and expect to continue to incur substantial costs for the foreseeable future. We are not currently profitable, and we may never achieve or sustain profitability. Our financial situation creates doubt whether we will continue as a going concern.
We have incurred recurring losses since inception and had an accumulated deficit of approximately $73.0 million as of September 30, 2023. We expect continued operating losses and negative cash flow from operations for the foreseeable future. We have never generated revenue from operations, nor do we have any revenue under contract or any immediate sales prospects. We may never be able to obtain regulatory approval for the marketing of our drug candidates in any indication in the United States or internationally. Even if we obtain regulatory approval for any drug candidates, development expenses will continue to increase. These conditions raise substantial doubt about our ability to continue as a going concern, meaning that we may be unable to continue operations for the foreseeable future or realize assets and discharge liabilities in the ordinary course of operations. If we are unable to obtain funding, we will be forced to delay, reduce or eliminate some or all of our research and development programs, or we may be unable to continue operations. Although we continue to pursue these plans, there can be no assurance that we will be successful in obtaining sufficient funding on terms acceptable to us to fund continuing operations, if at all.
We will continue to expend substantial cash resources for the foreseeable future for the clinical development of our product candidates and development of any other indications and product candidates we may choose to pursue. These expenditures will include costs associated with manufacturing and clinical development, such as conducting clinical trials, manufacturing operations and product candidate supply. Because the conduct and results of any clinical trial are highly uncertain, we cannot reasonably estimate the actual amounts necessary to successfully complete the development of our current and any future product candidates.
This offering is being made on a best efforts basis and we may sell fewer than all of the securities offered hereby and may receive significantly less in net proceeds from this offering. We believe that the net proceeds from this offering, together with our cash on hand, will satisfy our capital needs into ● under our current business plan. Following this offering, we will need to raise additional capital to fund our operations and continue to support our planned development and commercialization activities.
| 11 |
Risks Related to This Offering and Ownership of Our Common Stock
Because management has broad discretion as to the use of the net proceeds from this offering, you may not agree with how we use them, and such proceeds may not be applied successfully.
Our management will have considerable discretion over the use of proceeds from this offering. We currently intend to use the net proceeds from this offering for continued research and development for NCG-Cap, and for working capital, capital expenditures, and general corporate purposes, including investing further in research and development efforts. However, our management will have broad discretion in the application of the net proceeds from this offering and could spend the proceeds in ways that do not necessarily improve our operating results or enhance the value of our securities, or that you otherwise do not agree with. You will be relying on the judgment of our management concerning these uses and you will not have the opportunity, as part of your investment decision, to assess whether the proceeds are being used appropriately. The failure of our management to apply these funds effectively could, among other things, result in unfavorable returns and uncertainty about our prospects, each of which could cause the price of our securities to decline.
Pending their use, we may invest the net proceeds from this offering in short- and intermediate-term, interest-bearing obligations, investment-grade instruments, certificates of deposit or direct or guaranteed obligations of the U.S. government. These investments may not yield a favorable return to our stockholders. If we do not invest or apply the net proceeds from this offering in ways that enhance stockholder value, we may fail to achieve expected financial results, which could cause our stock price to decline.
If you purchase our securities sold in this offering, you will experience immediate and substantial dilution in the net tangible book value of your shares. In addition, we may issue additional equity or convertible debt securities in the future, which may result in additional dilution to investors.
Based on an assumed public offering price of $● per share and accompanying Common Warrant, the last reported sale price of our common stock on The Nasdaq Capital Market on ●, 202●, and our as adjusted net tangible book value per share as of September 30, 2023, if you purchase securities in this offering, you will experience an increase of $● per share in the net tangible book value of the common stock you purchase representing the difference between our as adjusted net tangible book value per share after giving effect to this offering and the assumed public offering price per share of common stock. The exercise of outstanding stock options and warrants, including those sold in this offering, will, however, result in dilution of your investment. In addition, to the extent we need to raise additional capital in the future and we issue additional shares of common stock or securities exercisable, convertible or exchangeable for our common stock, our then existing stockholders may experience dilution and the new securities may have rights senior to those of our common stock offered in this offering. See the section titled “Dilution” below for a more detailed illustration of the dilution you would incur if you participate in this offering.
There is no public market for the Pre-Funded Warrants or Common Warrants offered by us.
There is no established public trading market for the Pre-Funded Warrants or Common Warrants being offered in this offering, and we do not expect such a market to develop. In addition, we do not intend to apply to list the Pre-Funded Warrants or Common Warrants on any national securities exchange or other nationally recognized trading system. Without an active trading market, the liquidity of the Pre-Funded Warrants and Common Warrants will be limited.
Holders of Pre-Funded Warrants and Common Warrants purchased in this offering will have no rights as common stockholders until such holders exercise their Pre-Funded Warrants or Common Warrants and acquire our common stock.
Until holders of Pre-Funded Warrants or Common Warrants acquire shares of our common stock upon exercise of such warrants, holders of Pre-Funded Warrants and Common Warrants will have no rights with respect to the shares of our common stock underlying such Pre-Funded Warrants and Common Warrants. Upon exercise of the Pre-Funded Warrants and Common Warrants, the holders will be entitled to exercise the rights of a common stockholder only as to matters for which the record date occurs after the exercise date.
| 12 |
The Common Warrants are speculative in nature.
The Common Warrants do not confer any rights of common stock ownership on their holders, such as voting rights or the right to receive dividends, but rather merely represent the right to acquire shares of common stock at a fixed price for a limited period of time. Moreover, following this offering, the market value of the Common Warrants, if any, will be uncertain and there can be no assurance that the market value of the Common Warrants will equal or exceed their imputed offering price. The Common Warrants will not be listed or quoted for trading on any market or exchange. There can be no assurance that the market price of our common stock will ever equal or exceed the exercise price of the Common Warrants, and consequently, the Common Warrants may expire valueless.
The Common Warrants being offered may not have value.
The Common Warrants being offered by us in this offering have an exercise price of $● per share, subject to certain adjustments, and expire on the five-year anniversary of the Initial Exercise Date, upon which date such Common Warrants will expire and have no further value. In the event that the market price of our common stock does not exceed the exercise price of the Common Warrants during the period when they are exercisable, the Common Warrants may not have any value.
Purchasers who purchase our securities in this offering pursuant to a securities purchase agreement may have rights not available to purchasers that purchase without the benefit of a securities purchase agreement.
In addition to rights and remedies available to all purchasers in this offering under federal securities and state law, the purchasers that enter into a securities purchase agreement will also be able to bring claims of breach of contract against us. The ability to pursue a claim for breach of contract provides those investors with the means to enforce the covenants uniquely available to them under the securities purchase agreement including: (i) timely delivery of shares; (ii) agreement to not enter into variable rate financings for one year from closing, subject to certain exceptions; (iii) agreement to not enter into any financings for 60 days from closing; and (iv) indemnification for breach of contract.
This is a best efforts offering, with no minimum amount of securities required to be sold, and we may not raise the amount of capital we believe is required for our business plans, including our near-term business plans.
The placement agent has agreed to use its reasonable best efforts to solicit offers to purchase the securities in this offering. The placement agent has no obligation to buy any of the securities from us or to arrange for the purchase or sale of any specific number or dollar amount of the securities. There is no required minimum number of securities that must be sold as a condition to completion of this offering. Because there is no minimum offering amount required as a condition to the closing of this offering, the actual offering amount, placement agent fees and proceeds to us are not presently determinable and may be substantially less than the maximum amounts set forth above. We may sell fewer than all of the securities offered hereby, which may significantly reduce the amount of proceeds received by us, and investors in this offering will not receive a refund in the event that we do not sell a sufficient number of securities to support our continued operations, including our near-term continued operations. Thus, we may not raise the amount of capital we believe is required for our operations in the short term and may need to raise additional funds, which may not be available or available on terms acceptable to us.
Because there is no minimum required for the offering to close, investors in this offering will not receive a refund in the event that we do not sell a sufficient number of securities to pursue the business goals outlined in this prospectus.
We have not specified a minimum offering amount nor have or will we establish an escrow account in connection with this offering. Because there is no escrow account and no minimum offering amount, investors could be in a position where they have invested in our company, but we are unable to fulfill our objectives due to a lack of interest in this offering. Further, because there is no escrow account in operation and no minimum investment amount, any proceeds from the sale of securities offered by us will be available for our immediate use, despite uncertainty about whether we would be able to use such funds to effectively implement our business plan. Investor funds will not be returned under any circumstances whether during or after the offering.
| 13 |
If securities or industry analysts do not publish research or publish inaccurate or unfavorable research about our business, our stock price and trading volume could decline.
The trading market for our common stock and warrants will depend in part on the research and reports that securities or industry analysts publish about us or our business. We currently have limited research coverage by securities and industry analysts. If we fail to maintain adequate coverage by securities or industry analysts, the trading price for our stock would be negatively impacted. If one or more of the analysts who cover us downgrades our stock or publishes inaccurate or unfavorable research about our business, our stock price would likely decline. If one or more of these analysts ceases coverage of us or fails to publish reports on us regularly, demand for our stock could decrease, which could cause our stock price and trading volume to decline.
Future sales of our common stock, warrants, or securities convertible into our common stock may depress our stock price.
The price of our common stock or warrants could decline as a result of sales of a large number of shares of our common stock or warrants or the perception that these sales could occur. These sales, or the possibility that these sales may occur, also might make it more difficult for us to sell equity securities in the future at a time and at a price that we deem appropriate.
In addition, in the future, we may issue additional shares of common stock, warrants or other equity or debt securities convertible into common stock in connection with a financing, acquisition, litigation settlement, employee arrangements or otherwise. We may also issue additional shares of common stock to satisfy the exercise of outstanding warrants. Any such issuances could result in substantial dilution to our existing stockholders and could cause the price of our common stock to decline.
We do not anticipate paying any cash dividends on our common stock in the foreseeable future.
We do not anticipate declaring or paying any cash dividends on our common stock in the foreseeable future. We currently intend to retain any future earnings to finance the operation and expansion of our business. Consequently, stockholders must rely on sales of their common stock after price appreciation, which may never occur, as the only way to realize any future gains on their investment. There is no guarantee that shares of our common stock will appreciate in value or even maintain the price at which stockholders have purchased their shares.
If we fail to regain compliance with the continued listing requirements of Nasdaq, our common stock and/or warrants may be delisted and the price of our common stock and/or warrants and our ability to access the capital markets could be negatively impacted.
On March 16, 2023 we received notice from Nasdaq indicating that we are not in compliance with the requirement to maintain a minimum bid price of $1.00 per share for continued listing on Nasdaq. We were provided a compliance period of 180 calendar days from the date of the notice, or until September 16, 2023, to regain compliance with the minimum closing bid requirement, pursuant to Nasdaq Listing Rule 5810(c)(3)(A). On September 19, 2023, Nasdaq notified us that that it granted an extension until March 18, 2024 to regain compliance with the minimum closing bid requirement. If we fail to evidence compliance by March 18, 2024, we may be subject to delisting. If Nasdaq determines to delist our securities, we will have the right to appeal to a Nasdaq hearings panel. There can be no assurance that we will be able to regain compliance with the applicable Nasdaq listing requirements.
We will continue to monitor the closing bid price of our common stock and may, if appropriate, consider available options, including implementation of a reverse stock split of our common stock, to regain compliance with the minimum closing bid requirement. If we seek to implement a reverse stock split in order to remain listed on Nasdaq, the announcement or implementation of such a reverse stock split could negatively affect the price of our common stock and/or warrants.
We must satisfy Nasdaq’s continued listing requirements, including, among other things, a minimum stockholders’ equity of $2.5 million and a minimum closing bid price of $1.00 per share or risk delisting, which could have a material adverse effect on our business. If our common stock and warrants are delisted from Nasdaq, it could materially reduce the liquidity of our common stock and warrants and result in a corresponding material reduction in the price of our common stock and warrants as a result of the loss of market efficiencies associated with Nasdaq and the loss of federal preemption of state securities laws. In addition, delisting could harm our ability to raise capital through alternative financing sources on terms acceptable to us, or at all, and may result in the potential loss of confidence by investors, suppliers, customers and employees and fewer business development opportunities. If our common stock and warrants are delisted, it could be more difficult to buy or sell our common stock and warrants or to obtain accurate quotations, and the price of our common stock and warrants could suffer a material decline. Delisting could also impair our ability to raise capital on acceptable terms, if at all.
| 14 |
The sale of our common stock in this offering, including any shares issuable upon exercise of any Pre-Funded Warrants or Common Warrants, and any future sales of our common stock, or the perception that such sales could occur, may depress our stock price and our ability to raise funds in new stock offerings.
We may from time-to-time issue additional shares of common stock at a discount from the current trading price of our common stock. As a result, our stockholders would experience immediate dilution upon the purchase of any shares of our common stock sold at such a discount. In addition, as opportunities present themselves, we may enter into financing or similar arrangements in the future, including the issuance of debt securities, preferred stock or common stock. Sales of shares of our common stock in this offering, including any shares issuable upon exercise of any Pre-Funded Warrants or Common Warrants issued in this offering and in the public market following this offering, or the perception that such sales could occur, may lower the market price of our common stock and may make it more difficult for us to sell equity securities or equity-related securities in the future at a time and price that our management deems acceptable, or at all.
Significant holders or beneficial holders of our common stock may not be permitted to exercise Pre-Funded Warrants that they hold.
A holder of a Pre-Funded Warrant will not be entitled to exercise any portion of any Pre-Funded Warrants which, upon giving effect to such exercise, would cause the aggregate number of shares of our common stock beneficially owned by the holder (together with its affiliates) to exceed 4.99% (or, at the election of the purchaser, 9.99%) of the number of shares of our common stock outstanding immediately after giving effect to the exercise. Such percentage may be increased or decreased by written notice by the holder of the Pre-Funded Warrants to any other percentage not in excess of 9.99%. Such increase or decrease will not be effective until the sixty-first (61st) day after such notice is delivered to us. As a result, you may not be able to exercise your Pre-Funded Warrants for shares of our common stock at a time when it would be financially beneficial for you to do so. In such circumstances, you could seek to sell your Pre-Funded Warrants to realize value, but you may be unable to do so in the absence of an established trading market for the Pre-Funded Warrants.
| 15 |
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus and the documents incorporated by reference herein contain forward-looking statements. All statements other than statements of historical facts contained in this prospectus and the documents incorporated by reference herein are forward-looking statements, including statements regarding our future results of operations and financial position, business strategy, regulatory developments, research and development costs, the timing and likelihood of commercial success, the potential to develop future product candidates, plans and objectives of management for future operations, and future results of current and anticipated products. These statements involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. This prospectus and the documents incorporated by reference herein also contain estimates and other statistical data made by independent parties and by us relating to market size and growth and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. In addition, projections, assumptions and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk.
In some cases, you can identify forward-looking statements by terms such as “may,” “will,” “should,” “expect,” “plan,” “anticipate,” “could,” “intend,” “target,” “project,” “contemplates,” “believes,” “estimates,” “predicts,” “potential” or “continue” or the negative of these terms or other similar expressions. The forward-looking statements in this prospectus and the documents incorporated by reference herein are only predictions. We have based these forward-looking statements largely on our current expectations and projections about future events and financial trends that we believe may affect our business, financial condition and results of operations. These forward-looking statements speak only as of the date of this prospectus and are subject to a number of risks, uncertainties and assumptions, which we discuss in greater detail in the documents incorporated by reference herein, including under the heading “Risk Factors” and elsewhere in this prospectus. The events and circumstances reflected in our forward-looking statements may not be achieved or occur and actual results could differ materially from those projected in the forward-looking statements. Moreover, we operate in an evolving environment. New risk factors and uncertainties may emerge from time to time, and it is not possible for management to predict all risk factors and uncertainties. Given these risks and uncertainties, you should not place undue reliance on these forward-looking statements. Except as required by applicable law, we do not plan to publicly update or revise any forward-looking statements contained in this prospectus or the documents incorporated by reference herein, whether as a result of any new information, future events, changed circumstances or otherwise. For all forward-looking statements, we claim the protection of the safe harbor for forward-looking statements contained in the Private Securities Litigation Reform Act of 1995.
You should read this prospectus and the documents that we reference in this prospectus and have filed with the SEC as exhibits to the registration statement of which this prospectus is a part with the understanding that our actual future results, levels of activity, performance, and events and circumstances may be materially different from what we expect.
| 16 |
We estimate that the net proceeds from this offering will be approximately $●, based on an assumed public offering price of $● per share of common stock, the last reported sale price of our common stock on the Nasdaq Capital Market on ●, 202●, and assuming the sale of ● Common Warrants and no sale of any Pre-Funded Warrants in this offering after deducting the placement agent fees and estimated offering expenses payable by us. However, because this is a best efforts offering with no minimum number of securities or amount of proceeds as a condition to closing, the actual offering amount, the placement agent’s fees and net proceeds to us are not presently determinable and may be substantially less than the maximum amounts set forth on the cover page of this prospectus, and we may not sell all or any of the securities we are offering. As a result, we may receive significantly less in net proceeds.
We currently intend to use the net proceeds from this offering, together with our existing cash and cash equivalents, for continued research and development for NCG-Cap, and working capital and general corporate purposes. We may also use a portion of the net proceeds, together with our existing cash and cash equivalents, to in-license, acquire, or invest in complementary businesses, technologies, products or assets; however, we have no current commitments or obligations to do so.
We believe, based on our current operating plan, that our existing cash and cash equivalents together with the net proceeds from this offering and assuming no sale of any pre-funded warrants and no exercise of the warrants, will be sufficient to fund our operations into ●. However, the amounts and timing of our actual expenditures will depend on numerous factors, including the cost and timing to complete our Phase 1B trial; costs associated with the first part of our planned Phase 2 trial for NGC-Cap; any costs we incur related to NGC-Gem and NGC-Iri; for general and administrative costs to support operations; and other factors as described under “Risk Factors” in this prospectus and in the documents incorporated by reference herein. We therefore cannot estimate with certainty the amount of net proceeds to be used for the purposes described above. We may find it necessary or advisable to use the net proceeds for other purposes, and we will have broad discretion in the application of the net proceed and it may be necessary to reallocate funds. Accordingly, our management will have flexibility in applying the net proceeds from this offering. An investor will not have the opportunity to evaluate the economic, financial or other information on which we base our decisions on how to use the proceeds.
If we have based our estimates on assumptions that are incorrect, or we increase our anticipated clinical trials, then we could use our available capital resources sooner than we currently expect. We may satisfy our future cash needs through the sale of equity securities, debt financings, working capital lines of credit, corporate collaborations or license agreements, grant funding, interest income earned on invested cash balances or a combination of one or more of these sources.
Pending our use of the net proceeds from this offering, we intend to invest the net proceeds in a variety of capital preservation investments, including short-term, investment-grade, interest-bearing instruments and U.S. government securities.
Each $● increase (decrease) in the assumed public offering price of $● per share, the last reported sale price of our common stock on the Nasdaq Capital Market on ●, 202●, and accompanying Common Warrant, would increase (decrease) the net proceeds to us by approximately $● million, assuming that the number of shares of common stock, Common Warrants and Pre-Funded Warrants offered by us, as set forth on the cover page of this prospectus, remains the same, and after deducting the estimated placement agent discounts and commissions and estimated offering expenses payable by us. We may also increase or decrease the number of shares we are offering. Each increase (decrease) of ● shares in the number of shares of common stock (or common stock underlying Pre-Funded Warrants) and accompanying Common Warrants offered by us, as set forth on the cover page of this prospectus, would increase (decrease) the net proceeds to us by approximately $● million, assuming the assumed public offering price per share of common stock and accompanying Common Warrant remains the same, and after deducting the estimated placement agent discounts and commissions and estimated offering expenses payable by us.
| 17 |
We have never declared or paid any cash dividends on our capital stock. We intend to retain future earnings, if any, to finance the operation of our business and do not anticipate paying any cash dividends in the foreseeable future. Any future determination related to our dividend policy will be made at the discretion of our Board of Directors after considering our financial condition, results of operations, capital requirements, business prospects and other factors our Board of Directors deems relevant, and subject to the restrictions contained in any future financing instruments.
| 18 |
The following table sets forth our cash and cash equivalents and capitalization as of September 30, 2023 as follows:
| ● | on an actual basis; and | |
| ● | on as adjusted basis to give effect to the issuance by us of ● shares of our common stock in this offering at an assumed public offering price of $● per share, based on the last reported sale price of our common stock on the Nasdaq Capital Market on ●, 202●, assuming no sale of any Pre-Funded Warrants in this offering, after deducting the placement agent fees and estimated offering expenses payable by us. |
You should read this information in conjunction with our consolidated financial statements and notes thereto incorporated by reference into this prospectus.
| As of September 30, 2023 | ||||||||
| Actual | As adjusted | |||||||
| Cash and cash equivalents | $ | 6,860,672 | $ | |||||
| Preferred stock, $0.0001 par value: 1,000,000 shares authorized, no shares issued or outstanding | - | - | ||||||
| Common stock $0.0001 par value: 100,000,000 shares authorized, 24,731,474 issued and 24,631,474 outstanding, actual; ● shares issued and outstanding, as adjusted(1) | 2,473 | |||||||
| Additional paid-in capital | 80,429,556 | |||||||
| Treasury stock at cost – 100,000 shares | (300,000 | ) | (300,000 | ) | ||||
| Accumulated equity | (72,964,150 | ) | ||||||
| Total stockholders’ equity | 7,167,879 | |||||||
| Total capitalization | $ | $ | ||||||
(1) The foregoing tables and calculations (other than the historical net tangible book value calculation) are based on 24,631,474 shares of common stock outstanding as of September 30, 2023, and exclude:
| ● | 141,611 shares of our common stock issuable upon exercise of outstanding options, which have a weighted average exercise price of $18.22 per share; | |
| ● | 4,554,867 shares of common stock issuable for restricted stock units (RSUs) (of which 2,291,923 are vested) issuable upon meeting distribution restrictions; | |
| ● | 3,366,480 shares of common stock issuable upon exercise of vested outstanding common warrants at a weighted-average exercise price of $1.61 per share; | |
| ● | 467,735 shares of common stock reserved for issuance and available for future grant under our 2019 Omnibus Incentive Plan; and | |
| ● | the exercise of the Pre-Funded Warrants and Common Warrants issued in this offering. |
In addition, to the extent that any outstanding options, RSUs, or warrants described above are exercised, new options are issued, or we issue additional shares of common stock or other equity or convertible debt securities in the future, there will be further dilution to investors participating in this offering.
| 19 |
If you invest in our securities in this offering, your ownership interest will be immediately diluted to the extent of the difference between the combined public offering price per share and accompanying Common Warrant and the as adjusted net tangible book value per share of our common stock immediately after this offering, assuming no value is attributed to the warrants.
Historical net tangible book value (deficit) per share is determined by dividing our total tangible assets less our total liabilities by the total number of shares of common stock outstanding. Our historical net tangible book value (deficit) as of September 30, 2023 was approximately ($7.2) million, or ($0.27) per share, based on 26,848,397 shares of common stock outstanding (which includes vested but unissued RSUs and excludes unvested RSAs) as of that date.
After giving effect to the sale of shares of common stock and accompanying Common Warrants in this offering, at an assumed sale by us of ● shares in this offering at an assumed public offering price of $● per share, based on the last reported sale price of our common stock on the Nasdaq Capital Market on ●, 202●, assuming no sale of any Pre-Funded Warrants in this offering, after deducting the placement agent fees and estimated offering expenses payable by us, our as adjusted net tangible book value as of September 30, 2023 would have been approximately $● million, or $● per share, which excludes the Common Warrants to purchase shares of our common stock to be issued to investors in this offering.. This represents an immediate increase in net tangible book value of $● per share to our existing stockholders and an immediate dilution of $● per share to new investors purchasing shares of our securities in this offering.
The following table illustrates this dilution to new investors on a per share basis:
| Assumed combined public offering price per share and accompanying Common Warrant | $ | |||||||
| Historical net tangible book value (deficit) per share as of September 30, 2023 | $ | (0.27 | ) | |||||
| Increase in net tangible book value per share attributable to new investors participating in this offering | ||||||||
| As adjusted net tangible book value per share after this offering | ||||||||
| Dilution in as adjusted net tangible book value per share to new investors participating in this offering | $ |
This table does not take into account further dilution to new investors that could occur upon the exercise of the warrants offered hereby or outstanding options and warrants having a per share exercise price less than the public offering price per share in this offering. To the extent that outstanding options or warrants are exercised, or restricted stock units vest and settle, investors purchasing our common stock will experience further dilution. In addition, we may choose to raise additional capital due to market conditions or strategic considerations even if we believe we have sufficient funds for our current or future operating plans. To the extent that additional capital is raised through the sale of equity or convertible debt securities, the issuance of these securities could result in further dilution to our stockholders.
The foregoing tables and calculations (other than the historical net tangible book value calculation) are based on 24,631,474 shares of common stock outstanding as of September 30, 2023, and exclude:
| ● | 141,611 shares of our common stock issuable upon exercise of outstanding options, which have a weighted average exercise price of $18.22 per share; | |
| ● | 4,554,867 shares of common stock issuable for restricted stock units (RSUs) (of which 2,291,923 are vested) issuable upon meeting distribution restrictions; | |
| ● | 3,366,480 shares of common stock issuable upon exercise of vested outstanding common warrants at a weighted-average exercise price of $1.61 per share; | |
| ● | 467,735 shares of common stock reserved for issuance and available for future grant under our 2019 Omnibus Incentive Plan; and | |
| ● | the exercise of the Pre-Funded Warrants and Common Warrants issued in this offering. |
| 20 |
The following description of the material terms of our capital stock and the provisions of our amended and restated certificate of incorporation and amended and restated bylaws are summaries and are qualified by reference to copies of the amended and restated certificate of incorporation and bylaws, which are filed with the SEC as exhibits to our registration statement of which this prospectus forms a part.
We have the authority to issue an aggregate of 100,000,000 shares of $0.0001 par value common stock and 1,000,000 shares of $0.0001 par value preferred stock. As of December ●, 2023, there were 24,631,474 shares of common stock outstanding and no shares of preferred stock outstanding.
Common Stock
Dividend Rights. Subject to the rights of holders of preferred stock of any series that may be issued and outstanding from time to time, holders of our common stock are entitled to receive such dividends and other distributions as may be declared by our Board of Directors from time to time.
Voting Rights. Each outstanding share of our common stock is entitled to one vote on all matters submitted to a vote of stockholders generally. In the event we issue one or more series of preferred or other securities in the future such preferred stock or other securities may be given rights to vote, either together with the common stock or as a separate class on one or more types of matters. The holders of our common stock do not have cumulative voting rights.
Liquidation Rights. In the event of any liquidation, dissolution or winding up of the Company, the holders of our common stock will be entitled, subject to any preferential or other rights of any then outstanding preferred stock, to receive all assets of the Company available for distribution to stockholders.
Preemptive Rights. As of the date hereof, the holders of our common stock have no preemptive rights in their capacities as such holders.
Board of Directors. Holders of common stock do not have cumulative voting rights with respect to the election of directors. At any meeting to elect directors by holders of our common stock, the presence, in person or by proxy, of the holders of a majority of the voting power of shares of our capital stock then outstanding will constitute a quorum for such election. Directors may be elected by a plurality of the votes of the shares present and entitled to vote on the election of directors, except for directors whom the holders of any then outstanding preferred stock have the right to elect, if any.
Preferred Stock
Our Board is authorized, subject to certain limitations prescribed by law, without further stockholder approval, to issue from time to time up to an aggregate of 1,000,000 shares of preferred stock in one or more series and to fix or alter the designations, preferences, rights and any qualifications, limitations or restrictions of the shares of each such series thereof, including the dividend rights, dividend rates, conversion rights, voting rights and terms of redemption of shares constituting any series or designations of such series. The rights of holders of our common stock may be subject to, and adversely affected by, the rights of the holders of any preferred stock that may be issued in the future. The issuance of preferred stock may have the effect of delaying, deferring or preventing a change of control and may adversely affect the voting and other rights of holders of our common stock.
Indemnification of Directors and Officers
Our amended and restated certificate of incorporation provides that, to the fullest extent permitted by the Delaware General Corporate Law (“DGCL”) as it may hereafter be amended, none of our directors will be personally liable to us or our stockholders for monetary damages for breach of fiduciary duty as a director. Under the DGCL as it now reads, such limitation of liability is not permitted:
| ● | for any breach of the director’s duty of loyalty to us or our stockholders; |
| 21 |
| ● | for acts or omissions not in good faith or that involve intentional misconduct or a knowing violation of law; | |
| ● | for payments of unlawful dividends or unlawful stock purchases or redemptions under Section 174 of the DGCL; or | |
| ● | for any transaction from which the director derived an improper personal benefit. |
These provisions will have no effect on the availability of equitable remedies such as an injunction or rescission based on a director’s breach of his or her duty of care.
Our amended and restated certificate of incorporation and our amended and restated bylaws include provisions that require us to indemnify and advance expenses, to the fullest extent allowable under the DGCL as it now exists or may hereafter be amended, to our directors or officers for actions taken as a director or officer of us, or for serving at our request as a director or officer at another corporation or enterprise, as the case may be.
Section 145 of the DGCL provides that a corporation may indemnify directors and officers, as well as other employees and individuals, against expenses, including attorneys’ fees, judgments, fines and amounts paid in settlement, that are incurred in connection with various actions, suits or proceedings, whether civil, criminal, administrative or investigative, other than an action by or in the right of the corporation, known as a derivative action, if they acted in good faith and in a manner they reasonably believed to be in or not opposed to the best interests of the corporation, and, with respect to any criminal action or proceeding, if they had no reasonable cause to believe their conduct was unlawful. A similar standard is applicable in the case of derivative actions, except that indemnification only extends to expenses, including attorneys’ fees, incurred in connection with the defense or settlement of such actions, and the statute requires court approval before there can be any indemnification if the person seeking indemnification has been found liable to the corporation. The statute provides that it is not exclusive of other indemnification that may be granted by a corporation’s bylaws, disinterested director vote, stockholder vote, agreement or otherwise.
Our amended and restated bylaws require us to indemnify any person who was or is a party or is threatened to be made a party to, or was otherwise involved in, a legal proceeding by reason of the fact that he or she is or was a director or officer of the Company or is or was serving at our request as a director or officer of another corporation or enterprise, as the case may be, to the fullest extent authorized by the DGCL as it now exists or may hereafter be amended, against all expense, liability and loss (including attorneys’ fees, judgments, fines, Employee Retirement Income Security Act excise taxes or penalties and amounts paid in settlement) reasonably incurred or suffered by such director or officer in connection with such service. The right to indemnification in our amended and restated bylaws includes the right to be paid by the Company the expenses incurred in defending any proceeding for which indemnification may be sought in advance of the final disposition of such proceeding, subject to certain limitations. We carry directors’ and officers’ insurance protecting us, any director, officer, employee or agent of ours or who was serving at the request of the Company as a director, officer, employee or agent of another corporation or enterprise, as the case may be, against any expense, liability or loss, whether or not we would have the power to indemnify the person under the DGCL.
The limitation of liability and indemnification and advancement provisions in our amended and restated certificate of incorporation and our amended and restated bylaws may discourage stockholders from bringing a lawsuit against our directors for breach of fiduciary duty. These provisions also may reduce the likelihood of derivative litigation against our directors and officers, even though such an action, if successful, might otherwise benefit us and our stockholders. In addition, your investment in our common stock may be adversely affected to the extent we pay the costs of settlement and damage awards under these indemnification provisions.
Certain Anti-Takeover Effects
Provisions of Delaware Law. We are a Delaware corporation and Section 203 of the DGCL applies to us. It is an anti-takeover statute that is designed to protect stockholders against coercive, unfair or inadequate tender offers and other abusive tactics and to encourage any person contemplating a business combination with us to negotiate with our Board of Directors for the fair and equitable treatment of all stockholders.
| 22 |
Under Section 203 of the DGCL, a Delaware corporation is not permitted to engage in a “business combination” with an “interested stockholder” for a period of three years following the date that the stockholder became an interested stockholder. As defined for this purpose, the term “business combination” includes a merger, consolidation, asset sale or other transaction resulting in a financial benefit to the interested stockholder. The term “interested stockholder” is defined to mean a person who, together with affiliates and associates, owns, or within three years did own, 15% or more of the corporation’s outstanding voting stock. This prohibition does not apply if:
| ● | prior to the time that the stockholder became an interested stockholder, the Board of Directors of the corporation approved either the business combination or the transaction resulting in the stockholder becoming an interested stockholder; | |
| ● | upon completion of the transaction resulting in the stockholder becoming an interested stockholder, the stockholder owns at least 85% of the outstanding voting stock of the corporation, excluding voting stock owned by directors who are also officers and by certain employee stock plans; or | |
| ● | at or subsequent to the time that the stockholder became an interested stockholder, the business combination is approved by the Board and authorized at an annual or special meeting of stockholders, and not by written consent, by the affirmative vote of at least two-thirds of the outstanding voting stock that the interested stockholder does not own. |
A Delaware corporation may elect not to be governed by these restrictions. We have not opted out of Section 203.
Advance Notice Procedures. Our bylaws establish an advance notice procedure for stockholder nominations of persons for election to our Board of Directors and for any proposals to be presented by stockholders at an annual meeting. Stockholders at an annual meeting will only be able to consider nominations and other proposals specified in the notice of meeting or brought before the meeting by or at the direction of our Board of Directors or by a stockholder who was a stockholder of record on the record date for the meeting, who is entitled to vote at the meeting and who has given our corporate secretary timely written notice, in proper form, of the stockholder’s intention to nominate a person for election as a director or to bring a proposal for action at the meeting.
Potential Effects of Authorized but Unissued Stock
Pursuant to our amended and restated certificate of incorporation, we have shares of common stock and preferred stock available for future issuance without stockholder approval. We may utilize these additional shares for a variety of corporate purposes, including future public offerings to raise additional capital, to facilitate corporate acquisitions or payment as a dividend on the capital stock.
The existence of unissued and unreserved common stock and preferred stock may enable our board of directors to issue shares to persons friendly to current management or to issue preferred stock with terms that could render more difficult or discourage a third-party attempt to obtain control of us by means of a merger, tender offer, proxy contest or otherwise, thereby protecting the continuity of our management. In addition, the board of directors has the discretion to determine designations, rights, preferences, privileges and restrictions, including voting rights, dividend rights, conversion rights, redemption privileges and liquidation preferences of each series of preferred stock, all to the fullest extent permissible under the Delaware General Corporation Law and subject to any limitations set forth in our certificate of incorporation. The purpose of authorizing the board of directors to issue preferred stock and to determine the rights and preferences applicable to such preferred stock is to eliminate delays associated with a stockholder vote on specific issuances. The issuance of preferred stock, while providing desirable flexibility in connection with possible financings, acquisitions and other corporate purposes, could have the effect of making it more difficult for a third-party to acquire, or could discourage a third-party from acquiring, a majority of our outstanding voting stock.
| 23 |
Choice of Forum
Unless we consent in writing to the selection of an alternative forum, the Court of Chancery of the State of Delaware shall be the sole and exclusive forum for any stockholder to bring (i) any derivative action or proceeding brought on behalf of the Company, (ii) any action asserting a claim of breach of fiduciary duty owed by any director, officer or other employee of the Company or the Company’s stockholders, (iii) any action asserting a claim against the Company or any director or officer of the Company arising pursuant to, or a claim against the Company or any director or officer of the Company, with respect to the interpretation or application of any provision of the DGCL, our certificate of incorporation or bylaws, or (iv) any action asserting a claim governed by the internal affairs doctrine, except for, in each of the aforementioned actions, any claims to which the Court of Chancery of the State of Delaware determines it lacks jurisdiction. This provision will not apply to claims arising under the Exchange Act, or for any other federal securities laws which provide for exclusive federal jurisdiction. However, the exclusive forum provision provides that unless we consent in writing to the selection of an alternative forum, the federal district courts of the United States of America will be the exclusive forum for the resolution of any complaint asserting a cause of action arising under the Securities Act. Therefore, this provision could apply to a suit that falls within one or more of the categories enumerated in the exclusive forum provision and that asserts claims under the Securities Act, inasmuch as Section 22 of the Securities Act creates concurrent jurisdiction for federal and state courts over all suits brought to enforce any duty or liability created by the Securities Act or the rules and regulations thereunder. There is uncertainty as to whether a court would enforce such an exclusive forum provision with respect to claims under the Securities Act.
We note that there is uncertainty as to whether a court would enforce the provision and that investors cannot waive compliance with the federal securities laws and the rules and regulations thereunder. Although we believe this provision benefits us by providing increased consistency in the application of Delaware law in the types of lawsuits to which it applies, the provision may have the effect of discouraging lawsuits against our directors and officers.
| 24 |
DESCRIPTION OF SECURITIES WE ARE OFFERING
We are offering ● shares of our common stock at an assumed combined public offering price of $● per share and accompanying Common Warrants (the last reported sale price of our common stock on Nasdaq on ●, 202●). We are also offering Pre-Funded Warrants to those purchasers whose purchase of shares of our common stock in this offering would result in the purchaser, together with its affiliates and certain related parties, beneficially owning more than 4.99% (or, at the election of the purchaser, 9.99%) of our outstanding shares of common stock following the consummation of this offering in lieu of the shares of common stocks that would result in such excess ownership. For each Pre-Funded warrant we sell, the number of shares of common stock we sell in this offering will be decreased on a one-for-one basis. Each share of our common stock or Pre-Funded Warrant is being sold together with one Common Warrant to purchase one share of common stock. The shares of our common stock and/or Pre-Funded Warrants and related warrants will be issued separately. We are also registering the shares of our common stock issuable from time to time upon exercise of the Pre-Funded Warrants and Common Warrants offered hereby.
Common Stock
The material terms and provisions of our common stock are described under the caption “Description of Capital Stock” in this prospectus.
Common Warrants
The following summary of certain terms and provisions of the Common Warrants that are being offered hereby is not complete and is subject to, and qualified in its entirety by, the provisions of Common Warrant, the form of which is filed as an exhibit to the registration statement of which this prospectus forms a part. Prospective investors should carefully review the terms and provisions of the form of Common Warrant for a complete description of the terms and conditions of such warrant.
Duration and Exercise Price
The Common Warrants will have an exercise price of $● per share (●% of the combined public offering price per share of common stock and accompanying warrants) and will be exercisable beginning on the effective date of the Warrant Stockholder Approval, provided however, if the Pricing Conditions are met, the Common Warrants will be exercisable upon Initial Exercise Date. The Common Warrants will expire on the five-year anniversary of the Initial Exercise Date. The exercise price and number of shares of common stock issuable upon exercise of the warrants is subject to appropriate adjustment in the event of stock dividends, stock splits, reorganizations or similar events affecting our common stock and the exercise price. The Common Warrants will be issued separately from the common stock and Pre-Funded Warrants and may be transferred separately immediately thereafter. The Common Warrants will be issued in certificated form only.
Exercisability
The Common Warrants will be exercisable, at the option of each holder, in whole or in part, by delivering to us a duly executed exercise notice accompanied by payment in full for the number of shares of our common stock purchased upon such exercise (except in the case of a cashless exercise as discussed below). A holder (together with its affiliates) may not exercise any portion of such holder’s Common Warrants to the extent that the holder would own more than 4.99% of the outstanding common stock immediately after exercise, except that upon at least 61 days’ prior notice from the holder to us, the holder may increase the amount of ownership of outstanding stock after exercising the holder’s Common Warrants up to 9.99% of the number of shares of our common stock outstanding immediately after giving effect to the exercise, as such percentage ownership is determined in accordance with the terms of the Common Warrants.
Cashless Exercise
If, at the time a holder exercises its Common Warrants, a registration statement registering the issuance or resale of the shares of common stock underlying the Common Warrants under the Securities Act is not then effective or available for the issuance of such shares, then in lieu of making the cash payment otherwise contemplated to be made to us upon such exercise in payment of the aggregate exercise price, the holder may elect instead to receive upon such exercise (either in whole or in part) the net number of shares of common stock determined according to a formula set forth in the Common Warrant.
| 25 |
Fundamental Transactions
In the event of a fundamental transaction, as described in the Common Warrants and generally including any reorganization, recapitalization or reclassification of our shares of common stock, the sale, transfer or other disposition of all or substantially all of our properties or assets, our consolidation or merger with or into another person, the acquisition of more than 50% of the voting power represented by our outstanding shares of capital stock, any person or group becoming the beneficial owner of more than 50% of the voting power represented by our outstanding shares of capital stock, any merger with or into another entity or a tender offer or exchange offer approved by more than 50% of the voting power represented by our outstanding shares of capital, then upon any subsequent exercise of a Common Warrant, the holder will have the right to receive as alternative consideration, for each share of our common stock that would have been issuable upon such exercise immediately prior to the occurrence of such fundamental transaction, the number of shares of common stock of the successor or acquiring corporation or of our company, if it is the surviving corporation, and any additional consideration receivable upon or as a result of such transaction by a holder of the number of shares of our common stock for which the Common Warrant is exercisable immediately prior to such event. Notwithstanding the foregoing, in the event of a fundamental transaction, the holders of the Common Warrants have the right to require us or a successor entity to redeem the Common Warrants for cash in the amount of the Black-Scholes Value (as defined in each warrant) of the unexercised portion of the Common Warrants concurrently with or within 30 days following the consummation of a fundamental transaction.
However, in the event of a fundamental transaction which is not in our control, including a fundamental transaction not approved by our board of directors, the holders of the Common Warrants will only be entitled to receive from us or our successor entity, as of the date of consummation of such fundamental transaction the same type or form of consideration (and in the same proportion), at the Black Scholes Value of the unexercised portion of the Common Warrant that is being offered and paid to the holders of our common stock in connection with the fundamental transaction, whether that consideration is in the form of cash, stock or any combination of cash and stock, or whether the holders of our common stock are given the choice to receive alternative forms of consideration in connection with the fundamental transaction.
Transferability
Subject to applicable laws, a Common Warrant may be transferred at the option of the holder upon surrender of the Common Warrant to us together with the appropriate instruments of transfer.
Fractional Shares
No fractional shares of common stock will be issued upon the exercise of the Common Warrants. Rather, the number of shares of common stock to be issued will, at our election, either be rounded up to the next whole share or we will pay a cash adjustment in respect of such final fraction in an amount equal to such fraction multiplied by the exercise price.
Trading Market
There is no established trading market for the Common Warrants, and we do not expect such a market to develop. We do not intend to apply to list the Common Warrants on any securities exchange or other nationally recognized trading system. Without an active trading market, the liquidity of the Common Warrants will be extremely limited.
Right as a Stockholder
Except as otherwise provided in the Common Warrants or by virtue of the holder’s ownership of shares of our common stock, such holder of warrants does not have the rights or privileges of a holder of our common stock, including any voting rights, until such holder exercises such holder’s Common Warrants. The Common Warrants will provide that the holders of the Common Warrants have the right to participate in distributions or dividends paid on our shares of common stock.
| 26 |
Waivers and Amendments
The Common Warrants may be modified or amended or the provisions of such Common Warrants waived with our consent and the consent of the holders of at least a majority of the outstanding Common Warrants.
Pre-Funded Warrants
The following summary of certain terms and provisions of the Pre-Funded Warrants that are being offered hereby is not complete and is subject to, and qualified in its entirety by, the provisions of the Pre-Funded Warrant, the form of which will be filed as an exhibit to the registration statement of which this prospectus forms a part. Prospective investors should carefully review the terms and provisions of the form of the Pre-Funded Warrant for a complete description of the terms and conditions of the Pre-Funded Warrants.
Duration and Exercise Price
Each Pre-Funded Warrant offered hereby will have an initial exercise price per share of common stock equal to $0.0001. The Pre-Funded Warrants will be immediately exercisable and will expire when exercised in full. The exercise price and number of shares of common stock issuable upon exercise is subject to appropriate adjustment in the event of share dividends, share splits, reorganizations or similar events affecting our shares of common stock and the exercise price.
Exercisability
The Pre-Funded Warrants will be exercisable, at the option of each holder, in whole or in part, by delivering to us a duly executed exercise notice accompanied by payment in full for the number of shares of common stock purchased upon such exercise (except in the case of a cashless exercise as discussed below). A holder (together with its affiliates) may not exercise any portion of the Pre-Funded Warrant to the extent that the holder would own more than 4.99% of the outstanding shares of common stock immediately after exercise, except that upon at least 61 days’ prior notice from the holder to us, the holder may increase the amount of beneficial ownership of outstanding shares after exercising the holder’s Pre-Funded Warrants up to 9.99% of the number of our shares of common stock outstanding immediately after giving effect to the exercise, as such percentage ownership is determined in accordance with the terms of the Pre-Funded Warrants. Purchasers of Pre-Funded Warrants in this offering may also elect prior to the issuance of the Pre-Funded Warrants to have the initial exercise limitation set at 9.99% of our outstanding shares of common stock.
Cashless Exercise
In lieu of making the cash payment otherwise contemplated to be made to us upon such exercise in payment of the aggregate exercise price, the holder may elect instead to receive upon such exercise (either in whole or in part) the net number of shares of common stock determined according to a formula set forth in the Pre-Funded Warrants.
Fractional Shares
No fractional shares of common stock will be issued upon the exercise of the Pre-Funded Warrants. Rather, at the Company’s election, the number of shares of common stock to be issued will be rounded up to the next whole share or the Company will pay a cash adjustment in an amount equal to such fraction multiplied by the exercise price.
Transferability
Subject to applicable laws, a Pre-Funded Warrant may be transferred at the option of the holder upon surrender of the Pre-Funded Warrants to us together with the appropriate instruments of transfer.
| 27 |
Trading Market
There is no established trading market for the Pre-Funded Warrants, and we do not expect such a market to develop. We do not intend to apply to list the Pre-Funded Warrants on any securities exchange or other nationally recognized trading system. Without an active trading market, the liquidity of the Pre-Funded Warrants will be extremely limited.
Right as a Shareholder
Except as otherwise provided in the Pre-Funded Warrants or by virtue of such holder’s ownership of shares of common stock, the holders of the Pre-Funded Warrants do not have the rights or privileges of holders of our shares of common stock, including any voting rights, until they exercise their Pre-Funded Warrants. The Pre-Funded Warrants will provide that the holders of the Pre-Funded Warrants have the right to participate in distributions or dividends paid on our shares of common stock.
Fundamental Transaction
In the event of a fundamental transaction, as described in the Pre-Funded Warrants and generally including any reorganization, recapitalization or reclassification of our shares of common stock, the sale, transfer or other disposition of all or substantially all of our properties or assets, our consolidation or merger with or into another person, the acquisition of more than 50% of the voting power represented by our outstanding shares of capital stock, any person or group becoming the beneficial owner of more than 50% of the voting power represented by our outstanding shares of capital stock, any merger with or into another entity or a tender offer or exchange offer approved by more than 50% of the voting power represented by our outstanding shares of capital, then upon any subsequent exercise of a Pre-Funded Warrant, the holder will have the right to receive as alternative consideration, for each share of our common stock that would have been issuable upon such exercise immediately prior to the occurrence of such fundamental transaction, the number of shares of common stock of the successor or acquiring corporation or of our company, if it is the surviving corporation, and any additional consideration receivable upon or as a result of such transaction by a holder of the number of shares of our common stock for which the Pre-Funded Warrant is exercisable immediately prior to such event.
Placement Agent Warrants
We have also agreed to issue to the placement agent (or its designees) Placement Agent Warrants to purchase up to ● shares of common stock. The Placement Agent Warrants will be exercisable six months after this offering and will have substantially the same terms as the Common Warrants described above, except that the Placement Agent Warrants will have an exercise price of $● per share (representing 125 % of the offering price per share and accompanying warrants) and a termination date that will be ● years from the commencement of the sales pursuant to this offering. See “Plan of Distribution” below.
| 28 |
MATERIAL U.S. FEDERAL INCOME TAX CONSEQUENCES
The following discussion describes certain material U.S. federal income tax consequences relating to the acquisition, ownership and disposition of our common stock, pre-funded warrants and common warrants by a U.S. Holder or Non-U.S. Holder (as each term is defined below). This discussion is based on the current provisions of the Internal Revenue Code of 1986, as amended (referred to as the “Code”), existing and proposed U.S. Treasury regulations promulgated thereunder, and administrative rulings and court decisions in effect as of the date hereof, all of which are subject to change at any time, possibly with retroactive effect. No ruling has been or will be sought from the Internal Revenue Service, or IRS, with respect to the matters discussed below, and there can be no assurance the IRS will not take a contrary position regarding the tax consequences of the acquisition, ownership or disposition of our common stock, pre-funded warrants or common warrants, or that any such contrary position would not be sustained by a court.
We assume in this discussion that the shares of our common stock, pre-funded warrants and common warrants will be held as capital assets (generally, property held for investment). This discussion does not address all aspects of U.S. federal income taxes and does not address state or local taxes or U.S. federal gift and estate tax laws, or any non-U.S. tax consequences that may be relevant to holders in light of their particular circumstances. This discussion also does not address the special tax rules applicable to particular holders, such as:
| ● | a bank, insurance company, or other financial institution; | |
| ● | a tax-exempt entity, organization, or arrangement; | |
| ● | a government or any agency, instrumentality, or controlled entity thereof; | |
| ● | a real estate investment trust; | |
| ● | an S corporation or other pass-through entity (or an investor in an S corporation or other pass-through entity); | |
| ● | a regulated investment company; | |
| ● | a “controlled foreign corporation” or a “passive foreign investment company”; | |
| ● | a dealer or broker in stocks and securities, or currencies; | |
| ● | a trader in securities that elects mark-to-market treatment or any other holder subject to mark-to-market treatment; | |
| ● | a holder of our common stock, pre-funded warrants, or common warrants that is liable for the alternative minimum tax; | |
| ● | a holder of our common stock, pre-funded warrants, or common warrants that received such security through the exercise of options, warrants, or similar derivative securities or otherwise as compensation; | |
| ● | a holder of our common stock, pre-funded warrants, or common warrants that holds such security in a tax-deferred account (such as an individual retirement account or a plan qualifying under Section 401(k) of the Code); | |
| ● | a holder of our common stock, pre-funded warrants, or common warrants that has a functional currency other than the U.S. dollar; | |
| ● | a holder of our common stock, pre-funded warrants, or common warrants that holds such security as part of a hedge, straddle, constructive sale, conversion or other integrated transaction; | |
| ● | a holder of our common stock, pre-funded warrants, or common warrants required to accelerate the recognition of any item of gross income with respect to such security, as a result of such income being recognized on an applicable financial statement; | |
| ● | a holder of our common stock, pre-funded warrants, or common warrants that is a U.S. expatriate or former citizen or long-term resident of the United States; | |
| ● | a holder of our common stock, pre-funded warrants, or common warrants that does not hold such security as a capital asset within the meaning of Section 1221 of the Code (generally, for investment purposes); | |
| ● | a holder of our common stock, pre-funded warrants, or common warrants whose security may constitute “qualified small business stock” under Section 1202 of the Code or “Section 1244 stock” for purposes of Section 1244 of the Code; or | |
| ● | a holder of our common stock, pre-funded warrants, or common warrants that acquired such security in a transaction subject to the gain rollover provisions of Section 1045 of the Code; |
| 29 |
In addition, this discussion does not address the tax treatment of partnerships or other pass-through entities or of persons who hold our common stock, pre-funded warrants or common warrants through partnerships or other entities which are pass-through entities for U.S. federal income tax purposes. A partner in a partnership or other pass-through entity that will hold our common stock, pre-funded warrants or common warrants should consult his, her or its own tax advisor regarding the tax consequences of the ownership and disposition of our common stock, pre-funded warrants or common warrants through a partnership or other pass-through entity, as applicable.
For the purposes of this discussion, a “U.S. Holder” means a beneficial owner of our common stock, pre-funded warrants or common warrants that is for U.S. federal income tax purposes (a) an individual citizen or resident of the United States, (b) a corporation (or other entity treated as a corporation for U.S. federal income tax purposes), organized in or under the laws of the United States, any state thereof or the District of Columbia, (c) an estate the income of which is includable in gross income for U.S. federal income tax purposes regardless of its source, or (d) a trust if it (1) is subject to the primary supervision of a court within the United States and one or more U.S. persons (within the meaning of Section 7701(a)(30) of the Code) has the authority to control all substantial decisions of the trust or (2) has a valid election in effect under applicable U.S. Treasury regulations to be treated as a U.S. person. A “Non-U.S. Holder” is, for U.S. federal income tax purposes, a beneficial owner of common stock, pre-funded warrants or common warrants (other than a partnership or other entity or arrangement classified as a partnership for U.S. federal income tax purposes) that is not a U.S. Holder.
The discussion of U.S. federal income tax considerations is for information purposes only and is not tax advice. Investors should consult their own tax advisors regarding the U.S. federal, state, local and non-U.S. income and other tax considerations of acquiring, holding and disposing of our common stock, pre-funded warrants and common warrants.
Allocation of Purchase Price to Common Stock, Pre-Funded Warrants and Common Warrants
For U.S. federal income tax purposes, a holder’s acquisition of the common warrants and common stock or prefunded warrants, as applicable, should be treated as the acquisition of an “investment unit” consisting of one share of common stock or one pre-funded warrant, as applicable, and a warrant to acquire one share of our common stock, subject to adjustment. The purchase price for each investment unit will be allocated between these two components in proportion to their relative fair market values at the time the unit is purchased by the holder. This allocation of the purchase price for each unit will establish the holder’s initial tax basis for U.S. federal income tax purposes in the common stock or pre-funded warrant, as applicable, and the common stock warrant included in each unit. We do not intend to advise holders of the common warrants and common stock or prefunded warrants, as applicable, with respect to this determination, and holders are advised to consult their tax and financial advisors with respect to the relative fair market values of the common stock or pre-funded warrant, as applicable, and the common warrants for U.S. federal income tax purposes.
Tax Treatment of Pre-Funded Warrants
Although it is not entirely free from doubt, we believe a pre-funded warrant should be treated as a share of our common stock for U.S. federal income tax purposes and a holder of pre-funded warrants should generally be taxed in the same manner as a holder of common stock as described below. Accordingly, for U.S. federal income tax purposes, no gain or loss should be recognized upon the exercise of a pre-funded warrant, and upon exercise, the holding period of the share of common stock received should include the holding period of the pre-funded warrant. Similarly, the tax basis of a share of common stock received upon exercise of a pre-funded warrant should include the tax basis of the pre-funded warrant (discussed below) increased by the exercise price of $0.0001. The balance of this discussion generally assumes that the characterization described above is respected for U.S. federal income tax purposes.
| 30 |
Tax Considerations Applicable to U.S. Holders
Exercise and Expiration of the Pre-Funded Warrants and Common Warrants
In general, a U.S. Holder will not recognize gain or loss for U.S. federal income tax purposes upon exercise of a pre-funded warrant or common warrant (collectively for the balance of this discussion a “warrant”), except to the extent such U.S. Holder receives a cash payment for a fractional share that would otherwise have been issuable upon exercise of the warrant, which will be treated as a sale subject to the rules described below under “U.S. Holders — Disposition of Our Common Stock, Pre-Funded Warrants or Common Warrants.” The U.S. Holder will take a tax basis in the shares acquired on the exercise of a warrant equal to the exercise price of the warrant, increased by the U.S. Holder’s adjusted tax basis in the warrant exercised. A U.S. holder’s holding period in the common stock received upon exercise of a pre-funded warrant generally should include such U.S. holder’s holding period in the pre-funded warrants exchanged therefor. The U.S. Holder’s holding period in the shares of our common stock acquired on exercise of the common warrant will begin on the date of exercise of the common warrant (or possibly, the day after), and will not include any period for which the U.S. Holder held the common warrant.
In certain limited circumstances, a U.S. Holder may be permitted to undertake a cashless exercise of warrants into our common stock. The U.S. federal income tax treatment of a cashless exercise of warrants into our common stock is unclear, and the tax consequences of a cashless exercise could differ from the consequences upon the exercise of a warrant described in the preceding paragraph. Due to the absence of authority on the U.S. federal income tax treatment of a cashless exercise, there can be no assurance as to the tax treatment that would be adopted by the IRS or a court of law. U.S. Holders should consult their own tax advisors regarding the U.S. federal income tax consequences of a cashless exercise of warrants.
The lapse or expiration of a warrant will be treated as if the U.S. Holder sold or exchanged the warrant and recognized a capital loss equal to the U.S. Holder’s tax basis in the warrant. The deductibility of capital losses is subject to limitations.
Certain Adjustments to and Distributions on the Pre-Funded Warrants and Common Warrants
The exercise terms of the warrants may be adjusted in certain circumstances. An adjustment to the number of shares of common stock that will be issued on the exercise of the warrants or an adjustment to the exercise price of the warrants may be treated as a constructive distribution to a U.S. Holder of the warrants even if such holder does not receive any cash or other property in connection with the adjustment. If the exercise price is adjusted in certain other circumstances (or in certain circumstances, there is a failure to make adjustments), such adjustments may also result in a constructive distribution to a U.S. Holder. U.S. Holders should consult their tax advisors regarding the proper treatment of any adjustments to the warrants. Any constructive distribution will generally be taxed in the same manner as an actual distribution received by a U.S. Holder as discussed below under “Distributions.”
Distributions
We currently anticipate that we will retain all available funds and any future earnings for use in the operation of our business and do not anticipate declaring or paying any cash dividends on our common stock for the foreseeable future. In the event that we do make distributions to a U.S. Holder, those distributions generally will constitute dividends for U.S. tax purposes to the extent paid out of our current or accumulated earnings and profits (as determined under U.S. federal income tax principles). Distributions to a U.S. Holder that are not derived from our current or accumulated earnings and profits will constitute a return of capital that is applied against and reduces, but not below zero, the U.S. Holder’s adjusted tax basis in our common stock, common warrants or pre-funded warrants, as applicable, and to the extent in excess of such basis, will be treated as gain realized on the sale or exchange of our common stock, common warrants or pre-funded warrants, as applicable, as described below.
The U.S. Holder generally will recognize capital gain or loss in an amount equal to the difference between the amount realized and the U.S. Holder’s adjusted tax basis in the applicable common stock, pre-funded warrants or common warrants. Capital gain or loss will constitute long-term capital gain or loss if the U.S. Holder’s holding period for the applicable common stock, pre-funded warrant or common warrant exceeds one year. The deductibility of capital losses is subject to certain limitations. U.S. Holders who recognize losses with respect to a disposition of our common stock, pre-funded warrants or common warrants should consult their own tax advisors regarding the tax treatment of such losses.
| 31 |
Medicare Tax
Certain U.S. Holders that are individuals, estates or trusts are subject to a 3.8% tax on all or a portion of their “net investment income,” which may include all or a portion of their dividend income and net gains from the disposition of securities. Each U.S. Holder that is an individual, estate or trust is urged to consult its tax advisors regarding the applicability of the Medicare tax to its income and gains in respect of its investment in our securities.
Information Reporting and Backup Withholding
Information reporting requirements generally will apply to payments of dividends (including constructive dividends) on our common stock, pre-funded warrants and common warrants and to the proceeds of a sale or other disposition of common stock, common warrants and pre-funded warrants by a U.S. Holder unless such U.S. Holder is an exempt recipient, such as a corporation. Backup withholding will apply to those payments if the U.S. Holder fails to provide the holder’s taxpayer identification number, or certification of exempt status, or if the holder otherwise fails to comply with applicable requirements to establish an exemption. Backup withholding is not an additional tax. Rather, amounts withheld as backup withholding may be credited against a person’s U.S. federal income tax liability, and a holder generally may obtain a refund of any excess amounts withheld under the backup withholding rules by timely filing the appropriate claim for refund with the IRS and furnishing any required information.
Tax Considerations Applicable to Non-U.S. Holders
Exercise and Expiration of Pre-Funded Warrants and Common Warrants
In general, a Non-U.S. Holder will not be subject to U.S. federal income tax on the exercise of the warrants into shares of common stock. As described under “U.S. Holders - Exercise and Expiration of the Pre-Funded Warrants and Common Warrants” the U.S. federal income tax treatment of a cashless exercise of warrants into our common stock is unclear. A Non-U.S. Holder should consult his, her, or its own tax advisor regarding the U.S. federal income tax consequences of a cashless exercise of warrants.
The expiration of a warrant will be treated as if the Non-U.S. Holder sold or exchanged the warrant and recognized a capital loss equal to the Non-U.S. Holder’s tax basis in the warrant. However, a Non-U.S. Holder will not be able to utilize a loss recognized upon expiration of a warrant against the Non-U.S. Holder’s U.S. federal income tax liability unless the loss is effectively connected with the Non-U.S. Holder’s conduct of a trade or business within the United States (and, if an income tax treaty applies, is attributable to a permanent establishment or fixed base in the United States) or is treated as a U.S.-source loss and the Non-U.S. Holder is an individual nonresident and present 183 days or more in the taxable year of disposition in the United States and certain other conditions are met.
Certain Adjustments to and Distributions on the Pre-Funded Warrants and Common Warrants
As described under “U.S. Holders - Certain Adjustments to and Distributions on the Pre-Funded Warrants and Common Warrants” an adjustment to the warrants could result in a constructive distribution to a Non-U.S. Holder, which would be treated as described under “Distributions” below, and the tax treatment of a distribution on a warrant is unclear. Any resulting withholding tax attributable to deemed dividends would be collected from other amounts payable or distributable to the Non-U.S. Holder. Non-U.S. Holders should consult their tax advisors regarding the proper treatment of any adjustments to or distributions on the warrants.
Distributions
We currently anticipate that we will retain all available funds and any future earnings for use in the operation of our business and do not anticipate declaring or paying any cash dividends on our common stock for the foreseeable future. In the event that we do make distributions on our common stock or warrants to a Non-U.S. Holder, those distributions generally will be treated as dividends, as return of capital or as gain on the sale or exchange of common stock or warrants for U.S. federal income tax purposes as described in “U.S. Holders - Distributions.”
| 32 |
Subject to the discussions below under the sections titled “Information Reporting and Backup Withholding” and “Foreign Accounts,” any distribution (including constructive distributions) on our common stock or warrants that is treated as a dividend paid to a Non-U.S. Holder that is not effectively connected with the holder’s conduct of a trade or business in the United States will generally be subject to withholding tax at a 30% rate or such lower rate as may be specified by an applicable income tax treaty between the United States and the Non-U.S. Holder’s country of residence. To obtain a reduced rate of withholding under a treaty, a Non-U.S. Holder generally will be required to provide the applicable withholding agent with a properly executed IRS Form W-8BEN, IRS Form W-8BEN-E or other appropriate form, certifying the Non-U.S. Holder’s entitlement to benefits under that treaty. Such form must be provided prior to the payment of dividends and must be updated periodically and when otherwise required by law.
We generally are not required to withhold tax on dividends paid (or constructive dividends deemed paid) to a Non-U.S. Holder that are effectively connected with such holder’s conduct of a trade or business within the United States (and, if required by an applicable income tax treaty, are attributable to a permanent establishment or fixed base that the holder maintains in the United States) if a properly executed IRS Form W-8ECI, stating that the dividends are so connected, is furnished to us. In general, such effectively connected dividends will be subject to U.S. federal income tax on a net income basis at the regular rates applicable to U.S. persons. A corporate Non-U.S. Holder receiving effectively connected dividends may also be subject to an additional “branch profits tax,” which is imposed, under certain circumstances, at a rate of 30% (or such lower rate as may be specified by an applicable treaty) on the corporate Non-U.S. Holder’s effectively connected earnings and profits, subject to certain adjustments.
Distributions to a Non-U.S. Holder that are not derived from our current or accumulated earnings and profits generally will be treated as a return of capital that will be applied against and reduce (but not below zero) the Non-U.S. Holder’s basis in its common stock or warrants, as applicable, and to the extent in excess of such basis, will be treated as gain from the sale or exchange of such common stock or warrants, as applicable, as described under “Disposition of Our Common Stock, Pre-Funded Warrants or Common Warrants” below.
If a Non-U.S. Holder holds stock through a financial institution or other agent acting on the holder’s behalf, the holder will be required to provide appropriate documentation to such agent. The holder’s agent may then be required to provide certification to the applicable withholding agent, either directly or through other intermediaries.
Disposition of Our Common Stock, Pre-Funded Warrants or Common Warrants
Subject to the discussions below under the sections titled “Information Reporting and Backup Withholding” and “Foreign Accounts,” a Non-U.S. Holder generally will not be subject to U.S. federal income or withholding tax with respect to gain realized on a sale or other disposition of our common stock, pre-funded warrants or common warrants unless:
| ● | the gain is effectively connected with the Non-U.S. Holder’s conduct of a trade or business in the United States (and, if required by an applicable income tax treaty between the United States and such Non-U.S. Holder’s country of residence, the gain is attributable to a permanent establishment or fixed base maintained by the Non-U.S. Holder in the U.S.), in which case the Non-U.S. Holder will be taxed on a net income basis at the regular rates and in the manner applicable to U.S. persons, and if the Non-U.S. Holder is a corporation, an additional branch profits tax at a rate of 30%, or a lower rate as may be specified by an applicable income tax treaty, may also apply; | |
| ● | the Non-U.S. Holder is a nonresident alien present in the United States for 183 days or more in the taxable year of the disposition and certain other requirements are met, in which case the Non-U.S. Holder will be subject to a 30% tax (or such lower rate as may be specified by an applicable income tax treaty between the United States and such holder’s country of residence) on the net gain derived from the disposition, which may be offset by certain U.S.-source capital losses of the Non-U.S. Holder, if any, provided that the Non-U.S. Holder has timely filed U.S. federal income tax returns reporting those losses; or |
| 33 |
| ● | we are, or have been, a “United States real property holding corporation,” or USRPHC, for U.S. federal income tax purposes during the five-year period preceding such disposition (or the Non-U.S. Holder’s holding period, if shorter). We do not believe that we are or have been a USRPHC and, even if we are or were a USRPHC, as long as our common stock is regularly traded on an established securities market, dispositions will not be subject to tax for a Non-U.S. Holder that has not held more than 5% of our common stock, actually or constructively, during the five-year period preceding such Non-U.S. Holder’s disposition (or the Non-U.S. Holder’s holding period, if shorter). Special rules may apply to the determination of the 5% threshold in the case of a holder of a pre-funded warrant or common warrant. |
See the sections titled “Information Reporting and Backup Withholding” and “Foreign Accounts” below for additional information regarding withholding rules that may apply to proceeds of a disposition of our common stock, pre-funded warrants or common warrants paid to foreign financial institutions or non-financial foreign entities.
Information Reporting and Backup Withholding
We must report annually to the IRS and to each Non-U.S. Holder the gross amount of the distributions (including constructive distributions) on our common stock, pre-funded warrants or common warrants paid to such holder and the tax withheld, if any, with respect to such distributions. Non-U.S. Holders may have to comply with specific certification procedures to establish that the holder is not a U.S. person (as defined in the Code) in order to avoid backup withholding at the applicable rate, currently 24%. Generally, a Non-U.S. Holder will comply with such procedures if it provides a properly executed applicable IRS Form W-8 or by otherwise establishing an exemption. Dividends paid to Non-U.S. Holders subject to withholding of U.S. federal income tax, as described above under the heading “Distributions,” will generally be exempt from U.S. backup withholding.
Information reporting and backup withholding generally will apply to the proceeds of a disposition of our common stock, pre-funded warrants or common warrants by a Non-U.S. Holder effected by or through the U.S. office of any broker, U.S. or foreign, unless the Non-U.S. Holder certifies its status as a Non-U.S. Holder and satisfies certain other requirements, or otherwise establishes an exemption. Generally, information reporting and backup withholding will not apply to a payment of disposition proceeds to a Non-U.S. Holder where the transaction is effected outside the United States through a non-U.S. office of a non-U.S. broker. However, for information reporting purposes, dispositions effected through a non-U.S. office of a broker with substantial U.S. ownership or operations generally will be treated in a manner similar to dispositions effected through a U.S. office of a broker. Non-U.S. Holders should consult their own tax advisors regarding the application of the information reporting and backup withholding rules to them.
Copies of information returns may be made available to the tax authorities of the country in which the Non-U.S. Holder resides or is incorporated under the provisions of a specific treaty or agreement.
Backup withholding is not an additional tax. Any amounts withheld under the backup withholding rules from a payment to a Non-U.S. Holder can be refunded or credited against the Non-U.S. Holder’s U.S. federal income tax liability, if any, provided that an appropriate claim is timely filed with the IRS.
Foreign Accounts
Legislation commonly referred to as the “Foreign Account Tax Compliance Act,” or “FATCA,” generally imposes a 30% withholding tax on dividends on common stock, pre-funded warrants and common warrants if paid to a non-U.S. entity unless (i) if the non-U.S. entity is a “foreign financial institution,” the non-U.S. entity undertakes certain due diligence, reporting, withholding, and certification obligations, (ii) if the non-U.S. entity is not a “foreign financial institution,” the non-U.S. entity identifies certain of its U.S. investors, if any, or (iii) the non-U.S. entity is otherwise exempt under FATCA.
Intergovernmental agreements between the United States and foreign countries with respect to FATCA may significantly modify the requirements described in this section for Non-U.S. Holders. Holders should consult their own tax advisors regarding the possible implications of FATCA on their investment in our common stock, pre-funded warrants or common warrants.
| 34 |
The preceding discussion of material U.S. federal tax considerations is for information only. It is not tax advice. Prospective investors should consult their own tax advisors regarding the particular U.S. federal, state, local and non-U.S. tax consequences of purchasing, holding and disposing of our common stock, pre-funded warrants or common warrants, including the consequences of any proposed changes in applicable laws.
We have engaged ●, or the placement agent, to act as our exclusive placement agent to solicit offers to purchase the shares of our common stock, pre-funded warrants and common stock purchase warrants offered by this prospectus. The placement agent is not purchasing or selling any such securities, nor is it required to arrange for the purchase and sale of any specific number or dollar amount of such securities, other than to use its “reasonable best efforts” to arrange for the sale of such securities by us. Therefore, we may not sell all of the shares of common stock, pre-funded warrants and common stock purchase warrants being offered. The terms of this offering are subject to market conditions and negotiations between us, the placement agent and prospective investors. The placement agent will have no authority to bind us by virtue of the engagement letter. This is a best efforts offering and there is no minimum offering amount required as a condition to the closing of this offering. The placement agent may retain sub-agents and selected dealers in connection with this offering.
Investors purchasing securities offered hereby will have the option to execute a securities purchase agreement with us. Investors who do not enter into a securities purchase agreement shall rely solely on this prospectus in connection with the purchase of our securities in this offering. In addition to rights and remedies available to all purchasers in this offering under federal securities and state law, the purchasers which enter into a securities purchase agreement will also be able to bring claims of breach of contract against us. The ability to pursue a claim for breach of contract is material to larger purchasers in this offering as a means to enforce the following covenants uniquely available to them under the securities purchase agreement, including but not limited to: (i) a covenant to not enter into variable rate financings for a period of ● following the closing of the offering, subject to exceptions; and (ii) a covenant to not enter into any equity financings for ● from closing of the offering, subject to certain exceptions.
The nature of the representations, warranties and covenants in the securities purchase agreements shall include, but are not limited to:
| ● | standard issuer representations and warranties on matters such as organization, qualification, authorization, no conflict, no governmental filings required, current in SEC filings, no litigation, labor or other compliance issues, environmental, intellectual property and title matters and compliance with various laws such as the Foreign Corrupt Practices Act; and |
| ● | covenants regarding matters such as registration of shares issued and issuable upon exercise of the common stock purchase warrants, no integration with other offerings, no shareholder rights plans, use of proceeds, indemnification of purchasers, reservation and listing of common stock, and no subsequent equity sales for ●. |
Delivery of the shares of common stock, pre-funded warrants and common stock purchase warrants offered hereby is expected to occur on or ●, 2024, subject to satisfaction of certain customary closing conditions.
Fees and Expenses
The following table shows the per share price and total cash fees we will pay to the placement agent in connection with the sale of the securities pursuant to this prospectus.
| Per share of Common Stock and accompanying Common Warrant | Per Pre-Funded Warrant and accompanying Common Warrant | Total | ||||||||||
| Public offering price | $ | $ | $ | |||||||||
| Placement agent fees (1) | $ | $ | $ | |||||||||
| Proceeds to us, before expenses (2) | $ | $ | $ | |||||||||
| (1) | We have agreed to pay the placement agent a cash fee equal to 7.0% of the gross proceeds raised in this offering (other than proceeds received from the Company’s current directors and officers). We have also agreed to reimburse the placement agent for certain of its offering related expenses, including reimbursement for non-accountable expenses in legal fees and expenses in the amount of up to $112,500, and for its clearing expenses in the amount of $15,950. In addition, we have agreed to issue the placement agent or its designees warrants (“Placement Agent Warrants”) to purchase a number of shares of common stock equal to 4.0% of the shares of common stock sold in this offering (including the shares of common stock issuable upon the exercise of the Pre-Funded Warrants but not including shares of common stock sold to the Company’s current directors and officers), at an exercise price of $● per share, which represents 125% of the public offering price per share and accompanying warrant. | |
| (2) | Because there is no minimum number of securities or amount of proceeds required as a condition to closing in this offering, the actual public offering amount, placement agent fees, and proceeds to us, if any, are not presently determinable and may be substantially less than the total maximum offering amounts set forth above. |
We estimate the total expenses of this offering paid or payable by us, exclusive of the placement agent’s cash fee and expenses payable by us, will be approximately $●. After deducting the fees and expenses due to the placement agent and our estimated expenses in connection with this offering, assuming we sell all of the shares and accompanying warrants offered hereby, we expect the net proceeds from this offering will be approximately $●.
| 35 |
Placement Agent Warrants
In addition, we have agreed to issue to the placement agent or its designees warrants, or the placement agent warrants, to purchase up to 4% of the aggregate number of shares of common stock sold in this offering (including shares underlying any pre-funded warrants), except in connection with proceeds in this offering raised from the Company’s current directors and officers, at an exercise price equal to 125% of the public offering price per share and accompanying common stock purchase warrant to be sold in this offering. The placement agent warrants will be exercisable upon issuance and will expire three (3) years from the commencement of sales under this offering. The placement agent warrants provide for customary anti-dilution provisions (for share dividends, splits and recapitalizations and the like) consistent with FINRA Rule 5110. The placement agent warrants and the shares of common underlying the placement agent warrants are registered on the registration statement of which this prospectus is a part. The form of the placement agent warrant is included as an exhibit to this registration statement of which this prospectus forms a part.
Tail
We have also agreed to pay the placement agent a tail fee equal to the cash and warrant compensation in this offering, if any investor, who was contacted by the placement agent or who was introduced to us during the term of its engagement, provides us with capital in any public or private offering or other financing or capital raising transaction of any kind other than at-the-market facilities or equity line offerings during the nine (9) month period following expiration or termination of our engagement of the placement agent.
Right of First Refusal
Subject to consummation of the offering, we have granted a right of first refusal to the placement agent pursuant to which it has the right to act as the sole book-runner, manager, underwriter or placement agent, as applicable, if we decide to raise capital through a public offering (including an at-the-market facility) or private placement or any other capital-raising financing of equity, equity-linked or debt securities pursuant to which we engage an investment bank or broker/dealer at any time prior to the eight (8) months following the consummation of this offering.
Lock-Up Agreements
Our officers and directors have agreed with the placement agent to be subject to a lock-up period of ● following the closing of this offering. This means that, during the applicable lock-up period, such persons may not offer for sale, contract to sell, sell, distribute, grant any option, right or warrant to purchase, pledge, hypothecate or otherwise dispose of, directly or indirectly, any shares of our common stock or any securities convertible into, or exercisable or exchangeable for, shares of our common stock. Certain limited transfers are permitted during the lock-up period if the transferee agrees to these lock-up restrictions. We have also agreed to similar lock-up restrictions on the issuance and sale of our securities for ● following the closing of this offering, subject to certain exceptions. The placement agent may, in its sole discretion and without notice, waive the terms of any of these lock-up agreements.
In addition, subject to certain exceptions, we have agreed to not issue any securities that are subject to a price reset based on the trading prices of our common stock or upon a specified or contingent event in the future, or enter into any agreement to issue securities at a future determined price for a period of one year following the closing date of this offering.
Indemnification
We have agreed to indemnify the placement agent against certain liabilities, including certain liabilities under the Securities Act, or to contribute to payments that the placement agent may be required to make in respect of those liabilities.
In addition, we will indemnify the purchasers of securities in this offering against liabilities arising out of or relating to (i) any breach of any of the representations, warranties, covenants or agreements made by us in the securities purchase agreement or related documents or (ii) any action instituted against a purchaser by a third party (other than a third party who is affiliated with such purchaser) with respect to the securities purchase agreement or related documents and the transactions contemplated thereby, subject to certain exceptions
Other Relationships
The placement agent and its affiliates have engaged, and may in the future engage, in investment banking transactions and other commercial dealings in the ordinary course of business with us or our affiliates. The placement agent has received, or may in the future receive, customary fees and commissions for these transactions.
In addition, in the ordinary course of their business activities, the placement agent and its affiliates may make or hold a broad array of investments and actively trade debt and equity securities (or related derivative securities) for their own account and for the accounts of their customers. Such investments and securities activities may involve securities and/or instruments of ours or our affiliates. The placement agent and its affiliates may also make investment recommendations and/or publish or express independent research views in respect of such securities or financial instruments and may hold, or recommend to clients that they acquire, long and/or short positions in such securities and instruments.
| 36 |
Electronic Distribution
A prospectus in electronic format may be made available on a website maintained by the placement agent and the placement agent may distribute prospectuses electronically. Other than the prospectus in electronic format, the information on these websites is not part of this prospectus or the registration statement of which this prospectus forms a part, has not been approved and/or endorsed by us or the placement agent and should not be relied upon by investors.
Participation in this Offering
● and ●, members of our board of directors and ●, our [officer title] have expressed interest in purchasing up to ●, ● and ●, respectively, of shares of our common stock being offered in this offering for investment purposes. As of November 30, 2023, ●, ● and ● respectively, owned ●, ● and ● shares of our common stock prior to this offering. Such purchases, if any, would be made at the public offering price.
Transfer Agent
The transfer agent and registrar for our common stock is Continental Stock Transfer & Trust Company, LLC (“Continental”). Continental will act as the registrar and transfer agent for the common stock purchase warrants and the pre-funded warrants.
Nasdaq listing
Our shares of common stock are listed on the NASDAQ Capital Market under the symbol “PCSA.”
Certain legal matters relating to this offering and the validity of the securities offered by this prospectus will be passed upon for us by Foley & Lardner, LLP, Jacksonville, Florida. ●, New York, New York is acting as counsel for ● in connection with this offering.
The consolidated financial statements as of and for the fiscal years ended December 31, 2022 and 2021, incorporated by reference into this prospectus from the Company’s Annual Report on Form 10-K for the year ended December 31, 2022, have been so incorporated in reliance on the report of BD & Company, an independent registered public accounting firm, as stated in their report which is incorporated by reference herein, and has been so incorporated in reliance upon such report and upon the authority of such firm as experts in accounting and auditing.
INFORMATION INCORPORATED BY REFERENCE
The SEC allows us to “incorporate by reference” information that we file with it into this prospectus, which means that we can disclose important information to you by referring you to those documents. The information incorporated by reference is an important part of this prospectus. The information incorporated by reference is considered to be a part of this prospectus, and information that we file later with the SEC will automatically update and supersede information contained in this prospectus.
We incorporate by reference the documents listed below and any future filings made with the SEC under Sections 13(a), 13(c), 14, or 15(d) of the Exchange Act made after the date of the initial registration statement of which this prospectus forms a part and prior to effectiveness of the registration statement and subsequent to the date of this prospectus until the termination of the offering of the securities described in this prospectus (other than information in such filings that was “furnished,” under applicable SEC rules, rather than “filed”). We incorporate by reference the following documents or information that we have filed with the SEC:
| ● | Our Annual Report on Form 10-K for the fiscal year ended December 31, 2022, filed with the SEC on March 30, 2023; | |
| ● | Our Quarterly Report on Form 10-Q for the quarters ended March 31, 2023, June 30, 2023 and September 31, 2023, filed with the SEC on May 15, 2023, August 10, 2023 and November 13, 2023, respectively; | |
| ● | Our Current Reports on Form 8-K, filed with the SEC on February 6, 2023, February 13, 2023; March 22, 2023; June 29, 2023; August 8, 2023 (excluding Item 7.01 and the exhibit related thereto); September 21, 2023; November 13, 2023; and November 15, 2023 | |
| ● | Our Definitive Proxy Statement on Schedule 14A, filed with the SEC on May 1, 2023; and | |
| ● | The description of our common stock contained in our Registration Statement on Form 8-A, filed on September 17, 2020 pursuant to Section 12(b) of the Exchange Act, which incorporates by reference the description of the shares of our common stock contained in the “Description of Securities.” |
We will furnish without charge to you, on written or oral request, a copy of any or all of the documents incorporated by reference in this prospectus, including exhibits to these documents. You should direct any requests for documents to Wendy Guy, Chief Administrative Officer at Processa Pharmaceuticals, Inc. 7380 Coca Cola Drive, Suite 106, Hanover, Maryland 21076 or at (443) 776-3133.
| 37 |
You also may access these filings on our website at www.processapharmaceuticals.com. We do not incorporate the information on our website into this prospectus or any supplement to this prospectus and you should not consider any information on, or that can be accessed through, our website as part of this prospectus or any supplement to this prospectus (other than those filings with the SEC that we specifically incorporate by reference into this prospectus or any supplement to this prospectus).
Any statement contained in a document incorporated or deemed to be incorporated by reference in this prospectus will be deemed modified, superseded or replaced for purposes of this prospectus to the extent that a statement contained in this prospectus modifies, supersedes or replaces such statement.
WHERE YOU CAN FIND MORE INFORMATION
This prospectus is part of a registration statement we filed with the SEC. This prospectus does not contain all of the information set forth in the registration statement and the exhibits to the registration statement. For further information with respect to us and the securities we are offering under this prospectus, we refer you to the registration statement and the exhibits and schedules filed as a part of the registration statement. You should rely only on the information contained in this prospectus or incorporated by reference into this prospectus. We have not authorized anyone else to provide you with different information. We are not making an offer of these securities in any jurisdiction where the offer is not permitted. You should assume that the information contained in this prospectus, or any document incorporated by reference in this prospectus, is accurate only as of the date of those respective documents, regardless of the time of delivery of this prospectus or any sale of our securities.
We file annual, quarterly and current reports, proxy statements and other information with the SEC under the Exchange Act. Our SEC filings are available to the public from commercial document retrieval services and over the Internet at the SEC’s website at http://www.sec.gov.
We maintain a website at www.processapharmaceuticals.com. You may access our proxy statements, annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act with the SEC free of charge at our website as soon as reasonably practicable after such material is electronically filed with, or furnished to, the SEC. The information contained in, or that can be accessed through, our website is not incorporated by reference into, and is not part of, this prospectus.
You may also request a copy of these filings, at no cost to you, by writing or telephoning us at the following address:
Processa Pharmaceuticals, Inc.
Attn: Corporate Secretary
7380 Coca Cola Drive, Suite 106
Hanover, Maryland 21076
Telephone (443) 776-3133
| 38 |
Processa Pharmaceuticals, Inc.
Up to ● Shares of Common Stock
Up to ● Pre-Funded Warrants to Purchase up to ● Shares of Common Stock
Up to ● Common Warrants to Purchase up to ● Shares of Common Stock
Up to ● Placement Agent Warrants to Purchase up to ● Shares of Common Stock
Up to ● Shares of Common Stock underlying such Pre-Funded Warrants, Common Warrants and Placement Agent Warrants
PRELIMINARY PROSPECTUS
●, 202●
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution.
The following table sets forth all costs and expenses, other than underwriting discounts and commissions, paid or payable by the Registrant in connection with the issuance and sale of the common stock being registered. All amounts shown are estimates except for the SEC registration fee and the FINRA filing fee:
| Amount | ||||
| SEC registration fee | $ | ● | ||
| FINRA filing fee | ● | |||
| Legal fees and expenses | ● | |||
| Accounting fees and expenses | ● | |||
| Transfer agent and registrar fees and expenses | ● | |||
| Miscellaneous expenses | ● | |||
| Total | $ | ● | ||
Item 14. Indemnification of Directors and Officers.
Processa Pharmaceuticals, Inc. is incorporated under the laws of the State of Delaware.
Section 102(b)(7) of the General Corporation Law of the State of Delaware, or the “DGCL,” permits a Delaware corporation to include a provision in its certificate of incorporation eliminating or limiting the personal liability of directors to the corporation or its stockholders for monetary damages for breach of fiduciary duty as a director. This provision, however, may not eliminate or limit a director’s liability (1) for breach of the director’s duty of loyalty to the corporation or its stockholders, (2) for acts or omissions not in good faith or involving intentional misconduct or a knowing violation of law, (3) under Section 174 of the DGCL, or (4) for any transaction from which the director derived an improper personal benefit. The amended and restated certificate of incorporation of Processa contains such a provision.
Section 145(a) of the DGCL provides that a corporation may indemnify any person who was or is a party or is threatened to be made a party to any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative (other than an action by or in the right of the corporation), by reason of the fact that the person is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise, against expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred by the person in connection with such action, suit or proceeding if the person acted in good faith and in a manner the person reasonably believed to be in or not opposed to the best interests of the corporation, and, with respect to any criminal action or proceeding, had no reasonable cause to believe the person’s conduct was unlawful.
Section 145(b) of the DGCL provides that a corporation may indemnify any person who was or is a party or is threatened to be made a party to any threatened, pending or completed action or suit by or in the right of the corporation to procure a judgment in its favor by reason of the fact that the person is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise, against expenses (including attorneys’ fees) actually and reasonably incurred by the person in connection with the defense or settlement of such action or suit if the person acted in good faith and in a manner the person reasonably believed to be in or not opposed to the best interests of the corporation and except that no indemnification shall be made in respect of any claim, issue or matter as to which such person shall have been adjudged to be liable to the corporation unless and only to the extent that the Delaware Court of Chancery or the court in which such action or suit was brought shall determine upon application that, despite the adjudication of liability but in view of all of the circumstances of the case, such person is fairly and reasonably entitled to indemnity for such expenses which the Delaware Court of Chancery or such other court shall deem proper.
| II-1 |
Section 145(c) of the DGCL provides that to the extent that a present or former director or officer of a corporation has been successful on the merits or otherwise in defense of any action, suit or proceeding referred to in subsections (a) and (b) of Section 145 of the DGCL, or in defense of any claim, issue or matter therein, such person shall be indemnified against expenses (including attorneys’ fees) actually and reasonably incurred by such person in connection therewith.
Section 145(e) of the DGCL permits a Delaware corporation to advance litigation expenses, including attorneys’ fees, incurred by present and former directors and officers prior to the final disposition of the relevant proceedings. The advancement of expenses to a present director or officer is conditioned upon receipt of an undertaking by or on behalf of such director or officer to repay the advancement if it is ultimately determined that such director or officer is not entitled to be indemnified by the corporation. Advancement to former officers and directors may be conditioned upon such terms and conditions, if any, as the corporation may deem appropriate.
Section 145(g) of the DGCL specifically allows a Delaware corporation to purchase liability insurance on behalf of its directors and officers and to insure against potential liability of such directors and officers regardless of whether the corporation would have the power to indemnify such directors and officers under Section 145 of the DGCL.
The amended and restated certificate of incorporation and the amended and restated bylaws of Processa authorize the corporation to indemnify its directors and officers to the fullest extent permitted by law.
The foregoing summaries are necessarily subject to the complete text of the DGCL and Processa’s amended and restated certificate of incorporation and amended and restated bylaws.
Item 15. Recent Sales of Unregistered Securities.
In the three years preceding the filing of this registration statement, the Company has issued the following securities that were not registered under the Securities Act:
| ● | On February 24, 2021, the Company closed a private placement for the sale of 1,321,132 shares of common stock at a purchase price of $7.75 per share to accredited and institutional investors for gross proceeds of $10.2 million. | |
| ● | On March 23, 2022, the Company issued 123,609 shares of common stock (valued at $450,000) to Lincoln Park Capital Fund, LLC as a commitment fee in connection with entering into a Purchase Agreement. | |
| ● | On November 18, 2023, the Company issued 300,000 warrants to purchase shares of common stock for $0.37 per share to an accredited investor pursuant to the terms of an Investor Relations Agreement. |
All sales of securities described above were exempt from the registration requirements of the Securities Act in reliance on Section 4(a)(2) of the Securities Act, Rule 701 promulgated under the Securities Act or Regulation D promulgated under the Securities Act, relating to transactions by an issuer not involving a public offering. All of the foregoing securities are deemed restricted securities for purposes of the Securities Act.
Item 16. Exhibits and Financial Statement Schedules.
(a) Exhibits.
| II-2 |
+ | Indicates a management contract or compensatory plan or arrangement. |
| * | Filed herewith |
| ** | To be filed by amendment |
(b) Financial Statement Schedules.
All other schedules are omitted because they are not required, are not applicable, or the information is included in the financial statements or the related notes to financial statements thereto.
| II-3 |
Item 17. Undertakings.
The undersigned registrant hereby undertakes:
(a) To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
(i) To include any prospectus required by Section 10(a)(3) of the Securities Act;
(ii) To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the SEC pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than 20 percent change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement; and
(iii) To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement;
provided, however, that paragraphs (1)(i), (1)(ii) and (1)(iii) above do not apply if the information required to be included in a post-effective amendment by those paragraphs is contained in reports filed with or furnished to the Securities and Exchange Commission by the registrant pursuant to Section 13 or Section 15(d) of the Securities Exchange Act of 1934 that are incorporated by reference in the registration statement.
(b) that, for the purpose of determining any liability under the Securities Act, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(c) to remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering.
(d) that, for the purpose of determining liability under the Securities Act of 1933 to any purchaser, each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use.
(e) that, for the purpose of determining liability of the registrant under the Securities Act to any purchaser in the initial distribution of the securities, the undersigned registrant hereby undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser:
(i) Any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424 (§ 230.424 of this chapter);
(ii) Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant;
(iii) The portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and
(iv) Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser.
| II-4 |
(f) Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the Registrant pursuant to the foregoing provisions, or otherwise, the Registrant has been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the Registrant of expenses incurred or paid by a director, officer or controlling person of the Registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the Registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Act and will be governed by the final adjudication of such issue.
(g) That:
| (1) | For purposes of determining any liability under the Securities Act of 1933, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the Registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective. | |
| (2) | For the purpose of determining any liability under the Securities Act of 1933, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
(h) That, for purposes of determining any liability under the Securities Act of 1933, each filing of the Registrant’s annual report pursuant to section 13(a) or section 15(d) of the Securities Exchange Act of 1934 (and, where applicable, each filing of an employee benefit plan’s annual report pursuant to section 15(d) of the Securities Exchange Act of 1934) that is incorporated by reference in the registration statement shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
| II-5 |
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, as amended, the Registrant has duly caused this Registration Statement on Form S-1 to be signed on its behalf by the undersigned, thereunto duly authorized, in Hanover, Maryland, on the 29th day of December, 2023.
| Processa Pharmaceuticals, Inc. | |
| /s/ George Ng. | |
| George Ng. | |
| Chief Executive Officer and Director |
Each person whose signature appears below constitutes and appoints George Ng and James Stanker as his or her true and lawful attorneys-in-fact and agents, with full power of substitution and resubstitution, for such person and in his or her name, place and stead, in any and all capacities, to sign any or all further amendments (including post-effective amendments) to this registration statement (and any additional registration statement related hereto permitted by Rule 462(b) promulgated under the Securities Act of 1933, as amended (and all further amendments, including post-effective amendments, thereto)), and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or their substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the dates indicated.
| Signature | Title | Date | ||
| /s/ George Ng | Chief Executive Officer | December 29, 2023 | ||
| George Ng. | (principal executive officer) | |||
| /s/ James Stanker | Chief Financial Officer | December 29, 2023 | ||
| James Stanker | (principal accounting officer and principal financial officer) | |||
| /s/ Khoso Baluch | Director | December 29, 2023 | ||
| Khoso Baluch | ||||
| /s/ James Neal | Director | December 29, 2023 | ||
| James Neal | ||||
| /s/ Geraldine Pannu | Director | December 29, 2023 | ||
| Geraldine Pannu | ||||
| /s/ Justin Yorke | Director | December 29, 2023 | ||
| Justin Yorke | ||||
| /s/ Dr. David Young | Director | December 29, 2023 | ||
| Dr. David Young |
| II-6 |