UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
☒ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For The Year Ended December 31, 2023
OR
☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For The Transition Period From To
Commission file number 001-13795
AMERICAN VANGUARD CORPORATION
| | |
Delaware | | 95-2588080 |
(State or other jurisdiction of Incorporation or organization) | | (I.R.S. Employer Identification Number) |
| | |
4695 MacArthur Court, Newport Beach, California | | 92660 |
(Address of principal executive offices) | | (Zip Code) |
(949) 260-1200
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
| | | | |
Title of each class | | Trading Symbol(s) | | Name of each exchange on which registered |
Common Stock, $0.10 par value | | AVD | | New York Stock Exchange |
Securities registered pursuant to Section 12(g) of the Act:
NONE
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☒
Indicate by check mark whether the registrant is not required to file reports pursuant to Section 13 or Section 15 (d) of the Act. Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15 (d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| | | | | | |
Large accelerated filer |
| ☐ |
| Accelerated filer |
| ☒ |
Non-accelerated filer |
| ☐ |
| Smaller reporting company |
| ☐ |
| | | | Emerging growth company | | ☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☒
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ☐
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b).
☐Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes ☐ No ☒
The aggregate market value of the voting stock of the registrant held by non-affiliates is $503.9 million. This figure is estimated as of June 30, 2023 at which date the closing price of the registrant’s Common Stock on the New York Stock Exchange was $17.87 per share. For purposes of this calculation, shares owned by executive officers, directors, and 5% stockholders known to the registrant have been deemed to be owned by affiliates. The number of shares of $0.10 par value Common Stock outstanding as of June 30, 2023, was 34,643,674. The number of shares of $.10 par value Common Stock outstanding as of March 5, 2024 was 28,795,082.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the Registrant’s definitive proxy statement relating to its 2024 annual meeting of shareholders are incorporated by reference into Part III of this Annual Report on Form 10-K where indicated. The Registrant’s definitive proxy statement will be filed with the U.S. Securities and Exchange Commission within 120 days after the end of the fiscal year to which this report relates.
AMERICAN VANGUARD CORPORATION
ANNUAL REPORT ON FORM 10-K
December 31, 2023
AMERICAN VANGUARD CORPORATION
(Dollars in thousands, except per share data)
PART I
Unless otherwise indicated or the context otherwise requires, the terms “Company,” “we,” “us,” and “our” refer to American Vanguard Corporation and its consolidated subsidiaries (“AVD”).
Forward-looking statements in this report, including without limitation, statements relating to the Company’s plans, strategies, objectives, expectations, intentions, and adequacy of resources, are made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. Investors are cautioned that such forward-looking statements involve risks and uncertainties. (Refer to Part I, Item 1A, Risk Factors and Part II, Item 7, Management’s Discussion and Analysis of Financial Condition and Results of Operation, included in this Annual Report.)
All dollar amounts reflected in the consolidated financial statements are expressed in thousands, except per share data.
ITEM 1 BUSINESS
American Vanguard Corporation (“AVD”) was incorporated under the laws of the State of Delaware in January 1969 and operates as a holding company. Unless the context otherwise requires, references to the “Company” or the “Registrant” in this Annual Report refer to AVD. The Company conducts its business through its principle operating subsidiaries, including AMVAC Chemical Corporation (“AMVAC”) for its domestic business and AMVAC Netherlands BV (“AMVAC BV”) for its international business.
The operating subsidiaries in the U.S. include: AMVAC, GemChem, Inc. (“GemChem”), Envance Technologies, LLC (“Envance”), TyraTech Inc. (“TyraTech”) and OHP Inc. (“OHP”).
Internationally, the Company operates its business through the following subsidiaries: AMVAC BV, AMVAC Hong Kong Limited (“AMVAC Hong Kong”), AMVAC Mexico Sociedad de Responsabilidad Limitada (“AMVAC M”), AMVAC de Costa Rica Sociedad de Responsabilidad Limitada (“AMVAC CR Srl”), Grupo AgriCenter (including the parent AgriCenter S.A. and its subsidiaries) (“AgriCenter”), AMVAC do Brasil Representácoes Ltda (“AMVAC do Brasil”), AMVAC do Brazil 3p LTDA (“AMVAC 3p”), American Vanguard Australia PTY Ltd (“AVD Australia”), AgNova Technologies PTY Ltd (“AgNova”), and the Agrinos group entities (“Agrinos”).
The Company has one reportable segment.
AMVAC is a California corporation that traces its history from 1945 and is a manufacturer of chemical, biological and biorational products that develops and markets solutions for agricultural, commercial and consumer uses. It synthesizes and formulates chemicals and ferments and extracts microbial products for crops, turf, ornamental plants, and human and animal health protection. These products, which include insecticides, fungicides, herbicides, soil health, plant nutrition, molluscicides, growth regulators, soil fumigants, and biorationals, are marketed in liquid, powder, and granular forms. AMVAC primarily synthesizes, formulates, and distributes its own proprietary products or custom manufactures, formulates or distributes for others. In addition, the Company has carved out a leadership position in closed delivery systems, currently offers certain of its products in SmartBox, Lock ‘n Load and EZ Load systems, and its most recent commercial high technology packaging system known as SIMPAS (see “Intellectual Property” below) which permits the delivery of multiple products (from AMVAC and/or other companies) at variable rates in a single pass. AMVAC has historically expanded its business through both the acquisition of established chemistries, the development and commercialization of new formulations or compounds through licensing arrangements, the expansion of its global distribution network to gain broader market access, and self-funded research and development of precision application technology. Beginning in 2021, we have commenced basic molecular research and development of intellectual property related to our green solutions portfolio.
AMVAC BV is a Netherlands Corporation that was established in 2012 and is based in the Netherlands. AMVAC BV sells products both directly and through its network of subsidiaries in various international territories.
Below is a description of the Company’s acquisition/licensing during 2023. There were no acquisitions in 2022.
On October 5, 2023, the Company completed the purchase of all the outstanding shares of Punto Verde, a well-established distributor of agricultural products, located in Guayaquil, Ecuador. The acquired assets included product registration, trade names and trademarks, customer lists, workforce, fixed assets, and existing working capital.
On January 4, 2023, the Company completed the purchase of certain assets from American Bio-Systems, Inc. related to the proprietary-formula microbial cleaning products BioMopPlus® and DrainGel®. The assets acquired include end-use registrations, trademarks, formulation know-how, and books and records.
Seasonality
The agricultural chemical industry, in general, is cyclical in nature. The demand for AVD’s products tends to be seasonal. Seasonal usage, however, does not necessarily follow calendar dates, but more closely follows growing patterns, weather conditions, geography, weather related pressure from pests and customer marketing programs. Further, growing seasons vary by geographical region; thus, there is no single seasonal cycle affecting our sales. Rather, multiple seasons transpire over the course of the calendar year.
Backlog
AVD primarily sells its products based on purchase orders. The purchase orders are typically fulfilled within a short time frame. As a result, backlog is not considered a significant factor of, or a valid metric for, AVD’s business. The 2022 year, however, did end with a significantly larger than normal backlog of orders, primarily resulting from supply chain challenges on one specific product line in the last three months of 2022. This situation was fully rectified by the end of 2023.
Customers
The Company’s largest three customers accounted for 15%, 14% and 8% of the Company’s sales in 2023; 18%, 13% and 8% in 2022; and 17%, 14% and 8% in 2021.
Distribution
In the U.S. AMVAC predominantly distributes its products through national distribution companies and buying groups or co-operatives, which purchase AMVAC’s goods on a purchase order basis and, in turn, sell them to retailers/growers/end-users.
Internationally, AMVAC BV has sales offices or wholly owned distributors in Central America, Mexico, Brazil, Australia, and India, and sales force executives or sales agents in several other territories. The Company’s domestic and international distributors and agents typically have long-established relationships with retailers/end-users, far-reaching logistics and transportation capabilities, and/or customer service expertise. The markets for AVD’s products vary by region, target crop, use and type of distribution channel. AVD’s distributors and agents are experienced at addressing the needs of these various markets.
Competition
In its many marketplaces, AVD faces competition from both domestic and foreign manufacturers. Many of our competitors are larger and have substantially greater financial and technical resources than AVD. AVD’s capacity to compete depends on its ability to develop additional applications (including delivery systems and precision application technologies) for its current products and/or expand its product lines and customer base. AVD competes principally based on quality, product efficacy, price, technical service and customer support. In some cases, AVD has positioned itself in smaller niche markets, which are no longer addressed by larger companies. In other cases, for example in the Midwest corn and soybean markets, the Company competes directly against larger competitors.
Manufacturing
Through its six manufacturing facilities (see Item 2, Properties), AVD synthesizes many of the technical grade active ingredients that are in its end-use products. Further, the Company formulates and packages its end-use products at four of its own facilities or at the facilities of third-party formulators in the U.S. and at various international locations. Furthermore, the Company owns and operates two biological fermentation sites, one site in the U.S. and one in Mexico, and, in addition, has a certain biological product manufactured under a long-term arrangement at a third-party facility in India.
Raw Materials
AVD utilizes numerous companies to supply the various raw materials and components used in manufacturing its products. Many of these materials are readily available from domestic sources. In instances where there is a single source of supply, AVD seeks to secure its supply by either long-term (multi-year) arrangements or purchasing on long lead times from its suppliers. Further, where the availability or cost of certain raw materials may be subject to the effect of tariffs and/or supply chain disruption, the Company may order goods at times or in volumes out of the ordinary course to optimize pricing and to ensure supply.
Intellectual Property
AVD’s proprietary product formulations are protected, to the extent possible, as trade secrets and, to a lesser extent, by patents. Certain of the Company’s closed delivery systems are patented, and the Company has both pending and issued patents relating to its equipment portfolio, particularly with respect to its SIMPAS and ULTIMUS technology. In addition, the Company owns multiple issued patents relating to both its low-impact Envance solutions as well as its Agrinos biological and microbial solutions. The Company believes that AVD’s trademarks bring value to its products in both domestic and foreign markets. AVD considers that, in the aggregate, its product registrations, trademarks, licenses, customer lists and patents constitute valuable assets. While it does not regard its current business as being materially dependent upon any single product registration, trademark, license, or patent, it believes that patents will play an increasingly important role in its precision application technologies and green solutions portfolio.
EPA Registrations
In the U.S., AVD’s products also receive protection afforded by the terms of the Federal Insecticide, Fungicide and Rodenticide Act (“FIFRA”), pursuant to which it is unlawful to sell any pesticide in the U.S., unless such pesticide has first been registered by the U.S. Environmental Protection Agency (“USEPA”). Most of the Company’s products that are sold in the U.S. are subject to USEPA registration and periodic re-registration requirements and are registered in accordance with FIFRA. This registration by USEPA is based, among other things, on data demonstrating that the product will not cause unreasonable adverse effects on human health or the environment, when used according to approved label directions. In addition, each state requires a specific registration before any of AVD’s products can be marketed or used in that state. State registrations are predominantly renewed annually with a smaller number of registrations that are renewed on a multiple year basis. Foreign jurisdictions typically have similar registration requirements by statute.
In addition, certain of the Company’s biological products are labeled organic under the Organic Materials Review Institute (“OMRI”), Washington State Department of Agriculture (“WSDA”) and/or California Department of Food and Agriculture (“CDFA”) and, as such, are subject to the requirements of those certification standards, including with respect to raw materials and processes. As is the case with synthetic products, these biological products are also subject to specific labeling requirements that may vary from state to state.
The USEPA, state, and foreign agencies have required, and may require in the future, that certain scientific data requirements be performed on registered products sold by AVD. AVD, on its own behalf and in joint efforts with other registrants, has furnished, and is currently furnishing, required data relative to specific products. Under FIFRA, the federal government requires registrants to submit a wide range of scientific data to support U.S. registrations, including in the case of adding labeled uses. This requirement results in operating expenses in such areas as regulatory compliance, with USEPA and other such bodies in the markets in which the Company sells its products. In addition, at times, the Company is required to generate new formulations of existing products and/or to produce new products in order to remain compliant. The Company expensed $21,833, $18,081 and $16,643, during 2023, 2022 and 2021, respectively, on these activities. The costs are included in operating expenses in the Company’s consolidated statements of operations.
| | | | | | | | | | | | |
| | 2023 | | | 2022 | | | 2021 | |
Registration | | $ | 14,498 | | | $ | 11,979 | | | $ | 10,687 | |
Product development | | | 7,335 | | | | 6,102 | | | | 5,956 | |
Total | | $ | 21,833 | | | $ | 18,081 | | | $ | 16,643 | |
Environmental
American Vanguard is committed to be a part of the global solution to reduce the overall impact on the environment through our focused improvement efforts that minimize energy consumption, greenhouse gas emission, waste generation, and water consumption at our manufacturing and laboratory facilities. We cover our commitment and the specifics of our environmental stewardship program in our 2023 Corporate Sustainability Report, which can be found on the Company's website.
With respect to specific matters involving environmental considerations, during 2023, AMVAC continued activities to address environmental issues associated with its facility in Commerce, CA. (the “Facility”). An outline of the history of those activities follows.
In 1995, the California Department of Toxic Substances Control (“DTSC”) conducted a Resource Conservation and Recovery Act (“RCRA”) Facility Assessment (“RFA”) of those facilities having hazardous waste storage permits. In March 1997, the RFA culminated in DTSC accepting the Facility into its Expedited Remedial Action Program. Under this program, the Facility was required to conduct an environmental investigation and health risk assessment. This activity then took two paths: first, the RCRA permit closure and second, the larger site characterization.
With respect to the larger site characterization, soil and groundwater characterization activities began in December 2002 in accordance with the Site Investigation Plan that was approved by DTSC. Additional activities were conducted from 2003 to 2014, with oversight provided by DTSC. In 2014, the Company submitted a remedial action plan (“RAP”) to DTSC, under the provisions of which, the Company proposed not to disturb sub-surface contaminants, but to continue monitoring, maintain the cover above affected soil, enter into restrictive covenants regarding the potential use of the property in the future, and provide financial assurances relating to the requirements of the RAP. In January 2017, the RAP was circulated for public comment. DTSC responded to those comments and, on September 29, 2017, approved the RAP as submitted by the Company. The Company continues to conduct groundwater monitoring and maintain the existing cover above affected soil and throughout the Site. In 2022, the Company recorded land use covenants on certain affected parcels. In September 2023, DTSC approved the Company’s Remedial Action Completion Report and requested the Company prepare an estimate of costs for ongoing operations and maintenance to maintain the existing cover and conduct semi-annual groundwater monitoring. In 2024, DTSC requested the Company to prepare an Operations & Maintenance Plan (“O&M Plan”) to detail long-term monitoring requirements for the Site. After DTSC review and approval of the O&M Plan and associated cost estimate, the Company will be required to establish an approved financial assurance mechanism. At this stage, the Company does not believe that costs to be incurred in connection with the RAP will be material and has not recorded a liability for these activities.
AMVAC is subject to numerous federal and state laws and governmental regulations concerning environmental matters and employee health and safety at its six manufacturing facilities. The Company continually adapts its manufacturing process to the latest environmental control standards of the various regulatory agencies. The USEPA and other federal and state agencies have the authority to promulgate regulations that could have a material impact on the Company’s operations.
AMVAC expends substantial effort to minimize the risk of discharge of materials in the environment and to comply with the governmental regulations relating to protection of the environment. Wherever feasible, AMVAC recovers and recycles raw materials and increases product yield in order to partially offset increasing pollution abatement costs.
The Company is committed to a long-term environmental protection program that reduces emissions of hazardous materials into the environment, as well as to the remediation of identified existing environmental concerns.
Human Capital Resources
We believe that, beyond being essential to our operations, our people have inestimable worth independent of our business. As outlined in our Human Rights Policy (see, www.american-vanguard.com under ESG tab), we believe that it is fundamental to our corporate responsibility and, indeed, to our humanity, that we recognize, respect and nurture the freedom and dignity of all persons. Accordingly, we have insinuated that belief throughout the fabric of our operations in our approach toward our employees. Indeed, the first two core values underlying our commitment to sustainability (see, Update to Corporate Sustainability Report, www.american-vanguard.com under ESG tab) are “Safety First” – which is a culture that begins with highly-regulated manufacturing plants, continues into the design of science-backed products and extends into market-leading delivery systems – and “Making a Difference” – under which, by rewarding achievement and giving our employees a voice, we attract diverse employees who want to make a difference in their careers, in the company and in the communities that we serve.
During 2023, the Company hired its first Senior Vice President of Human Resources who will lead our Human Capital program, which consists of the following elements:
•Board Oversight – through our Nominating and Corporate Governance Committee (“N&CG”), our board of directors oversees human capital-related risks and opportunities. Annually, the N&CG Committee requires that management provides an update on succession planning for key executives, emphasizing a forward-looking approach with a commitment to fostering diversity, equity, and inclusion in future leadership planning. We are developing Human Capital programs that drive a culture of performance and engagement.
•Strategy – the Company’s human capital strategy has two primary elements: employee engagement and providing them with competitive benefits (including an outstanding health benefits plan). As we have covered in our Update to Corporate Sustainability Report, our Company is a destination for highly qualified employees who are drawn to a workplace where they can make a difference. Our management philosophy prioritizes collaborative and consistent execution to fulfill our commitments, fostering a performance-driven culture. This strategic approach has empowered the company to optimize retention, even amidst the challenges of a competitive employment market.
•Compensation – as highlighted in our strategy, compensation is a pivotal component of our human capital approach. We consistently motivate our workforce through competitive compensation and comprehensive welfare benefits. Additionally, we proactively educate our employees on the totality of their compensation, encompassing wages, stock options, health benefits, and paid time off.
•Employee Engagement –our management style is to solicit good ideas from employees, involve them in implementation and give them recognition for ideas that succeed. For example, personnel from virtually any department (be it sales, technology, product development or otherwise) can submit ideas to our Innovation Review Committee (“IRC”) for consideration and potential funding. The IRC continues to be a source of new product ideas that has enabled us to launch several new formulations and other solutions on an annual basis. Similarly, our Beekeeper platform is a company-only social media channel on which employees anywhere in the world can report on their accomplishments, commendations of others and local developments.
•Diversity, Equity and Inclusion (“DEI”) – the Company is actively broadening its DEI program, incorporating Employee Resource Groups like the AMVAC Women Network to serve as a support group for professional development. Three of nine members (33%) of our board of directors are female and two of nine (22%) are from underrepresented groups (LGBTQ and Latinx). Based upon the Company’s most current EEO-1 (“Equal Employment Opportunity”) Report, representation of African Americans in our domestic workforce exceeds the prevalence of that group in the national population, while representation of Hispanic personnel is slightly below the national average. Our average employee age is 43 and our gender diversity is at 27% female and 73% male.
The Company employed 845 employees as of December 31, 2023, and 822 employees as of December 31, 2022. From time to time, due to the seasonality of its business, AVD uses temporary contract personnel to perform certain duties primarily related to packaging of its products. None of the Company’s employees are subject to a collective bargaining agreement. The Company believes it maintains positive relations with its employees.
Domestic operations
AMVAC is a California corporation that was incorporated under the name of Durham Chemical in August 1945. The name of the corporation was subsequently changed to AMVAC in January 1971. As the Company’s main operating subsidiary, AMVAC owns and/or operates the Company’s domestic manufacturing facilities. AMVAC manufactures, formulates, packages and sells its products in the U.S. and is a wholly owned subsidiary of AVD.
GemChem is a California corporation that was incorporated in 1991 and was subsequently purchased by the Company in 1994. GemChem sells into the pharmaceutical, cosmetic and nutritional markets and, in addition, purchases key raw materials for the Company. GemChem is a wholly owned subsidiary of AVD.
2110 Davie Corporation ("DAVIE") owns real estate for corporate use only. The site is the home to the Company’s research center and provides accommodation for the Company’s production control team. DAVIE is a wholly owned subsidiary of AVD.
On October 2, 2017, AMVAC purchased substantially all the assets of OHP, a domestic distribution company specializing in products for the turf and ornamental market. OHP markets and sells end use products for third parties, either under third-party brands or else as its own label products.
Envance is a Delaware Limited Liability Company that was formed in 2012 by AMVAC and joint venture partner TyraTech. Envance and TyraTech became wholly owned subsidiaries of the Company on November 9, 2018. Envance has the rights to develop and commercialize pesticide products and technologies based on TyraTech’s intellectual property. Products are made from natural oils and are marketed in global consumer, commercial, professional, crop protection and seed treatment markets. Envance is taking products to market primarily by licensing its intellectual property to third parties.
International operations
AMVAC BV is a registered Dutch private limited liability company that was formed in July 2012 to manage foreign sales on behalf of the Company. AMVAC BV is located in the Netherlands and is a wholly owned subsidiary of AMVAC Hong Kong. AMVAC Hong Kong is a wholly owned subsidiary of AMVAC. During 2023 and 2022, the international business sold the Company’s products in 45 countries.
AMVAC M is a wholly owned subsidiary of AMVAC BV and was originally formed in 1998 (as Quimica Amvac de Mexico S.A. de C.V and subsequently changed to AMVAC Mexico Sociedad de Responsabilidad Limitada “AMVAC M”) to conduct the Company’s business in Mexico.
On October 27, 2017, AMVAC BV purchased 100% of the stock of AgriCenter with subsidiaries located in Costa Rica, Panama, Nicaragua, Honduras, the Dominican Republic, Mexico, Guatemala, and El Salvador. AgriCenter markets, sells and distributes end-use chemicals, including the Company’s own products, and biological products throughout Central America primarily for crop applications.
On January 10, 2019, AMVAC BV acquired 100% of the stock of Agrovant and Defensive, two distribution companies based in Brazil. Agrovant and Defensive marketed and distributed crop protection products and micronutrients with focus on the fruit and vegetable market segments throughout Brazil. On December 31, 2020, Agrovant and Defensive merged and the Company renamed the resulting entity AMVAC 3p.
On October 8, 2020, AVD Australia acquired 100% of the stock of AgNova, an Australian company that sources, develops, and distributes specialty crop protection and production solutions for agricultural and horticultural producers, and for selected non-crop users.
On October 2, 2020, the Company’s principal operating subsidiary, AMVAC, completed the purchase of all outstanding shares of Agrinos and certain intellectual property rights. Agrinos is a fully integrated biological input supplier with proprietary technology, manufacturing, and global distribution capabilities and has operating entities in the U.S., Mexico, India, Brazil, China, Ukraine, and Spain.
On July 27, 2023, AgNova established a new subsidiary in New Zealand called AgNova Technologies NZ Limited (“AgNova NZ”). This new entity is a wholly owned subsidiary of AgNova and was created to expand our business in the Australasia region.
On October 5, 2023, AgriCenter acquired Punto Verde, an Ecuadorian company that distributes crop protection products primarily in Ecuador.
The Company classifies as international sales all products bearing foreign labeling shipped to a foreign destination.
| | | | | | | | | | | | |
| | 2023 | | | 2022 | | | 2021 | |
International sales | | $ | 234,855 | | | $ | 244,282 | | | $ | 215,439 | |
Percentage of net sales | | | 40.5 | % | | | 40.1 | % | | | 38.6 | % |
Risk Management
The Company’s Environmental, Social and Corporate Governance (“ESG”) strategy is fully described on our website at https://www.american-vanguard.com/esg.
Available Information
The Company makes available free of charge (through its website, www.american-vanguard.com), its Annual Report on Form 10-K, Quarterly Reports on Form 10-Q, current reports on Form 8-K, and all amendments to those reports as soon as reasonably practicable after such material is electronically filed with the Securities and Exchange Commission (“SEC”). All reports filed with the SEC are available free of charge on the SEC website, www.sec.gov. Also available free of charge on the Company’s website are the Company’s Audit Committee, Compensation Committee, Finance Committee and Nominating and Corporate Governance Committee Charters, the Company’s Corporate Governance Guidelines, the Company’s Code of Conduct and Ethics, and the Company’s Employee Complaint Procedures for Accounting and Auditing Matters. Beneath the ESG tab at that site, you will also find links to the Company’s Corporate Sustainability Reports, Climate Change Commitment and Human Rights Policy. The Company’s Internet website and the information contained therein or incorporated therein are not intended to be incorporated into this Annual Report on Form 10-K.
ITEM 1A. RISK FACTORS
Supply Chain/Regulatory/Geopolitical/Tax Risks
Disruption in the global supply chain is creating delays, unavailability and adverse conditions for our industry. Despite improvement in container availability and freight costs, the global supply chain continues to present risk. At present, military activity in the Red Sea is disrupting shipping routes within that region. While not directly affecting the Company, there is no guarantee that this or other supply chain conditions will materially improve any time soon or that the Company will avoid material disruption. Such disruption could have a material adverse effect on the Company’s financial performance in future reporting periods.
The regulatory climate remains challenging to the Company’s interests both domestically and internationally. Various agencies within the U.S. (both federal and state) and foreign governments continue to exercise increased scrutiny in permitting continued uses (or the expansion of such uses) of many chemistries, including several of the Company’s products and, in some cases, have initiated or entertained challenges to these uses. The challenge of the regulatory climate is more pronounced in certain geographical regions (outside the U.S.) where the Company faces resistance to the continued use of certain of its products. For example, the European Union (“EU”) employs a hazard-based analysis when considering whether product registrations can be maintained; under this approach, EU regulatory authorities typically do not weigh benefit against risk in their assessments and routinely cancel products for which a safer alternative is available, notwithstanding the benefit of the cancelled product. There is no guarantee that this regulatory climate will change in the near term or that the Company will be able to maintain or expand the use of many of its products in the face of such regulatory challenges.
Several of the Company’s organophosphates are subject to a petition to revoke tolerances under the FFDCA which, if granted, could result in the limitation and/or cancellation of one or more registrations for such products. Presently, several of the Company’s organophosphate products are under registration review before the USEPA and, at the same time, subject to a petition to revoke tolerances under the Federal Food, Drug, and Cosmetic Act ("FFDCA"). The Company continues to provide data and other analysis to USEPA in support of its registrations and in response to that agency’s requests for clarification. Pursuant to a petition filed by NGOs NRDC and PANNA in 2007, USEPA revoked tolerances for chlorpyrifos (an OP not sold by the Company) finding that food residues from that product could not be deemed with reasonable certainty to cause no harm. Consequently, the agency cancelled the registrations for chlorpyrifos. The cancellation was appealed and subsequently overturned by the Eighth Circuit Court of Appeal in 2023, which noted that the agency had exceeded its authority in canceling even uses that had been shown to be within applicable safety tolerances. That said, there is no guarantee that USEPA will not make a similar finding with respect to one or more of the Company’s OPs, and that some or all uses of the Company’s OP products could be limited or cancelled. Accordingly, the Company intends to take all action necessary to defend its registrations. Such limitations and/or cancellations could have a material adverse effect on the Company’s financial performance in future reporting periods.
USEPA has issued a proposed final decision (“PFD”) to cancel PCNB. In mid-2022, the USEPA issued a “proposed final decision” (a variety of agency action theretofore unknown to registrants) to cancel the fungicide PCNB, which is registered by the Company for use on golf courses and potatoes, among other things. The basis for its PFD was the agency’s initial contention that the product is persistent, bioaccumulative and toxic (“PBT”). In light of further analysis and Company input, the agency has suggested that the compound may merely have characteristics of a PBT. At any rate, then, the Company must meet a truncated schedule for defending this product, all of which is posted on a public docket. The Company has submitted proposed mitigation measures, and discussion with the program office continues. There is no guarantee that the Company will succeed in persuading USEPA not to cancel the PCNB registration. AMVAC is the sole registrant of PCNB which it manufactures at its own facility. Loss of this product could have a material adverse effect on the Company's financial performance in future reporting periods.
The Company is dependent upon sole source or a limited number of suppliers for certain of its raw materials and active ingredients. There are a limited number of suppliers of certain important raw materials used by the Company in a number of its products. Certain of these raw materials are available solely from single or very few sources either domestically or overseas. In connection with supply chain disruptions in 2022 phosphorus and related compounds were increasingly difficult to source for our entire industry; ensuring a continuous supply required extraordinary efforts both with respect to sourcing and production planning. Similarly, in the first half of 2023, DCPA, the active ingredient in one of the Company’s high-margin herbicides, was unavailable from its overseas supplier. There is no guarantee that any of our suppliers will be willing or able to supply products to the Company reliably, continuously and at the levels anticipated by the Company or required by the market. If these sources prove to be unreliable and the Company is not able to supplant or otherwise second source these products, it is possible that the Company will not achieve its projected sales which, in turn, could have a material adverse effect on the Company’s financial performance in future reporting periods.
Public statements made by USEPA regarding their preliminary findings in connection with the registration review of the Company’s products could adversely affect product sales and/or commercial viability. Registrations for the Company’s products are subject to registration review by the USEPA from time to time. In the course of the review, the Company submits, and the USEPA reviews, data studies. At any stage in the course of the review, USEPA may reach preliminary findings that could impair the commercial viability of a product. For example, in connection with USEPA’s review of the DCPA registration, based upon a comparative thyroid assay study (which is comparatively rare and quite complex), based upon limited data points, the USEPA found an adverse effect upon neonate rodents. Consequently, in June 2023, the agency published preliminary findings, noting its concern that based upon current, permitted use patterns, the product could have an adverse effect upon human health and, in particular, pregnant women. At the same time, the agency invited the Company to examine mitigation measures to allay their concerns, which the Company has done. There is no guarantee that mitigation measures or additional data proffered by the Company will be sufficient to overcome USEPA’s conclusions. Further, it is possible that the agency could take more drastic measures to either reduce the use or cancel the registration of the product. Regulatory activities of this nature, whether in connection with DCPA or other products of significance, could have a material adverse effect on the Company’s financial performance in future reporting periods.
The Company benefits from customer early pay in meeting its working capital needs. As is the case with other companies in this industry, the Company receives cash from certain major customers at year-end in exchange for granting discounts on the Company’s products during the first half of the following year. The Company typically uses this cash to pay down secured debt and for other working capital needs. This flow of cash obviates the need for additional borrowing, which, in turn, preserves borrowing capacity used in part for paying customer programs at the end of the calendar year and, consequently, reduces interest expense. There is no guarantee that the Company’s customers will continue to support the early pay program at current levels. Further a material change in this program could have an adverse effect on the Company’s liquidity and its ability to meet working capital demands.
Product liability judgments on glyphosate and cases involving other pesticides by domestic courts present a litigation risk to companies in this industry. Multiple judgments have been rendered by domestic courts in product liability cases against Bayer/Monsanto in connection with injuries allegedly arising from exposure to the herbicide product, glyphosate. The basis was purported carcinogenicity based largely upon the findings of a certain international organization, despite significant scientific evidence to the contrary. While the Company does not sell glyphosate, the theory of these results could put one or more of the Company’s products at risk. There is no guarantee that one or more product liability actions would not be brought against the Company on a similar basis, and it is possible that adverse rulings in any such actions could have a material adverse effect on the Company’s financial performance in future reporting periods.
The trend of passing pesticide “ban-bills” in various states could put one or more of the Company’s products at risk. In certain states, including Maryland and New York, state and/or local legislatures have passed legislation banning the use of specific pesticides, such as chlorpyrifos, or pesticide in general, in spite of valid registrations at USEPA and/or the equivalent state agency. While the Company does not sell chlorpyrifos products, there is no guarantee that one or more of its registered products will not be targeted in state or local legislation of this nature. Further, such legislation could have a material adverse effect on the Company’s financial performance in future reporting periods.
Use of the Company’s products is subject to continuing challenges from activist groups. Use of agrochemical products, including the Company’s products, is regularly challenged by activist groups in many jurisdictions under a multitude of federal, state and foreign statutes, including FIFRA, the Food Quality Protection Act, Endangered Species Act (“ESA”) and the Clean Water Act, to name a few. These challenges typically take the form of lawsuits or administrative proceedings against the USEPA and/or other federal, state or foreign agencies, the filing of amicus briefs in pending actions, the introduction of legislation that is inimical to the Company’s interests, and/or adverse comments made in response to public comment invited by regulatory agencies in the course of registration, re-registration or label expansion. The most prominent of these actions include a line of cases under which environmental groups have sought to suspend, cancel or otherwise restrict the use of pesticides that have been approved by USEPA on the ground that that agency failed to confer with the National Marine Fishery Service and/or the Fish and Wildlife Service under the ESA with respect to biological opinions relating to the use of such products. While industry has been active in defending registrations and proposing administrative and legislative approaches to address serious resource issues at the affected agencies, these cases continue to be brought. It is possible that one or more of these challenges could succeed, resulting in a material adverse effect on the Company's financial performance in future reporting periods.
The distribution and sale of the Company’s products are subject to governmental approvals and thereafter ongoing governmental regulation. The Company’s products are subject to laws administered by federal, state and foreign governments, including regulations requiring registration, approval and labeling of its products. The labeling requirements restrict the use of, and type of, application for our products. More stringent restrictions could make our products less available, which would adversely affect our revenues and profitability and cash flows. Substantially all the Company’s products are subject to the USEPA (and/or similar agencies in the various territories or jurisdictions in which we do business) registration and re-registration requirements and are registered in accordance with FIFRA or similar laws. Such registration requirements are based, among other things, on data demonstrating that the product will not cause unreasonable adverse effects on human health or the environment when used according to approved label directions. All states, where any of the Company’s products are used, also require registration before products can be marketed or used in that state. Governmental regulatory authorities have required, and may require in the future, that certain scientific data requirements be fulfilled on the Company’s products. The Company, on its behalf and also in joint efforts with other registrants, has furnished, and is currently furnishing certain required data relative to its products. There can be no assurance, however, that the USEPA or similar agencies will not request that certain tests or studies be repeated, or that more stringent legislation or requirements will not be imposed in the future. The Company can provide no assurance that any testing approvals or registrations will be granted on a timely basis, if at all, or that its resources will be adequate to meet the costs of regulatory compliance.
The manufacturing of the Company’s products is subject to governmental regulations. The Company currently owns and operates six manufacturing facilities which are located in Commerce, California; Axis, Alabama; Hannibal, Missouri; Marsing, Idaho; Clackamas, Oregon; and Etchojoa, Mexico (the “Facilities”). The Facilities operate under the laws and regulations imposed by relevant country, state and local authorities. The manufacturing of key ingredients for certain of the Company’s products occurs at the Facilities. An inability to renew or maintain a license or permit, or a significant increase in the fees for such licenses or permits, could impede the Company’s manufacture of one or more of its products and/or increase the cost of production; this, in turn, would materially and adversely affect the Company’s ability to provide customers with its products in a timely and affordable manner.
A change in tax laws, treaties or regulations, or their interpretation or application, could have a negative impact on our business and results of operations. We operate in many different countries and in many states within the United States, and we are subject to changes in applicable tax laws, treaties or regulations in the jurisdictions in which we operate. A material change in these tax laws, treaties or regulations, or their interpretation or application, could have a material adverse effect on the Company’s financial performance in future reporting periods.
Climate Change may adversely affect the Company’s business. Over the course of the past several years, global climate conditions have become increasingly inconsistent, volatile and unpredictable. Many of the regions in which the Company does business have experienced excessive moisture, cold, drought and/or heat of an unprecedented nature at various times of the year. In some cases, these conditions have either reduced or obviated the need for the Company’s products, whether pre-plant, at-plant, post-emergent or at harvest. Further, climate change could disrupt our operations by causing loss of human life, impacting the availability and cost of materials needed for manufacturing, causing physical damage and partial or complete closure of our manufacturing sites or distribution centers, temporary or long-term disruption in the manufacturing and supply of products and services and disruption in our ability to deliver products and services to customers. In addition, these events and disruptions could increase insurance and other operating costs, including impacting our decisions regarding construction of new facilities to select areas less prone to climate change risks and natural disasters, which could result in indirect financial risks passed through the supply chain or other price modifications to our products and services.
The Company’s business may be adversely affected by weather effects and commodity prices. Demand for many of the Company’s products tends to vary with weather conditions and weather-related pressure from pests. Adverse weather conditions, then, may reduce the Company’s revenues and profitability. In light of the possibility of adverse seasonal effects, there can be no assurance that the Company will maintain sales performance at historical levels in any particular region. Similarly, demand for the Company’s products used in row crops tends to vary with the commodity prices of those crops, for instance, corn, soybeans and cotton. These prices may be driven in part by weather, pest pressure, the domestic farm economy and international markets (e.g., yield and pricing from similar crops grown in Brazil). There is no guarantee that the farm economy and row crop commodity prices will maintain sufficient strength and stability to support the Company’s products at or above historical levels.
The Company may be subject to environmental liabilities. The Company is fully committed toward minimizing the risk of discharge of materials into the environment and to complying with governmental regulations relating to protection of the environment, its neighbors and its workforce. Nevertheless, federal and state authorities may seek fines and penalties for any violation of the various laws and governmental regulations. In addition, while the Company continually adapts its manufacturing processes to the latest environmental control standards of regulatory authorities, it cannot entirely eliminate the risk of accidental contamination or injury from hazardous or regulated materials. In short, the Company may be held liable for significant damages or fines relating to any environmental contamination, injury, or compliance violation which could have a material adverse effect on the Company’s financial performance in future reporting periods.
Acquisition/Investment Risks
Newly acquired businesses or product lines may not generate forecasted results. While the Company conducts due diligence using a combination of internal and third-party resources and applies what it believes to be appropriate criteria for each transaction before making acquisitions, there is no guarantee that a business or product line acquired by the Company will generate results that meet or exceed results that were forecasted by the Company when evaluating the acquisition. There are many factors that could affect the performance of a newly acquired business or product line. While the Company uses assumptions that are based upon due diligence and other market information in valuing a business or product line prior to concluding an acquisition, actual results generated post-closing could vary widely from the Company’s forecast and, as such, could have a material adverse effect on the Company’s financial performance in future reporting periods.
The Company’s investment in foreign businesses may pose additional risks. With the expansion of its footprint internationally, the Company now carries on business at a material level in some jurisdictions that have a history of political, economic or currency-related instability and customers with a potentially higher risk profile regarding accounts receivable collectability, as compared to the Company’s legacy business. While such instability may not be present at the current time, there is no guarantee that conditions will not change in one or more jurisdictions quickly and without notice, nor is there any guarantee that the Company would be able to recoup its investment in such territories in light of such changes and potential losses due to political factors, economic factors, devaluation of local currencies, or the collectability risk from customers. Adverse changes of this nature could have a material adverse effect on the Company’s financial performance in future reporting periods.
The Company’s investment in technology may not generate forecasted returns. The Company has had a history of investing in technological innovation, including with respect to precision application technologies (such as SIMPAS and Ultimus), natural oil technology and biologicals, as part of its growth strategy. These investments are based upon the premise that new technology will allow for safer handling or lower overall toxicity profile of the Company’s product portfolio, appeal to regulatory agencies and the market we serve, gain commercial acceptance, and command a return that is sufficiently in excess of the investment. However, there is no guarantee that a new technology will be successfully commercialized, generate a material return or maintain market appeal. Further, many types of development costs must be expensed in the period in which they are incurred. This, in turn, tends to put downward pressure on period profitability. There can be no assurance that these expenses will be recovered through successful long-term commercialization of a new technology.
The Company’s growth has been fueled in part by acquisitions. Over the past few decades, the Company’s growth has been driven by acquisitions and licensing of both established and developmental products from third parties. There is no guarantee that acquisition targets or licensing opportunities meeting the Company’s investment criteria will remain available or will be affordable. If such opportunities do not present themselves, then the Company may be unable to duplicate historical growth rates in future years.
The Company faces competition from generic competitors that source product from countries having lower cost structures. The Company continues to face competition from competitors around the globe that may enter the market through either offers to pay data compensation, or similar means in foreign jurisdictions, and then subsequently source material from countries having lower cost structures (typically India and China). These competitors typically tend to operate at thinner gross margins and, with low costs of goods, tend to drive pricing and profitability of subject product lines downward. There is no guarantee that the Company will maintain market share and pricing when facing such generic competitors, or that such competitors will not offer generic versions of the Company’s products in the future.
The Company’s key customers typically carry competing product lines and may be influenced by the Company’s larger competitors. A significant portion of the Company’s products are sold to national distributors in the U.S., which also carry product lines of competitors that are much larger than the Company. Typically, revenues from the sales of these competitor product lines and related program incentives constitute a greater part of our distributors’ income than do revenues from sales and program incentives arising from the Company’s product lines. With the recent consolidation among domestic distribution companies, these considerations have become more pronounced. In light of these facts, there is no assurance that such customers will continue to market our products aggressively or successfully, or that the Company will be able to influence such customers to continue to purchase our products instead of those of our competitors.
Industry consolidation may threaten the Company’s position in various markets. The global agricultural chemical industry continues to undergo significant consolidation. Many of the Company’s competitors have grown or are expected to grow through mergers and acquisitions. As a result, these competitors will tend to be in position to realize greater economies of scale, offer more diverse portfolios and thereby exert greater influence throughout the distribution channels. Consequently, the Company may find it more difficult to compete in various markets. While such merger activity may generate acquisition opportunities for the Company, there is no guarantee that the Company will benefit from such opportunities. Further, there is a risk that the Company’s future performance may be hindered by the growth of its competitors through consolidation.
The Company is dependent on a limited number of customers, which makes it vulnerable to the continued relationship with and financial health of those customers. Our top three customers accounted for 37% of the Company’s sales in 2023, and 39% of the Company’s sales in both 2022 and 2021. The Company’s future prospects may depend on the continued business of such customers and on our continued status as a qualified supplier to such customers. The Company cannot guarantee that these key customers will continue to buy products from us at current levels. The loss of a key customer could have a material adverse effect on the Company’s financial performance in future reporting periods.
General Risks
The carrying value of certain assets on the Company’s consolidated balance sheets may be subject to impairment depending upon market trends and other factors. The Company regularly reviews the carrying value of certain assets, including long-lived assets, inventory, fixed assets and intangibles. Depending upon the class of assets in question, the Company takes into account various factors including, among others, sales, trends, market conditions, cash flows, profit margins and the like. Based upon this analysis, where circumstances warrant, the Company may leave such carrying values unchanged or adjust them as appropriate. There is no guarantee that these carrying values can be maintained indefinitely, and it is possible that one or more such assets could be subject to impairment which, in turn, could have a material adverse effect on the Company’s financial performance in future reporting periods.
The Company’s computing systems are subject to cyber security risks. In the course of its operations the Company relies on its computing systems, including access to the internet, the use of third-party applications and the storage and transmission of data through such systems. While the Company has implemented security measures to protect these systems, there is no guarantee that a third-party will not penetrate these defenses through hacking, phishing or otherwise and either compromise, corrupt or shut down these systems. Further, in the event of such incursion it is possible that confidential business information and private personal data could be taken and operations, including procurement, customer service, finance, and manufacturing, could be compromised. Such an event could adversely affect both the Company’s ability to operate, its reputation with key stakeholders and its overall financial performance.
Reduced financial performance may limit the Company’s ability to borrow under its credit facility. The Company has historically grown net sales and net income through the expansion of current product lines, the acquisition of product lines from third parties and the acquisition of both domestic and international distributors with strong niche market positions. In order to finance such acquisitions, the Company has drawn upon its senior credit facility. However, the Company’s borrowing capacity under the senior credit facility depends, in part, upon its satisfaction of a negative covenant that sets a maximum ratio of borrowed debt to earnings (as measured over the trailing 12-month period). There is no guarantee that the Company will continue to generate earnings necessary to ensure that it has sufficient borrowing capacity to support future acquisitions or that, when necessary, the lender group will amend the senior credit facility to provide for such borrowing capacity. Further, despite the Company’s long-standing relationship with its lenders, in light of the uncertainties in global financial markets, there is no guarantee that the Company’s lenders will be either willing or able to continue lending to the Company at such rates and in such amounts as may be necessary to meet the Company’s working capital needs.
To the extent that capacity utilization is not fully realized at its manufacturing facilities, the Company may experience lower profitability. While the Company endeavors continuously to maximize utilization of its manufacturing facilities, our success in these endeavors is dependent upon many factors, including fluctuating market conditions, product life cycles, weather conditions in our key markets, availability of raw materials, manufacturing equipment performance, retention of the workforce and regulatory constraints, among other things. There can be no assurance that the Company will be able to maximize the utilization of its manufacturing facilities. Underutilization of such manufacturing resources could have a material adverse effect on the Company’s financial performance in future reporting periods.
The Company’s continued success depends, in part, upon a limited number of key employees. Within certain functions, the Company relies heavily on a small number of key employees to manage ongoing operations and to perform strategic planning. In some cases, there are no internal candidates who are qualified to succeed these key personnel in the short term. In the event that the Company were to lose one or more key employees, there is no guarantee that the Company could replace them with people having comparable skills. Further, the loss of key personnel could adversely affect the operation of our business.
Domestic and regional inflation trends, increased interest rates and other factors could lead to the erosion of economies and adversely impact the Company. Both the US and many other countries are experiencing inflation, which, in turn, is leading to increased costs in multiple industry segments, including agriculture and related industries. The persistence of inflation has led central bankers to increase interest rates within their regions. There is no guarantee that these measures will arrest the inflationary trend. Further, these factors, taken together with reduced productivity and constraints on the labor supply, could lead to recessionary periods in the regions in which the Company does business. While the Company takes measures within its control to manage the effects of inflation, higher interest rates and other factors, ultimately, they are outside of the Company’s control. Further, the persistence and/or severity of one or more of them could have a material adverse effect on the Company’s financial performance in future reporting periods.
None
ITEM 1C. CYBERSECURITY
Risk Management & Strategy. AVD has adopted a comprehensive set of controls and processes to encourage a high level of awareness of, and responsiveness to, cybersecurity threats. The foundational document outlining the program is embodied in Registrant’s Enterprise Information Security Policy (the “REIS Policy”). The REIS Policy establishes a framework for the continuous monitoring of its computing resources, the maintenance and reporting of audit logs and the assessment of events that could form the basis of a threat. In addition, the REIS Policy sets forth requirements for employee awareness training, user authentication, software usage restrictions and boundary protection, among other things. The policy also establishes an incident response plan, including back-up hosting, alternate processing and system recovery, along with assignment of responsibility and resources for those activities. Within the exhibit of the REIS Policy, management either working alone or, in case of greater complexity, with consultants, will assess an incident or series of incidents for materiality, taking into account the nature of the incident,, duration, the nature of data compromised, and the nature of damages (including with respect to reputational, third party, share price, and business interruption) and all within the context of the Company's financial performance during the affected reporting period(s).
Furthermore, AVD has taken measures to prevent cybersecurity breaches, to minimize threats and, to the extent possible, to anticipate trends and identify vulnerabilities before arising to the level of an incident. In short, AVD is pro-active in its approach and has formulated a specific plan to investigate, respond and minimize loss of functionality or other damage from an incident. There are no cybersecurity threats, including as a result of prior incidents, that have materially affected the Company, including our business strategy, results of operations or financial condition as of the date hereof to our knowledge.
Governance. The REIS Policy has been drafted in collaboration with one of the largest IT solutions providers in the field and was modeled after NIST standards relating to governance, documentation and processes. The Company is implementing the REIS Policy through its Cyber and Privacy Risk Steering Committee (the “CPRSC”), which is chaired by the Chief Administrative Officer (who is also AVD’s Risk Manager) and includes cross functional business process owners from operations, sales, marketing, finance and Human Resources, as well as our Director of Information Technology, who alone has over 30 years’ experience in IT-related security and whose staff collectively has over 50 years’ experience in this area. In addition, the committee is advised by a virtual Corporate Information Security Officer who works with the third-party solutions provider.
AVD's Board of Directors maintains oversight of cybersecurity planning, response and reporting as follows. The Lead Director, Scott Baskin, who also serves as Chair of the Risk Committee and member of the Audit Committee, is Cybersecurity Liaison to AVD’s management team. The Chair of the CPRSC reports on cybersecurity preparedness, issues and incidents to the Cybersecurity Liaison regularly. Through this reporting structure, the cybersecurity team has direct interaction with the highest level of the Board and with both the Risk and Audit Committees. Cyber risk has been a subject of regular review and discussion at the Risk Committee for several years. With the advent of the CPRSC, the delineation of governance and responsibility has become that much more focused.
ITEM 2 PROPERTIES
AMVAC owns in fee the Facility constituting approximately 152,000 square feet of improved land in Commerce, California (“Commerce”) on which its West Coast manufacturing, some of its warehouse facilities and some of its manufacturing administrative offices are located.
DAVIE owns in fee approximately 72,000 square feet of warehouse, office and laboratory space on approximately 118,000 square feet of land in Commerce, California, which is leased to AMVAC. In 2013, the Company made a significant investment in the Glenn A. Wintemute Research Center, which houses the Company’s primary research laboratory supporting synthesis, formulation and other new product endeavors.
In 2001, AMVAC completed the acquisition of a manufacturing facility (the “Axis Facility”) from E.I. DuPont de Nemours and Company (“DuPont”). The Axis Facility was one of three such units located on DuPont’s 510-acre complex in Axis, Alabama. The acquisition consisted of a long-term ground lease of 25 acres and the
purchase of all improvements thereon. The facility is a multi-purpose plant designed for synthesis of active ingredients and formulation and packaging of finished products. In 2018, FMC Corporation acquired from DuPont a business unit, which held, among other things, the Axis Facility. Prior to expiration of the lease, AMVAC and FMC negotiated the terms of a new lease, which has a term of 15 years and the option to renew for two, 5-year periods.
On December 28, 2007, AMVAC purchased certain manufacturing assets relating to the production of Thimet and Counter and located at BASF’s multi-plant facility situated in Hannibal, Missouri (the “Hannibal Site”). Subject to the terms and conditions of the Agreement, AMVAC purchased certain buildings, manufacturing equipment, office equipment, fixtures, supplies, records, raw materials, intermediates and packaging constituting the “T/C Unit” of the Hannibal Site. The parties entered into a ground lease and a manufacturing and shared services agreement, under which BASF continues to supply various shared services to AMVAC for the Hannibal Site.
On March 7, 2008, AMVAC acquired from Bayer CropScience Limited Partnership, (“BCS LP”), a U.S. business of Bayer CropScience GmbH, a facility (the “Marsing Facility”) located in Marsing, Idaho, which consists of approximately 17 acres of improved real property. The Marsing Facility is engaged in the blending of liquid and powder raw materials and the packaging of some of the Company’s finished goods inventory in liquid, powder and pelletized formulations which are sold both in the U.S. and internationally. In addition, during 2019, the Company purchased approximately three acres of unimproved real estate immediately adjacent to the Marsing Facility for potential storage and operational use in the future.
On October 2, 2020, AMVAC completed the purchase of all outstanding shares of Agrinos which is a fully integrated biological input supplier with proprietary technology, internal manufacturing, and global distribution capabilities. Its High Yield Technology® product platform works in conjunction with other nutritional crop inputs to increase crop yield, improve soil health and reduce the environmental footprint of traditional agricultural practices. Agrinos has two primary biological production facilities, a state-of-the-art microbial fermentation facility based in Clackamas, Oregon, and a facility in Sonora, Mexico. The Clackamas and Sonora facilities are used as both manufacturing sites and operational centers for global supply chain and logistics.
AVD regularly adds chemical processing equipment to enhance or expand its production capabilities. The Company believes its facilities are in good operating condition, are suitable and adequate for current needs and have flexibility to change products. Facilities and equipment are insured against losses from fire as well as other usual business risks. The Company knows of no material defects in title to, or encumbrances on, any of its properties except that substantially all of the Company’s assets are pledged as collateral under the Company’s credit facility agreements with its lender group. For further information, refer to Note 3 of the Notes to the Consolidated Financial Statements in Part II, Item 8 of this Annual Report on Form 10-K.
AVD owns approximately 42 acres of unimproved land in Texas for possible future expansion.
AVD leases approximately 19,953 square feet of office space located at 4695 MacArthur Court in Newport Beach, California. In 2020, the lease was amended and was extended to June 30, 2026. The premises have served as the Company’s corporate headquarters since 1995.
The facilities occupied by GemChem, OHP, Envance and TyraTech (Envance and TyraTech are co-located), AMVAC BV, AMVAC M, AMVAC CR Srl, AgNova, Agrinos, AMVAC 3p and AgriCenter, consist of administration, development centers (in the case of Envance and TyraTech) and/or sales offices which are leased. In addition, AMVAC 3p leases warehouse space in Jaboticabal, Brazil.
ITEM 3 LEGAL PROCEEDINGS
Please refer to Note 5 – Litigation and Environmental of the Notes to the Consolidated Financial Statements in Part II, item 8 of this Annual Report on Form 10-K.
ITEM 4 MINE SAFETY DISCLOSURES
Not Applicable.
PART II
ITEM 5 MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Market Information
Effective March 7, 2006, the Company listed its $0.10 par value common stock (“Common Stock”) on the New York Stock Exchange under the ticker symbol AVD. From January 1998 through March 6, 2006, the Common Stock was listed on the American Stock Exchange under the ticker symbol AVD. The Company’s Common Stock traded on The NASDAQ Stock Market under the symbol AMGD from March 1987 through January 1998.
Holders
As of March 5, 2024, the number of stockholders of the Company’s Common Stock was approximately 10,089, which includes beneficial owners with shares held in brokerage accounts under street name and nominees.
Dividends
The Company has issued a cash dividend in each of the last twenty-seven years dating back to 1996. Cash dividends declared during the past three years are summarized in the table below.
| | | | | | | | | | | | |
Declaration Date | | Record Date | | Distribution Date | | Dividend
Per Share | | | Total
Paid | |
December 15, 2023 | | December 29, 2023 | | January 12, 2024 | | $ | 0.030 | | | $ | 834 | |
September 12, 2023 | | September 22, 2023 | | October 6, 2023 | | | 0.030 | | | | 834 | |
June 12, 2023 | | June 28, 2023 | | July 14, 2023 | | | 0.030 | | | | 848 | |
March 13, 2023 | | March 24, 2023 | | April 14, 2023 | | | 0.030 | | | | 851 | |
Total 2023 | | | | | | $ | 0.120 | | | $ | 3,367 | |
December 12, 2022 | | December 28, 2022 | | January 11, 2023 | | $ | 0.030 | | | $ | 851 | |
September 12, 2022 | | September 23, 2022 | | October 7, 2022 | | | 0.025 | | | | 715 | |
June 6, 2022 | | June 24, 2022 | | July 8, 2022 | | | 0.025 | | | | 742 | |
March 14, 2022 | | March 25, 2022 | | April 15, 2022 | | | 0.025 | | | | 736 | |
Total 2022 | | | | | | $ | 0.105 | | | $ | 3,044 | |
December 13, 2021 | | December 27, 2021 | | January 10, 2022 | | $ | 0.020 | | | $ | 594 | |
September 13, 2021 | | October 1, 2021 | | October 15, 2021 | | | 0.020 | | | | 594 | |
June 8, 2021 | | June 24, 2021 | | July 8, 2021 | | | 0.020 | | | | 600 | |
March 10, 2021 | | March 15, 2021 | | April 15, 2021 | | | 0.020 | | | | 596 | |
Total 2021 | | | | | | $ | 0.080 | | | $ | 2,384 | |
Share Repurchase Programs
The Company periodically repurchases shares of its common stock under board-authorized repurchase programs through a combination of open market transactions and accelerated share repurchase (“ASR”) arrangements. The Company did not repurchase any of its common stock during the three months ended December 31, 2023.
Pursuant to Amendment Number Six to the Third Amended Loan and Security Agreement, effective November 7, 2023, the Company is currently prevented from making stock repurchases.
Securities Authorized for Issuance under Equity Compensation Plans
| | | | | | | | | | | | |
Plan Category | | Number of securities to
be issued upon exercise
of outstanding options,
warrants, and rights | | | Weighted average
exercise price of
outstanding options,
warrants, rights | | | Number of securities
remaining available for
future issuance under
equity compensation plans | |
Equity compensation plans approved by security holders | | | 146,679 | | | $ | 11.49 | | | | 1,376,610 | |
Total | | | 146,679 | | | $ | 11.49 | | | | 1,376,610 | |
Stock Performance Graph
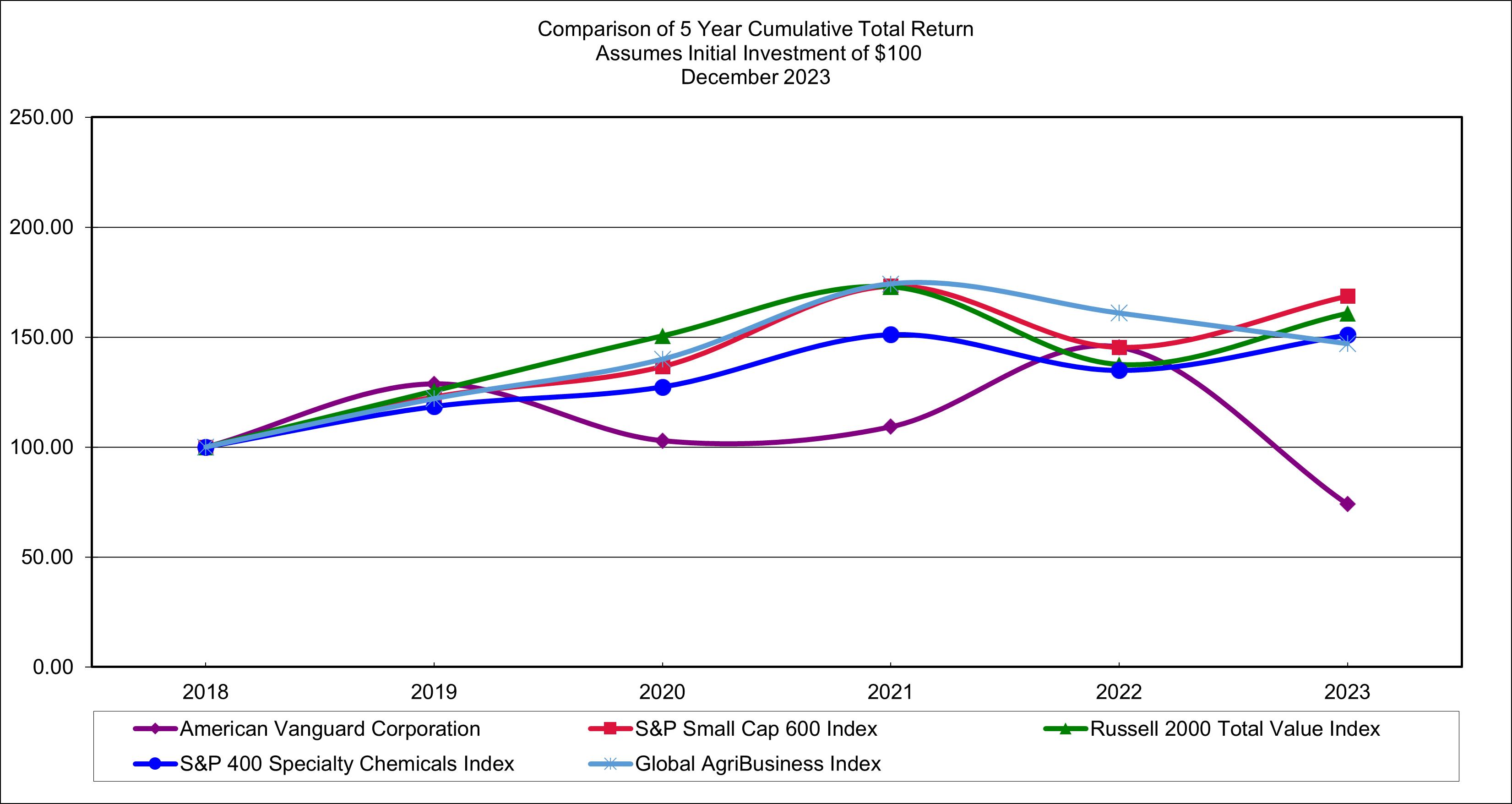
The following graph presents a comparison of the cumulative, five-year total return for the Company, the Russell 2000 Stock Index, and a peer group (S&P 400 Specialty Chemical Industry). The graph assumes that the beginning values of the investments in the Company, the Russell 2000 Stock Index, and the S&P 400 Specialty Chemical Index (a peer group of companies) each was $100 on December 31, 2018. All calculations assume reinvestment of dividends. Returns over the indicated period should not be considered indicative of future returns.
ITEM 6 RESERVED
Not applicable
ITEM 7 MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
FORWARD-LOOKING STATEMENTS/RISK FACTORS:
The Company, from time-to-time, may discuss forward-looking statements including assumptions concerning the Company’s operations, future results and prospects. Generally, “may,” “could,” “will,” “would,” “expect,” “believe,” “estimate,” “anticipate,” “intend,” “continue” and similar words identify forward-looking statements. Forward-looking statements appearing in this Report are made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. Forward-looking statements are based on our current expectations and are subject to risks and uncertainties that can cause actual results and events to differ materially from those set forth in or implied by the forward-looking statements and related assumptions contained in the entire Report. Such factors include, but are not limited to: product demand and market acceptance risks; the effect of economic conditions; weather conditions; changes in regulatory policy; the impact of competitive products and pricing; changes in foreign exchange rates; product development and commercialization difficulties; capacity and supply constraints or difficulties; availability of capital resources; general business regulations, including taxes and other risks as detailed from time-to-time in the Company’s reports and filings filed with the U.S. Security and Exchange Commission (“SEC”). It is not possible to foresee or identify all such factors. We urge you to consider these factors carefully in evaluating the forward-looking statements contained in this Report.
The discussion and analysis of our financial condition and results of operations for 2023, as compared to 2022, appears below. As permitted by SEC rules, we have omitted the discussion and analysis of our financial condition and results of operations for 2022 compared to 2021. See Item 7, “Management’s Discussions and Analysis of Financial Condition and Results of Operations”, in our Annual Report on Form 10-K for the year ended December 31, 2022, filed on March 16, 2023 for this discussion.
MANAGEMENT OVERVIEW
The Company’s performance in 2023 declined as compared to the prior year due primarily to three factors: first customers in many regions implemented destocking directives in order to limit their carrying costs of inventory, second, weakening economic conditions in China manifested themselves in a glut of low-priced generic products in many regions, and third, supply chain constraints affecting one key product.
Within this context, the Company experienced a drop in overall sales of approximately 5%, with domestic sales down 6% and international sales down 4%. The unavailability of two high margin products, Aztec and Dacthal, due to supply issues in the first half of 2023, accounted for much of the drop in consolidated sales. With the resumption of production of those products, the Company’s US crop business, and the Company as a whole, generated much stronger sales performance in the fourth quarter of 2023.
Cost of sales dropped by about 4% on lower sales in 2023 compared to 2022. Factory performance generated higher net expense as compared to 2022, due largely to the unavailability of raw materials and reduced demand from destocking activity early in the year. As a result, gross margin percent dropped to 31% in 2023 from 32% as compared to the prior year.
Operating expenses rose by about 2% in 2023, as compared to 2022, due primarily to increased selling expenses and regulatory costs, partially offset by reduced general and administrative costs including recording no short-term incentive compensation expense. Further, operating expenses as a percent of net sales rose to 27% in 2022, compared to 25% in 2022. With lower sales during the first three quarters of 2023 in the face of reduced demand and product unavailability, the Company’s average indebtedness rose to $167,976 ($117,705 last year) and, coupled with significantly higher interest rates, interest expense for the year rose sharply. Sales activity in the fourth quarter coupled with customer pre-pay activity enabled the Company to lower inventory and reduce indebtedness by year-end. On a full-year basis, the Company generated net income of $7,519 (or $0.26 per share) which, while down from that of the prior year ($27,404 or $0.92 per share), represents a significant rebound from the first three quarters of 2023. Details of our financial performance are set forth below.
Results of Operations
2023 Compared with 2022:
| | | | | | | | | | | | | | |
| | 2023 | | | 2022 | | | $ Change | | | % Change |
Net sales: | | | | | | | | | | | |
U.S. crop | | $ | 269,229 | | | $ | 288,624 | | | $ | (19,395 | ) | | -7% |
U.S. non-crop | | | 75,287 | | | | 76,709 | | | | (1,422 | ) | | -2% |
Total U.S. | | | 344,516 | | | | 365,333 | | | | (20,817 | ) | | -6% |
International | | | 234,855 | | | | 244,282 | | | | (9,427 | ) | | -4% |
Total net sales | | $ | 579,371 | | | $ | 609,615 | | | $ | (30,244 | ) | | -5% |
Total cost of sales | | $ | (400,207 | ) | | $ | (417,227 | ) | | $ | 17,020 | | | -4% |
Total gross profit | | $ | 179,164 | | | $ | 192,388 | | | $ | (13,224 | ) | | -7% |
Total gross margin | | 31% | | | 32% | | | | | | |
Net sales of our U.S. crop business were about 7% lower than those of the prior year. This performance was driven by several factors. The pacing of procurement in the domestic distribution channel relaxed from the strong purchasing practices of 2022 to more of a just-in-time approach in 2023, as higher interest rates increased the carrying cost of channel inventories, prompting distributors and retailers to respond with widespread destocking efforts. Additionally, our herbicide business was negatively affected by the supply-chain unavailability of Dacthal (for use on high-value crops, including onions) and increased competitive pressure on our Impact post-emergent corn herbicide brand caused by the steep decline in prices of many non-specific herbicides. Conversely, we experienced strong year-over-year sales of our granular soil insecticide products (including Aztec, Force, and Thimet). Our cotton products saw solid demand for our Bidrin foliar insecticides and a modest decline for our Folex harvest defoliant, as compared to 2022. We also recorded stronger sales of our soil fumigants (for use on a wide range of fruit and vegetables).
Net sales of our U.S. non-crop business were down about 2%. This decline was driven largely by a nationwide pattern of customer inventory destocking and purchasing control measures designed to deal with the challenge of high interest rate carrying costs. Net sales from our OHP nursery and ornamental business were slightly below those of 2022, as US consumer demand for plant materials softened. Sales of insecticides for commercial applications firmed during the year, as the professional pest-control market gradually regained more normal pre-pandemic levels.
Net sales of our international businesses decreased by 4% in 2023 vs. 2022. During 2023, our international group continued to integrate two important new businesses, AgNova in Australia and Agrinos biological products in more than a half dozen countries worldwide. We experienced a slight sales decline in our Central American businesses due to persistent drought conditions in Panama and a very significant influx of generic Chinese crop protection products which created a difficult pricing environment. Internationally, we experienced slightly lower demand for our soil fumigants due to increased Asian competition, partially offset by higher sales of herbicides. We saw a large increase in sales of our Counter nematicide in Brazil and in Mexico. Our Mocap brand improved sales in Mexico and Korea, while our Nemacur soil insecticide grew sales in Mexico, Argentina, and Taiwan.
Overall costs of sales declined by 4% across our U.S. crop, U.S. non-crop and international, which is roughly equivalent to the 5% average decline in net sales across those businesses.
Operating expenses increased by $4,132 in 2023, to $155,869 or 27% of net sales, as compared to $151,737 or 25% in 2022. The differences in operating expenses by department are as follows:
| | | | | | | | | | | | | | | | |
| | 2023 | | | 2022 | | | Change | | | % Change | |
Selling | | $ | 56,015 | | | $ | 52,512 | | | $ | 3,503 | | | | 7 | % |
General and administrative: | | | | | | | | | | | | |
Other | | | 47,049 | | | | 51,671 | | | | (4,622 | ) | | | -9 | % |
Proxy contest activities | | | 541 | | | | 1,785 | | | | (1,244 | ) | | | -70 | % |
Amortization | | | 13,282 | | | | 13,953 | | | | (671 | ) | | | -5 | % |
Transformation costs | | | 957 | | | | — | | | | 957 | | | | 100 | % |
Research, product development and regulatory | | | 38,025 | | | | 31,816 | | | | 6,209 | | | | 20 | % |
Total | | $ | 155,869 | | | $ | 151,737 | | | $ | 4,132 | | | | 3 | % |
•Selling expenses increased by 7% to $56,015 for the year ended December 31, 2023, as compared to $52,512 in 2022. This included inflation related increased wages, additional people and expenses associated with the newly acquired AgriCenter business in Ecuador, and increased costs associated with travel expenses as the sales teams work to grow the global business.
•General and administrative expenses decreased by 10% to $47,049 for the year ended December 31, 2023, as compared to $51,671 in 2022. During the year, we increased our accruals for potential bad debts primarily related to our Latin American business. That increase in cost was more than offset by reduced incentive compensation expense driven by the Company's financial performance and lower legal costs.
•The Company spent $541 in fees associated with our Proxy defense activities in 2023, as compared to $1,785 for the year ended December 31, 2022.
•Amortization expense declined in 2023 as compared to 2022 as a result of fully amortized assets.
•Research, product development and regulatory expenses increased by 19% to $38,025 in 2023, as compared to $31,816 in 2022. The main drivers were increases in our product development costs, primarily resulting from increased international regulatory activities and work in support of our SIMPAS/ULTIMUS technology platform. In addition, wages and travel expenses increased in support of our business.
•Transformation costs relate to the Company’s digital and structural transformation project. The digital transformation effort is intended to ensure that business process owners have access to current and complete data that has been generated through standardized processes. The structural transformation effort is intended to improve operating leverage by applying business analytics to current operations, structures, products and services. The Company hired a Chief Transformation Officer to steer and drive the project. In addition, the Company engaged a Human Resources consultant to help plan, overhaul and update the Company's Human Resources processes. Further, Kearney Management Consulting is assisting the Company in navigating the project to gain the maximum benefit at the earliest possible time.
In July 2020, the Company made a strategic investment in Clean Seed Inc. (Clean Seed) in the amount of $1,190. The investment is carried at fair value and is included in other assets on the Company’s consolidated balance sheets. The Company recorded losses related to Clean Seed’s change in fair value in the amount of $359 and $732 in 2023 and 2022, respectively. These losses are included in change in fair value of equity investments on the Company’s consolidated statements of operations.
Net interest expense was $12,639 in 2023, as compared to $3,954 in 2022. Interest costs are summarized in the following table:
| | | | | | | | | | | | | | | | | | | | | | | | |
| | 2023 | | | 2022 | |
Average Indebtedness and Interest expense | | Average
Debt | | | Interest
Expense, net | | | Interest
Rate | | | Average
Debt | | | Interest
Expense, net | | | Interest
Rate | |
Senior credit facility | | $ | 167,976 | | | $ | 12,136 | | | | 7.2 | % | | $ | 117,705 | | | $ | 3,977 | | | | 3.4 | % |
Interest expense, net | | | — | | | | (368 | ) | | | — | | | | — | | | | (167 | ) | | | — | |
Amortization of deferred loan fees | | | — | | | | 255 | | | | — | | | | — | | | | 261 | | | | — | |
Amortization of other deferred liabilities | | | — | | | | — | | | | — | | | | — | | | | 28 | | | | — | |
Other interest expense | | | — | | | | 1,183 | | | | — | | | | — | | | | 172 | | | | — | |
Subtotal | | | 167,976 | | | | 13,206 | | | | 7.9 | % | | | 117,705 | | | | 4,271 | | | | 3.6 | % |
Capitalized interest | | | — | | | | (567 | ) | | | — | | | | — | | | | (317 | ) | | | — | |
Total | | $ | 167,976 | | | $ | 12,639 | | | | 7.5 | % | | $ | 117,705 | | | $ | 3,954 | | | | 3.4 | % |
The Company’s average debt for the year ended December 31, 2023, was $167,976, as compared to $117,705 for the year ended December 31, 2022. The increase in average debt can be in large part attributed to the global agriculture market moving from buying early (during 2021 and 2022) to buying as close as possible to time of use. This effective destocking of the channel was a global Ag market reaction to high interest rates and the drive by distribution to push working capital back to manufacturers. Our effective interest rate on our senior credit facility increased to 7.4%, as compared to 3.6% in 2022.
Our provision for income taxes for 2023 was $2,778, as compared to $8,561 for 2022. The effective tax rate for 2023 was 27.0%, as compared to 23.8% in 2022. The increase of the effective tax rate in 2023, as compared to 2022, was primarily due to reductions in income before provision for income taxes, release of certain uncertain tax positions due to statute of limitation expirations in 2022 and an increase in the change in valuation allowance recorded through the income statement in 2023, partially offset by a one-time tax benefit associated with the migration of certain Intellectual Property to the U.S..
The Company is subject to U.S. federal income tax as well as to income tax in multiple state jurisdictions. Federal income tax returns of the Company are subject to Internal Revenue Service (“IRS”) examination for the 2020 through 2022 tax years. State income tax returns are subject to examination for the 2019 through 2022 tax years. The Company has other foreign income tax returns subject to examination.
Net income was $7,519 or $0.27 per basic share and $0.26 per diluted share in 2023, as compared to $27,404 or $0.94 per basic share and $0.92 per diluted share in 2022.
Liquidity and Capital Resources
The Company used $58,748 of cash in operating activities during the year ended December 31, 2023, as compared to cash provided by operating activities of $57,105 in the prior year. Included in the $58,748 are net income of $7,519, plus non-cash depreciation, amortization of intangibles and other long-term assets in the amount of $23,534, amortization of deferred loan fees and discounted liabilities of $254, and provision for bad debts in the amount of $1,935. In addition, stock-based compensation of $6,139, change in fair value of investments of $359, change in value of deferred income taxes of $9,710, change in value for uncertain tax positions or unrecognized tax benefits of $508, non-cash lease expense of $256 and net foreign currency adjustment of $581, resulted in net cash provided by operating activities (prior to changes in assets and liabilities associated with operations, net of business combinations) of $29,713 as compared to $55,529 for the same period of 2022.
The Company’s working capital increased by $79,560 at December 31, 2023, accounts receivable increased by $20,278, inventories increased by $27,315, tax receivable, net decreased by $3,568, and prepaid expenses decreased by $1,269. Deferred revenue decreased by $45,079, driven by customer decisions to make early payments in return for early cash incentive programs. Our accounts payable balances decreased by $2,287, program accruals decreased by $7,244 and other payables and accrued expenses decreased by $5,066.
With regard to our program accrual, the year-over-year change is primarily driven by the mix of product line sales and customers in 2023, as compared to the prior year. The Company accrues programs in line with the growing season upon which specific products are targeted. Most of our programs relate to domestic sales. Typically, domestic crops have a growing season that ends on August 31st of each year. During 2023, the Company made accruals in the amount of $93,264 and made payments in the amount of $85,931. During 2022, the Company made accruals in the amount of $111,649 and made payments in the amount of $114,151.
Cash used for investing activities amounted to $17,017 for the year ended December 31, 2023, as compared to $14,470 in 2022. In 2023, the Company spent $11,878 on capital expenditures primarily focused on continuing to invest in manufacturing infrastructure to expand production efficiency and capabilities, spent $186 on registrations and patents and received $242 in disposal of fixed assets. In 2023, the Company spent $5,195 in business acquisitions including intangible assets, goodwill, working capital and fixed assets as well as patent application costs and $650 in product acquisition.
During the year ended December 31, 2023, financing activities provided $66,737, as compared to $38,260 used during the prior year. This included making net borrowings of $86,600 on the Company’s senior credit facility during the year ended December 31, 2023, as compared to a net repayment of $1,000, in 2022. During 2023, the Company paid dividends to stockholders amounting to $3,384, as compared to $2,787 in 2022. Further, the Company used $15,539 to repurchase common stock in 2023, as compared to $34,002 in 2022. The Company also made payment of $68 in 2022 for contingent consideration where no such payment occurred in 2023.
The Company has long-term debt as of December 31, 2023 and 2022 relating to a senior credit facility as summarized in the following table:
| | | | | | | | |
Indebtedness | | 2023 | | | 2022 | |
Senior credit facility | | $ | 138,900 | | | $ | 52,300 | |
Deferred loan fees | | | (1,218 | ) | | | (823 | ) |
Total indebtedness | | $ | 137,682 | | | $ | 51,477 | |
The deferred loan fees as of December 31, 2023 are included in other assets on the consolidated balance sheets.
The Company and certain of its affiliates are parties to a senior credit facility agreement entitled the “Third Amended and Restated Loan and Security Agreement” dated as of August 5, 2021 (the “Credit Agreement”), which is a senior secured lending facility among AMVAC, the Company’s principal operating subsidiary, as Agent (including the Company and AMVAC BV), as "Borrowers", on the one hand, and a group of commercial lenders led by BMO Bank, N.A. (formerly Bank of the West) as administrative agent, documentation agent, syndication agent, collateral agent and sole lead arranger, on the other hand. The Credit Agreement consists of a line of credit of up to $275,000, an accordion feature of up to $150,000, a letter of credit and swingline sub-facility (each having limits of $25,000) and has a maturity date of August 5, 2026. The Credit Agreement amended and restated the previous credit facility, which had a maturity date of June 30, 2022. With respect to key financial covenants, the Credit Agreement contains two: namely, borrowers are required to maintain a Total Leverage (“TL”) Ratio of no more than 3.5-to-1, during the first three years, stepping down to 3.25-to-1 as of December 31, 2024, and a Fixed Charge Coverage Ratio ("FCCR") of at least 1.25-to-1. In addition, to the extent that it completes acquisitions totaling $15 million or more in any 90-day period, AMVAC may step-up the TL Ratio by 0.5-to-1, not to exceed 4.00-to-1, for the next three full consecutive quarters. Acquisitions below $50 million do not require Agent consent.
The Company’s borrowing capacity varies with its financial performance, measured in terms of Consolidated EBITDA as defined in the Credit Agreement, for the trailing twelve-month period. Under the Credit Agreement, revolving loans bear interest at a variable rate based, at borrower’s election with proper notice, on either (i) London Interbank Offered Rate ("LIBOR") plus the “Applicable Margin” which is based upon the Total Leverage (“TL”) Ratio (“LIBOR Revolver Loan”) or (ii) the greater of (x) the Prime Rate, (y) the Federal Funds Rate plus 0.5%, and (z) the Daily One-Month LIBOR Rate plus 1.00%, plus, in the case of (x), (y) or (z) the Applicable Margin (“Adjusted Base Rate Revolver Loan”). The Company and the Lenders entered into an amendment to the Credit Agreement, effective March 9, 2023, whereby LIBOR was replaced by the Secured Overnight Financing Rate ("SOFR") with a credit spread adjustment of 10.0 bps for all SOFR periods. The revolving loans now bear interest at a variable rate based at our election with proper notice, on either (i) SOFR plus 0.1% per annum and the “Applicable Margin” or (ii) the greater of (x) the Prime Rate, (y) the Federal Funds Rate plus 0.5%, and (z) the Daily One-Month SOFR Rate plus 1.10%, plus, in the case of (x), (y) or (z) the Applicable Margin (“Adjusted Base Rate Revolver Loan”). Interest payments for SOFR Revolver Loans are payable on the last day of each interest period (either one-, three- or six- month periods, as selected by the Company) and the maturity date, while interest payments for Adjusted Base Rate Revolver Loans are payable on the last business day of each month and the maturity date.
On November 7, 2023, the Company entered into Amendment Number Six to the Third Amended Loan and Security Agreement that provided relief in respect of both financial covenants. Specifically, with respect to the Maximum Total Leverage Ratio, the existing ratio of 3.5 through September 30, 2024 and 3.25 through December 31, 2024 and thereafter was changed to 5.5 through September 30, 2023, 4.5 for the periods ending December 31, 2023 and March 31, 2024, 4.0 for the period ending June 30, 2024, 3.5 through September 30, 2024 and returning to 3.25 from December 31, 2024 and thereafter. In addition, the Minimum Fixed Charge Coverage Ratio was changed from 1.25 to 1.0 for the periods ending September 30, 2023, December 31, 2023 and March 31, 2024 and returning to 1.25 for the period ending June 30, 2024 and thereafter. Further, after the delivery of financial statements and a covenant compliance certificate for the period ended December 31, 2023 assuming Total Leverage is less than 2.75, then Borrowers may terminate the covenant modification period (“CMP”) and revert to the terms of the existing Credit Agreement. Further, for the duration of the CMP, the Company is restricted from making share repurchases. Finally, the Applicable Margin (SOFR and Adjusted Base Rate) and Letter of Credit fees increase by 0.50 basis points for each tier of interest during CMP. The interest rate on December 31, 2023, was 8.33%.
As of December 31, 2023, the Company is deemed to be in compliance with its financial covenants.
According to the terms of the Credit Agreement, as amended, and based on our performance against the most restrictive covenant listed above, the Company had the capacity to increase its borrowings by up to $115,002 and $200,372 as of December 31, 2023 and December 31, 2022, respectively. Furthermore, at December 31, 2023, the Company’s leverage, as defined in the Credit Agreement, was 2.46.
The Company believes that the combination of its cash flows from future operations, current cash on hand and the availability under the Company’s credit facility will be sufficient to meet its working capital and capital expenditure requirements and will provide the Company with adequate liquidity to meet its anticipated operating needs for at least the next 12 months from the issuance of these consolidated financial statements. Although operating activities are expected to provide cash, to the extent of growth in the future, its operating and investing activities will use cash and, consequently, this growth may require the Company to access some or all of the availability under the credit facility. It is also possible that additional sources of finance may be necessary to support additional growth.
Recently Issued Accounting Guidance
Please refer to Note 1 of the Notes to the Consolidated Financial Statements in Part II, Item 8 of this Annual Report on Form 10-K for recently issued and adopted accounting standards.
CRITICAL ACCOUNTING ESTIMATES
The preparation of our consolidated financial statements requires us to make estimates that affect the reported amounts of assets, liabilities, sales and expenses, and related disclosure of contingent assets and liabilities. We base our estimates and judgments on historical experience and on various other assumptions that are believed to be reasonable under the circumstances. Actual results may differ from these estimates.
The Company’s critical accounting estimates include:
Current Expected Credit Losses —The Company maintains an allowance to cover its Current Expected Credit Losses ("CECL") on its trade receivables, other receivables and contract assets arising from the failure of customers to make contractual payments. The Company estimates credit losses expected over the life of its trade receivables, other receivables and contract assets based on historical information combined with current conditions that may affect a customer’s ability to pay and reasonable and supportable forecasts. In most instances, the Company’s policy is to write-off trade receivables when they are deemed uncollectible. The vast majority of the Company's trade receivables, other receivables and contract assets are less than 365 days. Under the CECL impairment model, the Company develops and documents its allowance for credit losses on its trade receivables based on multiple portfolios. The determination of portfolios is based primarily on geographical location, type of customer and receivables aging.
Inventories — The Company values its inventories at lower of cost or net realizable value. Cost is determined by the first-in, first-out (“FIFO”) or average cost method, including, as appropriate, raw materials, labor, factory overhead and subcontracting services. The Company writes down its inventory to the net realizable value following assessments of slow-moving and obsolete inventory and other annual adjustments to ensure that our standard costs continue to closely reflect actual manufacturing cost.
Intangible Assets—The primary identifiable intangible assets of the Company relate to assets associated with its product and business acquisitions. All of the Company’s intangible assets have finite lives and are amortized. The estimated useful life of an identifiable intangible asset is based upon a number of factors including the effects of demand, competition, and expected changes in the marketability of the Company’s products.
Business Combinations—The Company uses its best estimates and assumptions to assign fair value to the tangible and intangible assets acquired and liabilities assumed at the acquisition date. The Company’s estimates are inherently uncertain and subject to refinement. During the measurement period, which may be up to one year from the acquisition date, the Company may record adjustments to the fair value of these tangible and intangible assets acquired and liabilities assumed, with the corresponding offset to goodwill or an adjustment to the gain from a bargain purchase. In addition, uncertain tax positions and tax-related valuation allowances are initially recorded in connection with a business combination as of the acquisition date. The Company continues to collect information and re-evaluates these estimates and assumptions quarterly and records any adjustments to the Company’s preliminary estimates to goodwill provided that the Company is within the measurement period. Upon the conclusion of the measurement period or final determination of the fair value of assets acquired or liabilities assumed, whichever comes first, any subsequent adjustments are recorded in the Company’s consolidated statement of operations.
From time to time, certain of our acquisition agreements include contingent earn-out arrangements, which are generally based on the achievement of future income thresholds. The fair values of these earn-out arrangements are included as part of the purchase price of the acquired companies on their respective acquisition dates. For each transaction, we estimate the fair value of contingent earn-out payments as part of the initial purchase price and record the estimated fair value of contingent consideration as a liability on the consolidated balance sheets.
The Company reviews and re-assesses the estimated fair value of contingent consideration on a quarterly basis until the contingent period ends, and the updated fair value could be materially different from the initial estimates or prior quarterly amounts. Changes in the estimated fair value of our contingent earn-out liabilities are reported in operating results.
Asset Acquisitions—If an acquisition of an asset or group of assets does not meet the definition of a business, the transaction is accounted for as an asset acquisition rather than a business combination. An asset acquisition does not result in the recognition of goodwill and transaction costs are capitalized as part of the cost of the asset or group of assets acquired. The Company uses its best estimates and assumptions to assign fair value to the tangible and intangible assets acquired and liabilities assumed at the acquisition date. In most cases, the Company engages third party valuation specialists to assist the Company in its assessment. The acquisitions costs are allocated to the assets acquired on a relative fair value basis. From time to time, certain of our acquisition agreements include contingent earn-out arrangements, which are recognized only when the contingency is resolved, and the consideration is paid or becomes payable.
Goodwill—The Company reviews goodwill for impairment utilizing either a qualitative or quantitative assessment. If the Company decides that it is appropriate to perform a qualitative assessment and concludes that the fair value of a reporting unit more likely than not exceeds its carrying value, no further evaluation is necessary. If the Company performs a quantitative assessment, the Company compares the fair value of a reporting unit with its carrying value and recognizes an impairment charge for the amount that the carrying amount exceeds the reporting unit’s fair value. The Company annually tests goodwill for impairment at the beginning of the fourth quarter, or earlier if triggering events occur. Fair value determinations require considerable judgment and are sensitive to inherent uncertainties and changes in estimates and assumptions regarding revenue growth rates, gross margins, expenses, capital expenditures, working capital requirements, tax rates, terminal growth rates, discount rates, and synergies available to market participants. As of October 1, 2023, the Company conducted its most recent annual impairment test by quantitively testing goodwill assigned to its domestic and international reporting units. Based on the results of the quantitative test, the Company concluded that the fair value of both the domestic and international reporting units exceed their respective carrying value. The fair value of the domestic and international reporting units exceeded the carrying value by 18% and 9%, respectively. As of December 31, 2023, the goodwill related to the domestic and international reporting units amounted to $9,132 and $42,067, respectively. The carrying value of both reporting units is mainly sensitive to discount rates, the projected net sales growth rates, gross margin improvements, and terminal growth rates. Negative deviations from the Company’s projections and assumptions used in its quantitative impairment test may result in an impairment.
Impairment—The carrying values of long-lived assets other than goodwill are reviewed for impairment annually and/or whenever events or changes in circumstances indicate that the carrying value of such assets may not be recoverable. The Company evaluates recoverability of an asset group by comparing the carrying value to the future undiscounted cash flows that it expects to generate from the asset group. If the comparison indicates that the carrying value of an asset group is not recoverable, measurement of the impairment loss is based on the fair value of the asset. There were no circumstances that would indicate any impairment of the carrying value of these long-lived assets and no material impairment losses were recorded in 2023, 2022, or 2021.
Income taxes—Income tax expense, deferred tax assets and liabilities, and liabilities for unrecognized tax benefits reflect management’s best estimate of current and future taxes to be paid. The Company is subject to income taxes in the U.S. and several foreign jurisdictions. The Company assessed the ability to realize deferred tax assets and determined that based on the available evidence, including a history of taxable income and estimates of future taxable income, it is more likely than not that the net deferred tax assets relating to the Company’s operations in Brazil, Spain, Singapore and Ukraine will not be realized and a full valuation allowance has been recorded in those jurisdictions. Significant management judgment is required in determining the provision for income taxes and deferred tax assets and liabilities. In the event that actual results differ from these estimates, we will adjust these estimates in future periods, which may result in a change in the effective tax rate in a future period. Accounting for income taxes involves uncertainty and judgment on how to interpret and apply tax laws and regulations within the Company’s annual tax filings. Such uncertainties from time to time may result in a tax position that may be challenged and overturned by a tax authority in the future, which could result in additional tax liability, interest charges and possibly penalties. The Company classifies interest and penalties as a component of income tax expense.
ITEM 7A QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
The Company is exposed to market risk related to changes in interest rates, foreign exchange rates, and inflation.
Interest rate risk—The Company is primarily exposed to changes in interest rates related to its borrowing activities. The Company’s indebtedness to its primary group of lenders is evidenced by a line of credit with a variable rate of interest, which fluctuates with changes in the lender’s reference rate (SOFR). An increase or decrease in interest rates by 25 bps would impact the Company’s net income by approximately $315 based on the Company’s historical average borrowing in 2023. The Company may use derivative financial instruments to hedge its exposure to interest rate fluctuations.
Foreign exchange rate risk—The Company conducts business in various foreign currencies, primarily when doing business in Mexico, Central and South America, Australia, New Zealand and Europe. Therefore, changes in the value of the currencies of such countries or regions affect the Company’s financial position and cash flows when translated into U.S. Dollars. The Company has mitigated, and will continue to mitigate, a portion of its currency exchange exposure through natural hedges based on the operation of decentralized foreign operating companies in which the majority of all costs are local-currency-based. The remaining currency exchange exposure mainly pertains to intercompany loans granted to wholly owned international entities which have their local currency as their functional currency. A 10% change between the US Dollar and the respective local currencies of these entities would amount to approximately $6,400. The Company may use derivative financial instruments for trading purposes to protect trading performance from exchange rate fluctuations on material contracts, though there are no such instruments in place during any periods presented in this Annual Report on Form 10-K.
Inflation—The Company is working diligently with its critical raw material suppliers to control inflationary pressures, conducting contract negotiations with focus on the following: reducing or delaying price increases due to higher environmental costs from suppliers mainly in China and India, managing the tariff impacts by sourcing and leveraging alternate geographies where possible, and lastly, monitoring strengths of the U.S. dollar vs other currencies in order to secure benefits and balance tariff effects. The Company recognizes there is long-term pressure on demand for raw materials in the developing world and is utilizing its expertise to minimize inflationary pressure. The Company has been able to push back on many of the proposed price increases for actives and intermediates that are shipped to our U.S. factories, to either avoid, minimize or forestall them. In response to inflation and other factors that have increased the cost of goods and services, the Company has successfully implemented price increases on its products. However, inflation resulted in persistently high interest rates. As a result, customers in many regions implemented destocking directives in order to limit their carrying costs of inventory. This, in turn, led to an overall drop in demand in late 2022 through mid-2023. There can be no assurance that inflation will not have a material adverse effect on the Company’s financial performance in future periods.
As part of an on-going process of assessing business risk, management has identified risk factors which are disclosed in Item 1A. Risk Factors of this Annual Report on Form 10-K.
ITEM 8
| | |
Description | | Page No |
| | |
Financial Statements: | | |
Report of Independent Registered Public Accounting Firm Deloitte & Touche LLP; Costa Mesa, California, PCAOB ID#34 | | 28 |
Report of Independent Registered Public Accounting Firm BDO USA, P.C.; Costa Mesa, California, PCAOB ID#243 | | 31 |
Consolidated Balance Sheets as of December 31, 2023 and 2022 | | 32 |
Consolidated Statements of Operations for the Years Ended December 31, 2023, 2022 and 2021 | | 33 |
Consolidated Statements of Comprehensive Income for the Years Ended December 31, 2023, 2022 and 2021 | | 34 |
Consolidated Statements of Stockholders’ Equity for the Years Ended December 31, 2023, 2022 and 2021 | | 35 |
Consolidated Statements of Cash Flows for the Years Ended December 31, 2023, 2022 and 2021 | | 36 |
Notes to Consolidated Financial Statements | | 37 |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Stockholders and the Board of Directors of
American Vanguard Corporation
Newport Beach, California
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheet of American Vanguard Corporation and subsidiaries (the “Company”) as of December 31, 2023, the related consolidated statements of operations, comprehensive income, stockholders’ equity, and cash flows for the year then ended, and the related notes and the schedules listed in the Index at Item 15 (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2023, and the results of its operations and its cash flows for the year then ended, in conformity with accounting principles generally accepted in the United States of America.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the Company’s internal controls over financial reporting as of December 31, 2023, based on criteria established in Internal Control — Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission and our report dated March 27, 2024, expressed an unqualified opinion on the Company’s internal control over financial reporting.
Change in Accounting Principle
As discussed in Note 1 to the financial statements, the Company has elected to change its classification of warehousing, handling and outbound freight costs from operating expenses to cost of sales in the years ended December 31, 2023, 2022, and 2021.
Basis for Opinion
These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on the Company's financial statements based on our audit. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. Our audit included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audit also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audit provides a reasonable basis for our opinion.
Critical Audit Matters
The critical audit matters communicated below are matters arising from the current-period audit of the financial statements that were communicated or required to be communicated to the audit committee and that (1) relate to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the financial statements, taken as a whole, and we are not, by communicating the critical audit matters below, providing a separate opinion on the critical audit matters or on the accounts or disclosures to which they relate.
Accrued Program Costs — Refer to Note 1 to the financial statements
Critical Audit Matter Description
The Company offers discounts to its customers based on various programs, which are primarily volume-based incentives. As of December 31, 2023, accrued program costs were $68.1 million. These discount Programs represent variable consideration. Revenues from sales are recorded at the net sales price, which is the transaction price, less an estimate of variable consideration. The Company uses the expected value method for determining the estimated variable consideration, which includes amounts expected to be paid to customers. Quarterly, the Company compares individual sale transactions with programs to determine what, if any, Program liabilities have been incurred. Once the quarterly calculation is made, the Company will make an assessment of whether customers are tracking in a manner that indicates they will meet the requirements of the agreed upon terms and conditions of the applicable Programs, making adjustments to the accumulated accrual based on the liability at the balance sheet date.
Given the nature of the data utilized to analyze the Program accruals, and the volume of Programs data required to estimate total variable consideration, auditing the Program accrual required extensive audit effort when performing audit procedures and evaluating the results of those procedures.
How the Critical Audit Matter Was Addressed in the Audit
Our audit procedures related to accrued Program costs included the following, among others:
•We tested management’s controls over accrued Program costs, including those over the completeness and accuracy of data inputs and adjustments to the calculation.
•We tested the mathematical accuracy of management’s calculation of accrued Program costs.
•We assessed the reasonableness of management’s accrual rate used by performing a fluctuation analysis of the accrual rate compared to the prior period.
•We evaluated the accuracy of the accrued Programs costs by comparing the accrued Program costs calculation inputs and adjustments to supporting documentation.
•We performed inquiries of appropriate individuals outside of the finance organization to corroborate the completeness and accuracy of the inputs into the calculation.
•We evaluated management’s ability to accurately record accrued Programs costs by comparing actual Programs related payments made in 2023 to management’s historical accrual.
Goodwill — Refer to Notes 1 and 9 to the financial statements
Critical Audit Matter Description
The Company reviews goodwill for impairment utilizing either a qualitative or quantitative assessment. If the Company decides that it is appropriate to perform a qualitative assessment and concludes that the fair value of a reporting unit more likely than not exceeds its carrying value, no further evaluation is necessary. If the Company performs a quantitative assessment, the Company compares the fair value of a reporting unit with its carrying amounts and recognizes an impairment charge for the amount that the carrying amount exceeds the reporting unit’s fair value. The determination of a reporting unit’s fair value includes the Company’s use of a discounted cash flows model and a market approach. Key assumptions in the discounted cash flow include, but are not limited to, discount rates, future net sales growth, gross margins, expenses, capital expenditures, and terminal growth rates. The market approach key assumption relates to the earnings before interest, taxes, depreciation, and amortization (EBITDA) multiples. Negative deviations from the Company’s projections and assumptions used in its quantitative impairment test may result in an impairment. The goodwill balance was $51.2 million as of December 31, 2023. The fair value of each reporting unit exceeded its carrying value as of the measurement date and, therefore, no impairment was recognized
Given the significant judgments made by management to estimate the fair value of the reporting units, performing audit procedures to evaluate the reasonableness of management’s estimates and assumptions related to the future net sales growth and expenses, and the selection of EBITDA multiples and discount rates required a high degree of auditor judgment and an increased extent of effort, including the need to involve our fair value specialists.
How the Critical Audit Matter Was Addressed in the Audit
Our audit procedures related to future net sales growth and expenses, and the selection of EBITDA multiples (“forecasts”) and discount rates, to estimate the fair value of each reporting unit included the following procedures:
•We tested management’s controls over the goodwill impairment evaluation, including those over the determination of the fair value of each reporting unit, such as controls related to management’s forecasts, and selection of discount rates.
•We evaluated the reasonableness of management’s forecasted future net sales growth and expenses by comparing forecasted future net sales growth and expenses to (1) historical results of the Company, (2) contractual information, (3) internal communications to management and the board of directors, and (4) industry reports of the Company and comparable companies.
•We evaluated management’s ability to accurately forecast by comparing actual results to management’s historical forecasts.
•With the assistance of our fair value specialists, we evaluated discount rates, including testing the source information underlying the determination of discount rates, testing the mathematical accuracy of the calculations, and developing a range of independent estimates and comparing those to the discount rates selected by management.
•With the assistance of our fair value specialists, we evaluated the selection of EBITDA multiples from comparable companies, including testing the underlying source information and mathematical accuracy of the calculations, and evaluated the appropriateness of the Company’s selection of companies in its peer public company group.
/s/ Deloitte & Touche LLP
Costa Mesa, California
March 27, 2024
We have served as the Company’s auditor since 2023.
Report of Independent Registered Public Accounting Firm
Shareholders and Board of Directors
American Vanguard Corporation
Newport Beach, California
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated balance sheet of American Vanguard Corporation (the “Company”) as of December 31, 2022, the related consolidated statements of operations, comprehensive income, stockholders’ equity, and cash flows for the years ended December 31, 2022 and 2021, and the related notes and schedule (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company at December 31, 2022, and the results of its operations and its cash flows for the years ended December 31, 2022 and 2021, in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ BDO USA, P.C.
We served as the Company's auditor from 1991 to 2022.
Costa Mesa, California
March 16, 2023
CONSOLIDATED BALANCE SHEETS
December 31, 2023 and 2022
(In thousands, except share data)
| | | | | | | | |
| | 2023 | | | 2022 | |
Assets | | | | | | |
Current assets: | | | | | | |
Cash | | $ | 11,416 | | | $ | 20,328 | |
Receivables: | | | | | | |
Trade, net of allowance for credit losses of $7,107 and $5,136, respectively | | | 182,613 | | | | 156,492 | |
Other | | | 8,356 | | | | 9,816 | |
Total receivables, net | | | 190,969 | | | | 166,308 | |
Inventories | | | 219,551 | | | | 184,190 | |
Prepaid expenses | | | 6,261 | | | | 15,850 | |
Income taxes receivable | | | 3,824 | | | | 1,891 | |
Total current assets | | | 432,021 | | | | 388,567 | |
Property, plant and equipment, net | | | 74,560 | | | | 70,912 | |
Operating lease right-of-use assets, net | | | 22,417 | | | | 24,250 | |
Intangible assets, net of amortization | | | 172,508 | | | | 184,664 | |
Goodwill | | | 51,199 | | | | 47,010 | |
Deferred income tax assets | | | 2,849 | | | | 141 | |
Other assets | | | 11,994 | | | | 10,769 | |
Total assets | | $ | 767,548 | | | $ | 726,313 | |
Liabilities and Stockholders’ Equity | | | | | | |
Current liabilities: | | | | | | |
Accounts payable | | $ | 68,833 | | | $ | 69,000 | |
Customer prepayments | | | 65,560 | | | | 110,597 | |
Accrued program costs | | | 68,076 | | | | 60,743 | |
Accrued expenses and other payables | | | 16,354 | | | | 20,982 | |
Operating lease liabilities, current | | | 6,081 | | | | 5,279 | |
Income taxes payable | | | 5,591 | | | | — | |
Total current liabilities | | | 230,495 | | | | 266,601 | |
Long-term debt | | | 138,900 | | | | 51,477 | |
Operating lease liabilities, long-term | | | 17,113 | | | | 19,492 | |
Deferred income tax liabilities | | | 7,892 | | | | 14,597 | |
Other liabilities | | | 3,138 | | | | 4,167 | |
Total liabilities | | | 397,538 | | | | 356,334 | |
Commitments and contingent liabilities (Notes 5 and 11) | | | | | | |
Stockholders’ equity: | | | | | | |
Preferred stock, $0.10 par value per share; authorized 400,000 shares; none issued | | | — | | | | — | |
Common stock, $0.10 par value per share; authorized 40,000,000 shares; issued 34,676,787 shares in 2023 and 34,446,194 shares in 2022 | | | 3,467 | | | | 3,444 | |
Additional paid-in capital | | | 110,810 | | | | 105,634 | |
Accumulated other comprehensive loss | | | (5,963 | ) | | | (12,182 | ) |
Retained earnings | | | 332,897 | | | | 328,745 | |
| | | 441,211 | | | | 425,641 | |
Less treasury stock at cost, 5,915,182 shares in 2023 and 5,029,892 in 2022 | | | (71,201 | ) | | | (55,662 | ) |
Total stockholders’ equity | | | 370,010 | | | | 369,979 | |
Total liabilities and stockholders’ equity | | $ | 767,548 | | | $ | 726,313 | |
See summary of significant accounting policies and notes to consolidated financial statements.
CONSOLIDATED STATEMENTS OF OPERATIONS
Years ended December 31, 2023, 2022 and 2021
(In thousands, except per share data)
| | | | | | | | | | | | |
| | 2023 | | | 2022 | | | 2021 | |
Net sales | | $ | 579,371 | | | $ | 609,615 | | | $ | 557,676 | |
Cost of sales | | | (400,207 | ) | | | (417,227 | ) | | | (386,953 | ) |
Gross profit | | | 179,164 | | | | 192,388 | | | | 170,723 | |
Operating expenses | | | | | | | | | |
Selling, general and administrative | | | (117,844 | ) | | | (119,921 | ) | | | (111,093 | ) |
Research, product and regulatory | | | (38,025 | ) | | | (31,816 | ) | | | (28,855 | ) |
Bargain purchase gain on business acquisition | | | — | | | | — | | | | 171 | |
Operating income | | | 23,295 | | | | 40,651 | | | | 30,946 | |
Change in fair value of equity investments, net | | | (359 | ) | | | (732 | ) | | | (790 | ) |
Other income | | | — | | | | — | | | | 672 | |
Interest expense, net | | | (12,639 | ) | | | (3,954 | ) | | | (3,687 | ) |
Income before provision for income taxes and loss on equity method investment | | | 10,297 | | | | 35,965 | | | | 27,141 | |
Provision for income taxes | | | (2,778 | ) | | | (8,561 | ) | | | (8,166 | ) |
Income before loss on equity method investment | | | 7,519 | | | | 27,404 | | | | 18,975 | |
Loss from equity method investment | | | — | | | | — | | | | (388 | ) |
Net income | | $ | 7,519 | | | $ | 27,404 | | | $ | 18,587 | |
Earnings per common share—basic | | $ | 0.27 | | | $ | 0.94 | | | $ | 0.62 | |
Earnings per common share—assuming dilution | | $ | 0.26 | | | $ | 0.92 | | | $ | 0.61 | |
Weighted average shares outstanding—basic | | | 28,128 | | | | 29,234 | | | | 29,811 | |
Weighted average shares outstanding—assuming dilution | | | 28,533 | | | | 29,872 | | | | 30,410 | |
See summary of significant accounting policies and notes to consolidated financial statements.
CONSOLIDATED STATEMENTS OF COMPREHENSIVE INCOME
Years ended December 31, 2023, 2022 and 2021
(In thousands)
| | | | | | | | | | | | |
| | 2023 | | | 2022 | | | 2021 | |
Net income | | $ | 7,519 | | | $ | 27,404 | | | $ | 18,587 | |
Other comprehensive gain (loss) | | | | | | | | | |
Foreign currency translation adjustment, net of tax effects | | | 6,219 | | | | 1,602 | | | | (4,462 | ) |
Comprehensive income | | $ | 13,738 | | | $ | 29,006 | | | $ | 14,125 | |
See summary of significant accounting policies and notes to consolidated financial statements.
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY
Years ended December 31, 2023, 2022 and 2021
(In thousands, except share data)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | Accumulated | | | | | | | | | | | | | |
| | | | | | | | Additional | | | Other | | | | | | | | | | | | | |
| | Common Stock | | | Paid-in | | | Comprehensive | | | Retained | | | Treasury Stock | | | AVD | |
| | Shares | | | Amount | | | Capital | | | loss | | | Earnings | | | Shares | | | Amount | | | Total | |
Balance, January 1, 2021 | | | 33,922,433 | | | $ | 3,394 | | | $ | 96,642 | | | $ | (9,322 | ) | | $ | 288,182 | | | | 3,061,040 | | | $ | (18,160 | ) | | $ | 360,736 | |
Stocks issued under ESPP | | | 50,782 | | | | 4 | | | | 739 | | | | — | | | | — | | | | — | | | | — | | | | 743 | |
Cash dividends declared on common stock ($0.08
per share) | | | — | | | | — | | | | — | | | | — | | | | (2,384 | ) | | | — | | | | — | | | | (2,384 | ) |
Foreign currency translation adjustment, net | | | — | | | | — | | | | — | | | | (4,462 | ) | | | | | | | | | | | | (4,462 | ) |
Stock based compensation | | | — | | | | — | | | | 6,880 | | | | — | | | | — | | | | — | | | | — | | | | 6,880 | |
Stock options exercised, grants, termination,
and vesting of restricted stock units (net of
shares in lieu of taxes) | | | 275,003 | | | | 28 | | | | (2,811 | ) | | | — | | | | — | | | | — | | | | — | | | | (2,783 | ) |
Shares repurchased | | | — | | | | — | | | | — | | | | — | | | | — | | | | 300,000 | | | | (4,579 | ) | | | (4,579 | ) |
Net income | | | — | | | | — | | | | — | | | | — | | | | 18,587 | | | | — | | | | — | | | | 18,587 | |
Balance, December 31, 2021 | | | 34,248,218 | | | | 3,426 | | | | 101,450 | | | | (13,784 | ) | | | 304,385 | | | | 3,361,040 | | | | (22,739 | ) | | | 372,738 | |
Stocks issued under ESPP | | | 51,240 | | | | 4 | | | | 833 | | | | — | | | | — | | | | — | | | | — | | | | 837 | |
Cash dividends declared on common stock ($0.10
per share) | | | — | | | | — | | | | — | | | | — | | | | (3,044 | ) | | | — | | | | — | | | | (3,044 | ) |
Foreign currency translation adjustment, net | | | — | | | | — | | | | — | | | | 1,602 | | | | — | | | | — | | | | — | | | | 1,602 | |
Stock based compensation | | | — | | | | — | | | | 5,684 | | | | — | | | | — | | | | — | | | | — | | | | 5,684 | |
Stock options exercised, grants, termination,
and vesting of restricted stock units (net of
shares in lieu of taxes) | | | 146,736 | | | | 14 | | | | (1,254 | ) | | | — | | | | — | | | | — | | | | — | | | | (1,240 | ) |
Shares repurchased | | | — | | | | — | | | | (1,079 | ) | | | — | | | | — | | | | 1,668,852 | | | | (32,923 | ) | | | (34,002 | ) |
Net income | | | — | | | | — | | | | — | | | | — | | | | 27,404 | | | | — | | | | — | | | | 27,404 | |
Balance, December 31, 2022 | | | 34,446,194 | | | | 3,444 | | | | 105,634 | | | | (12,182 | ) | | | 328,745 | | | | 5,029,892 | | | | (55,662 | ) | | | 369,979 | |
Stocks issued under ESPP | | | 50,025 | | | | 5 | | | | 976 | | | | — | | | | — | | | | — | | | | — | | | | 981 | |
Cash dividends declared on common stock ($0.12
per share) | | | — | | | | — | | | | — | | | | — | | | | (3,367 | ) | | | — | | | | — | | | | (3,367 | ) |
Foreign currency translation adjustment, net | | | — | | | | — | | | | — | | | | 6,219 | | | | — | | | | — | | | | — | | | | 6,219 | |
Stock based compensation | | | — | | | | — | | | | 6,138 | | | | — | | | | — | | | | — | | | | — | | | | 6,138 | |
Stock options exercised, grants, termination,
and vesting of restricted stock units (net of
shares in lieu of taxes) | | | 180,568 | | | | 18 | | | | (1,938 | ) | | | — | | | | — | | | | — | | | | — | | | | (1,920 | ) |
Shares repurchased | | | — | | | | — | | | | — | | | | — | | | | — | | | | 885,290 | | | | (15,539 | ) | | | (15,539 | ) |
Net income | | | — | | | | — | | | | — | | | | — | | | | 7,519 | | | | — | | | | — | | | | 7,519 | |
Balance, December 31, 2023 | | | 34,676,787 | | | $ | 3,467 | | | $ | 110,810 | | | $ | (5,963 | ) | | $ | 332,897 | | | | 5,915,182 | | | $ | (71,201 | ) | | $ | 370,010 | |
See summary of significant accounting policies and notes to consolidated financial statements.
CONSOLIDATED STATEMENTS OF CASH FLOWS
Years ended December 31, 2023, 2022 and 2021
(In thousands)
| | | | | | | | | | | | |
| | 2023 | | | 2022 | | | 2021 | |
| | | | | | | | | |
Cash flows from operating activities: | | | | | | | | | |
Net income | | $ | 7,519 | | | $ | 27,404 | | | $ | 18,587 | |
Adjustments to reconcile net income to net cash (used in) provided by operating activities: | | | | | | | | | |
Depreciation and amortization of property, plant and equipment and intangible assets | | | 21,780 | | | | 22,138 | | | | 22,229 | |
Amortization of other long-term assets | | | 1,754 | | | | 3,573 | | | | 3,943 | |
Amortization and accretion of deferred loan fees and discounted liabilities | | | 254 | | | | 289 | | | | 359 | |
Loss on disposal of property, plant and equipment | | | — | | | | 268 | | | | 194 | |
Provision for bad debts | | | 1,935 | | | | 1,171 | | | | 649 | |
Loan principal and interest forgiveness | | | — | | | | — | | | | (672 | ) |
Fair value adjustment of contingent consideration | | | — | | | | 610 | | | | 758 | |
Decrease in environmental liability | | | — | | | | — | | | | (167 | ) |
Stock-based compensation | | | 6,138 | | | | 5,684 | | | | 6,880 | |
Deferred income taxes | | | (9,710 | ) | | | (5,278 | ) | | | (2,090 | ) |
Changes in liabilities for uncertain tax positions or unrecognized tax benefits | | | (508 | ) | | | (1,441 | ) | | | (1,783 | ) |
Change in equity investment fair value | | | 359 | | | | 732 | | | | 790 | |
Loss from equity method investment | | | — | | | | — | | | | 388 | |
Bargain purchase gain | | | — | | | | — | | | | (171 | ) |
Non-cash lease expense | | | 256 | | | | 68 | | | | 286 | |
Foreign currency transaction gains | | | (581 | ) | | | (29 | ) | | | (225 | ) |
Changes in assets and liabilities associated with operations, net of business combinations: | | | | | | | | | |
Increase in receivables | | | (20,278 | ) | | | (6,447 | ) | | | (24,347 | ) |
(Increase) decrease in inventories | | | (27,315 | ) | | | (29,220 | ) | | | 9,357 | |
(Increase) decrease in income tax receivable | | | 3,568 | | | | (4,910 | ) | | | 6,051 | |
(Increase) decrease in prepaid expenses and other assets | | | 1,269 | | | | (3,082 | ) | | | (4,581 | ) |
Increase (decrease) in accounts payable | | | (2,287 | ) | | | 1,704 | | | | 8,783 | |
(Decrease) Increase in customer prepayments | | | (45,079 | ) | | | 47,551 | | | | 19,280 | |
Increase (decrease) in accrued program costs | | | 7,244 | | | | (2,449 | ) | | | 17,877 | |
Increase (decrease) in accrued expenses and other payables | | | (5,066 | ) | | | 90 | | | | 3,986 | |
Decrease in contingent consideration | | | — | | | | (1,321 | ) | | | — | |
Net cash (used in) provided by operating activities | | | (58,748 | ) | | | 57,105 | | | | 86,361 | |
Cash flows from investing activities: | | | | | | | | | |
Capital expenditures | | | (11,878 | ) | | | (13,261 | ) | | | (9,518 | ) |
Proceeds from disposal of property, plant and equipment | | | 242 | | | | 84 | | | | — | |
Acquisitions of business and product line, net of cash acquired | | | (5,195 | ) | | | — | | | | (10,000 | ) |
Intangible assets | | | (186 | ) | | | (1,293 | ) | | | (524 | ) |
Net cash used in investing activities | | | (17,017 | ) | | | (14,470 | ) | | | (20,042 | ) |
Cash flows from financing activities: | | | | | | | | | |
Payments under line of credit agreement | | | (172,500 | ) | | | (254,000 | ) | | | (186,569 | ) |
Borrowings under line of credit agreement | | | 259,100 | | | | 253,000 | | | | 131,000 | |
Payment of contingent consideration | | | — | | | | (68 | ) | | | (1,301 | ) |
Net receipt from the issuance of common stock under ESPP | | | 981 | | | | 837 | | | | 743 | |
Net receipt from the exercise of stock options | | | 46 | | | | 827 | | | | 172 | |
Net payment from common stock purchased for tax withholding | | | (1,967 | ) | | | (2,067 | ) | | | (2,955 | ) |
Repurchase of common stock | | | (15,539 | ) | | | (34,002 | ) | | | (4,579 | ) |
Payment of cash dividends | | | (3,384 | ) | | | (2,787 | ) | | | (2,382 | ) |
Net cash provided by (used in) financing activities | | | 66,737 | | | | (38,260 | ) | | | (65,871 | ) |
Net (decrease) increase in cash | | | (9,028 | ) | | | 4,375 | | | | 448 | |
Effect of exchange rate changes on cash | | | 116 | | | | (332 | ) | | | (86 | ) |
Cash at beginning of year | | | 20,328 | | | | 16,285 | | | | 15,923 | |
Cash at end of year | | $ | 11,416 | | | $ | 20,328 | | | $ | 16,285 | |
See summary of significant accounting policies and notes to the consolidated financial statements.
AMERICAN VANGUARD CORPORATION
SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES AND
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Years Ended December 31, 2023, 2022 and 2021
(Dollars in thousands, except share data)
(1)Description of Business and Summary of Significant Accounting Policies
American Vanguard Corporation (the “Company” or “AVD”) is primarily a specialty solutions manufacturer that develops and markets safe synthetic, biological and biorational products for agricultural, commercial and consumer uses. The consolidated financial statements include the accounts of the Company and its subsidiaries. All significant intercompany accounts and transactions have been eliminated in consolidation. The Company’s Chief Executive Officer is the Chief Operating Decision Maker (CODM), and the Company operates within a single operating and single reporting segment. The Company’s CODM makes strategic decisions based on the Company’s consolidated financial statements, and market opportunities and synergies across the entire organization. Therefore, the Company’s CODM allocates resources and assesses financial performance on a consolidated basis.
All U.S. Dollar amounts reflected in the notes to the consolidated financial statements are presented in thousands, except per share data.
The Company believes that the combination of its cash flows from future operations, current cash on hand and the availability under the Company’s credit facility will be sufficient to meet its working capital and capital expenditure requirements and will provide the Company with adequate liquidity to meet its anticipated operating needs for at least the next 12 months from the issuance of these consolidated financial statements. Although operating activities are expected to provide cash, to the extent of growth in the future, its operating and investing activities will use cash and, consequently, this growth may require the Company to access some or all of the availability under the credit facility. It is also possible that additional sources of finance may be necessary to support additional growth.
Change in Accounting Principle—Historically, the Company included warehousing, handling and outbound freight costs in operating expenses on its consolidated statements of operations. Effective January 1, 2023, the Company elected to include these costs in cost of sales instead of operating expenses on its consolidated statements of operations. The effects of the change in accounting have been retrospectively applied to all periods presented. The Company believes that the change in accounting is preferable as it aligns the Company’s classification of this warehousing, handling and outbound freight costs in such a way as to present operational management with a clearer vision of the operational performance by business unit. This accounting change also increases the comparability of the Company’s financial performance with its peer companies as most peer companies include these warehousing, handling and outbound freight costs in cost of sales rather than operating expenses. As a result, this change is intended to help interested parties better understand the Company’s performance and facilitate comparisons with most of the Company’s peer companies. This change in accounting principle does not impact operating income, net income, and net income per share. The following table compares the Company’s historical classification with the classification after the adoption of the change in accounting for the three years ended December 31, 2023, 2022 and 2021.
| | | | | | | | | | | | | | | | |
| | Classification after adoption
of accounting change | | | Classification prior to adoption
of accounting change | |
| | 2022 | | | 2021 | | | 2022 | | | 2021 | |
Net sales | | $ | 609,615 | | | $ | 557,676 | | | $ | 609,615 | | | $ | 557,676 | |
Cost of sales | | | (417,227 | ) | | | (386,953 | ) | | | (368,263 | ) | | | (343,629 | ) |
Gross profit | | | 192,388 | | | | 170,723 | | | | 241,352 | | | | 214,047 | |
Operating expenses | | | | | | | | | | | | |
Selling, general and administrative | | | (119,921 | ) | | | (111,093 | ) | | | (168,885 | ) | | | (154,417 | ) |
Research, product and regulatory | | | (31,816 | ) | | | (28,855 | ) | | | (31,816 | ) | | | (28,855 | ) |
Bargain purchase gain on business acquisition | | | — | | | | 171 | | | | — | | | | 171 | |
Operating income | | $ | 40,651 | | | $ | 30,946 | | | $ | 40,651 | | | $ | 30,946 | |
Reclassifications—Certain prior years’ amounts have been reclassified to conform to the current year’s presentation.
Cost of Sales— Cost of sales primarily includes inventory procurement, production, warehousing, handling, and outbound freight. These costs include direct labor, materials, and manufacturing overhead. Depreciation and amortization expense included in cost of sales amounted to $6,599, $8,906, and $9,232 for the years ended December 31, 2023, 2022, and 2021, respectively.
Advertising Expense—The Company expenses advertising costs in the period incurred. Advertising expenses are recognized as selling expenses in the consolidated statements of operations and were $5,736, $5,836 and $5,201 in 2023, 2022 and 2021, respectively.
Cash—The Company maintains cash balances that exceed federally insured limits with a number of financial institutions.
Inventories—Inventory is stated at the lower of cost or net realizable value. Cost is determined by the average cost method, and includes material, labor, factory overhead and subcontracting services. Inventory reserves are recorded for excess and slow-moving inventory. The Company recorded an inventory reserve of $2,756 and $3,015 at December 31, 2023 and 2022, respectively.
The components of inventories, consist of the following:
| | | | | | | | |
| | 2023 | | | 2022 | |
Finished products | | $ | 198,935 | | | $ | 155,128 | |
Raw materials | | | 20,616 | | | | 29,062 | |
Total inventories | | $ | 219,551 | | | $ | 184,190 | |
Finished products consist of products that are sold to customers in their current form as well as intermediate products that require further formulation to be saleable to customers.
Leases— The Company has operating leases for warehouses, manufacturing facilities, offices, cars, railcars and certain equipment for which operating lease right-of-use (“ROU”) assets and corresponding lease liabilities are recorded. The Company measures ROU assets throughout the lease term at the carrying amount of the lease liability, plus initial direct costs, plus any prepaid lease payments, less the unamortized balance of lease incentives received. The lease liabilities are measured at the present value of the unpaid lease payments at the lease commencement date. Leases that include both lease and non-lease components are accounted for as a single lease component for each asset class, except for real estate leases.
The minimum payments under operating leases are recognized on a straight-line basis over the lease term in the consolidated statements of operations. Operating lease expenses related to variable lease payments are recognized in cost of sales or as operating expenses in a manner consistent with the nature of the underlying lease and as the events, activities, or circumstances in the lease agreement occur. Leases with a term of less than 12 months are not recognized on the consolidated balance sheets, and the related lease expenses are recognized in the consolidated statements of operations on a straight-line basis over the lease term.
Accounting for leases requires management to exercise judgment and make estimates in determining the applicable discount rate, lease term and payments due under a lease. Most of our leases do not provide an implicit interest rate, nor is it available to us from our lessors. As an alternative, the Company uses our estimated incremental borrowing rate, which is derived from information available at the lease commencement date, including publicly available data, in determining the present value of lease payments. The Company also estimated the fair value of the lease and non-lease components for some of our warehouse leases based on market data and cost data.
The lease term includes the non-cancellable period of the lease plus any additional periods covered by either an option to extend (or not terminate) that the Company is reasonably certain to exercise. The Company has leases with a lease term ranging from 1 year to 20 years.
The operating leases of the Company do not contain major restrictions or covenants such as those relating to dividends or additional financial obligations. Finance leases are immaterial to the consolidated financial statements. There were no lease transactions with related parties during 2023, 2022 and 2021.
The operating lease expense for the years ended December 31, 2023, 2022 and 2021 was $7,579, $6,531 and $6,053, respectively. Lease expenses related to variable lease payments and short-term leases were immaterial. Additional information related to operating leases are as follows:
| | | | | | | | | | | | |
| | Year Ended
December 31, 2023 | | | Year Ended
December 31, 2022 | | | Year Ended
December 31, 2021 | |
Cash paid for amounts included in the measurement of lease liabilities | | $ | 7,333 | | | $ | 6,450 | | | $ | 5,750 | |
ROU assets obtained in exchange for new lease liabilities | | $ | 4,466 | | | $ | 4,468 | | | $ | 18,521 | |
The weighted-average remaining lease term and discount rate related to the operating leases as of December 31, 2023 and 2022 were as follows:
| | | | | | | | |
| | December 31, 2023 | | | December 31, 2022 | |
Weighted-average remaining lease term (in years) | | | 5.04 | | | | 5.93 | |
Weighted-average discount rate | | | 4.60 | % | | | 4.00 | % |
Future minimum lease payments under non-cancellable operating leases as of December 31, 2023 were as follows:
| | | | |
2024 | | $ | 6,879 | |
2025 | | | 5,755 | |
2026 | | | 4,275 | |
2027 | | | 2,814 | |
2028 | | | 1,883 | |
Thereafter | | | 4,338 | |
Total lease payments | | $ | 25,944 | |
Less: imputed interest | | | (2,750 | ) |
Total | | $ | 23,194 | |
| | | |
Amounts recognized in the consolidated balance sheets: | | | |
Operating lease liabilities, current | | $ | 6,081 | |
Operating lease liabilities, long term | | $ | 17,113 | |
Revenue Recognition— The Company recognizes revenue when control of the ordered goods or services are transferred to its customers in an amount that reflects the consideration the Company expects to be entitled to in exchange for those goods or services. Amounts billed for shipping and handling activities after the transfer of control to the customer are considered fulfillment activities and are recognized as revenue. The costs are accrued when the related revenue is recognized. Taxes collected from customers relating to product sales and remitted to governmental authorities are excluded from revenues. The Company sells its products mainly to distributors and retailers. In addition, the Company also sells its products direct to end users internationally. The products include insecticides, herbicides, soil fumigants, fungicides and biologicals. In addition, the Company recognizes royalty income related to licensing arrangements which qualify as functional licenses rather than symbolic licenses. Upon signing a new licensing agreement, the Company typically receives up-front fees, which are generally characterized as non-refundable royalties. These fees are recognized as revenue upon the execution of the license agreements. Minimum royalty fees are recognized once the Company has an enforceable right for payment. Sales-based royalty fees are typically recognized when the sales occur. The Company calculates and accrues estimated royalties based on the contractual terms and correspondence with the licensees regarding actual sales. Selective enterprise information of sales disaggregated by category and geographic region is as follows:
| | | | | | | | | | | | |
| | 2023 | | | 2022 | | | 2021 | |
Net sales: | | | | | | | | | |
U.S. crop | | $ | 269,229 | | | $ | 288,624 | | | $ | 263,632 | |
U.S. non-crop | | | 75,287 | | | | 76,709 | | | | 78,605 | |
Total U.S. | | | 344,516 | | | | 365,333 | | | | 342,237 | |
International | | | 234,855 | | | | 244,282 | | | | 215,439 | |
Total net sales | | $ | 579,371 | | | $ | 609,615 | | | $ | 557,676 | |
Contract Assets— Contract assets relate to royalties earned on certain functional licenses granted for the use of the Company’s intellectual property. The Company did not have any contract assets as of December 31, 2023. At December 31, 2022, the contract assets amounted to $3,100. The respective short-term and long-term contract assets at December 31, 2022 of $2,098 and $1,002 are included in other receivables and other assets, respectively, on the consolidated balance sheets.
Accrued Program Costs— The Company offers various discounts to customers based on the volume purchased within a defined period, other pricing adjustments, some grower volume incentives or other key performance indicator driven payments made to distributors, retailers or growers, usually at the end of a growing season. The Company describes these payments as “Programs.” Programs are a critical part of doing business in both the U.S. crop and non-crop chemicals marketplaces. These discount Programs represent variable consideration. Revenues from sales are recorded at the net sales price, which is the transaction price, less an estimate of variable consideration. Variable consideration includes amounts expected to be paid to its customers using the expected value method. Each quarter management compares individual sale transactions with Programs to determine what, if any, Program liabilities have been incurred. Once this initial calculation is made for the specific quarter, sales and marketing management, along with executive and financial management, review the accumulated Program balance and, for volume driven payments, make assessments of whether or not customers are tracking in a manner that indicates that they will meet the requirements set out in agreed upon terms and conditions attached to each Program. Following this assessment, management adjusts the accumulated accrual to properly reflect the liability at the balance sheet date. Programs are paid out predominantly on an annual basis, usually in the final quarter of the financial year or the first quarter of the following year.
Customer Prepayments— From time to time, the Company receives prepayments from customers which are recorded as customer prepayments on the Company’s consolidated balance sheets. The Company does not recognize revenue on any such payments until the customer places binding purchase orders, the goods are shipped, and control is transferred to the customer. Revenue recognized for the years ended December 31, 2023, 2022, and 2021 that were included in the customer prepayments balance at the beginning of 2023, 2022, and 2021 was $88,097, $63,064, and $37,779, respectively. During 2023, the Company refunded $22,500 to customers.
Current Expected Credit Losses— The Company maintains an allowance to cover its Current Expected Credit Losses ("CECL") on its trade receivables, other receivables and contract assets arising from the possible failure of customers to make contractual payments. The Company estimates credit losses expected over the life of its trade receivables, other receivables and contract assets based on historical information combined with current conditions that may affect a customer’s ability to pay and reasonable and supportable forecasts. In most instances, the Company’s policy is to write off trade receivables when they are deemed uncollectible regarding likely future payments. The vast majority of the Company's trade receivables, other receivables and contract assets are less than 365 days. Under the CECL impairment model, the Company develops and documents its allowance for credit losses on its trade receivables based on multiple portfolios. The determination of portfolios is based primarily on geographical location, type of customer and accounts receivables aging.
Deferred Loan Fees— These fees in connection with the Company’s senior credit facility are capitalized and amortized on a straight-line basis over the life of the borrowing and included in interest expense, net.
Property, Plant and Equipment and Depreciation— Property, plant and equipment includes the cost of land, buildings, machinery and equipment, office furniture and fixtures, automobiles, construction projects and improvements to existing plant and equipment. Interest costs related to construction projects are capitalized at the Company’s current weighted average effective interest rate. Expenditures for minor repairs and maintenance are expensed as incurred. When property or equipment is sold or otherwise disposed of, the related cost and accumulated depreciation are removed from the respective accounts and the gain or loss realized on disposition is reflected in operations. All plant and equipment are depreciated using the straight-line method, utilizing the estimated useful property lives.
Business Combinations— The Company uses its best estimates and assumptions to assign fair value to the tangible and intangible assets acquired and liabilities assumed at the acquisition date. The Company’s estimates are inherently uncertain and subject to refinement. During the measurement period, which may be up to one year from the acquisition date, the Company may record adjustments to the fair value of these tangible and intangible assets acquired and liabilities assumed, with the corresponding offset to goodwill or an adjustment to the gain from a bargain purchase. In addition, when appropriate uncertain tax positions and tax-related valuation allowances are initially recorded in connection with a business combination as of the acquisition date. The Company continues to collect information and re-evaluates these estimates and assumptions quarterly and records any adjustments to the Company’s preliminary estimates to goodwill or an adjustment to the gain from a bargain purchase, provided that the Company is within the measurement period. Upon the conclusion of the measurement period or final determination of the fair value of assets acquired or liabilities assumed, whichever comes first, any subsequent adjustments are recorded to the Company’s consolidated statement of operations.
From time to time, certain of our acquisition agreements include contingent earn-out arrangements, which are generally based on the achievement of future income thresholds. The fair values of these earn-out arrangements are included as part of the purchase price of the acquired companies on their respective acquisition dates. For each transaction, the Company engages third-party valuation specialists to assist it in making estimates of the fair value of contingent earn-out payments, both as part of the initial purchase price and at each subsequent financial statement date until the end of the related performance period. The Company records the estimated fair value of contingent consideration as a liability on the consolidated balance sheets. The Company reviews and re-assesses the estimated fair value of contingent consideration on a quarterly basis, and the updated fair value could be materially different from the initial estimates or prior quarterly amounts. Changes in the estimated fair value of the contingent earn-out liabilities are reported in operating results.
Asset Acquisitions— If an acquisition of an asset or group of assets does not meet the definition of a business, the transaction is accounted for as an asset acquisition rather than a business combination. An asset acquisition does not result in the recognition of goodwill and transaction costs are capitalized as part of the cost of the asset or group of assets acquired. The Company uses its best estimates and assumptions to assign fair value to the tangible and intangible assets acquired and liabilities assumed at the acquisition date. The acquisitions costs are allocated to the assets acquired on a relative fair value basis.
Intangible Assets— The primary identifiable intangible assets of the Company relate to assets associated with its product and business acquisitions. All the Company’s intangible assets are amortizing assets with finite lives. The estimated useful life of an identifiable intangible asset is based upon several factors including the effects of demand, competition, and expected changes in the marketability of the Company’s products.
Impairment— The carrying values of long-lived assets other than goodwill are reviewed for impairment annually and/or whenever events or changes in circumstances indicate that the carrying value of such assets may not be recoverable. The Company evaluates recoverability of an asset group by comparing the carrying value to the future undiscounted cash flows that it expects to generate from the asset group. If the comparison indicates that the carrying value of an asset group is not recoverable, measurement of the impairment loss is based on the fair value of the asset. There were no circumstances that would indicate any impairment of the carrying value of these long-lived assets and no material impairment losses were recorded in 2023, 2022, or 2021.
The Company reviews goodwill for impairment utilizing either a qualitative or quantitative assessment. If the Company decides that it is appropriate to perform a qualitative assessment and concludes that the fair value of a reporting unit more likely than not exceeds its carrying value, no further evaluation is necessary. If the Company performs a quantitative assessment, the Company compares the fair value of a reporting unit with its carrying amounts and recognizes an impairment charge for the amount that the carrying amount exceeds the reporting unit’s fair value. The determination of a reporting units’ fair value includes the Company’s use of a discounted cash flows model and a market approach. Key assumptions in the discounted cash flow include, but are not limited to, discount rates, future net sales growth, gross margins, expenses, capital expenditures, and terminal growth rates. The market approach key assumption relates to the earnings before interest, taxes, depreciation, and amortization (EBITDA) multiples. The Company annually tests goodwill for impairment in the beginning of the fourth quarter, or earlier if triggering events occur. The Company did not record any impairment losses in 2023, 2022, or 2021.
Fair Value of Financial Instruments— The accounting standard for fair value measurements provides a framework for measuring fair value and requires expanded disclosures regarding fair value measurements. Fair value is defined as the price that would be received for an asset or the exit price that would be paid to transfer a liability in the principal or most advantageous market in an orderly transaction between market participants on the measurement date. This accounting standard established a fair value hierarchy, which requires an entity to maximize the use of observable inputs, where available. The following summarizes the three levels of inputs required:
•Level 1 – Quoted prices in active markets for identical assets or liabilities.
•Level 2 – Observable inputs other than quoted prices in active markets for identical assets and liabilities, quoted prices for identical or similar assets or liabilities in inactive markets, or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities.
•Level 3 – Inputs that are generally unobservable and typically reflect management’s estimate of assumptions that market participants would use in pricing the asset or liability.
The Company did not have any significant Level 1 investments as of December 31, 2023 and 2022, except for its equity investment in Clean Seed Capital Group Ltd. (see Note 16 – Equity Investments).
The carrying amount of the Company’s financial instruments, which principally include cash, accounts receivable, accounts payable and accrued expenses, approximates fair value because of the relatively short maturity of such instruments. The carrying amount of the Company’s borrowings, which are considered Level 2 liabilities, approximates fair value as they bear interest at a variable rate that represents current market rates.
The Company measures its contingent earn-out liabilities in connection with business acquisitions at fair value on a recurring basis using significant unobservable inputs classified within Level 3 of the fair value hierarchy. The Company may use various valuation techniques depending on the terms and conditions of the contingent consideration including a Monte-Carlo simulation. This simulation uses probability distribution for each significant input to produce thousands of possible outcomes and the results are analyzed to determine probabilities of different outcomes occurring. Refer to Note 10 for the Company’s earn-out liability movements.
Foreign Currency Translation— Certain international operations use the respective local currencies as their functional currency, while other international operations use the U.S. Dollar as their functional currency. The Company considers the U.S. Dollar as its reporting currency. Translation adjustments for subsidiaries where the functional currency is its local currency are included in other comprehensive gain (loss). Foreign currency transaction gains (losses) resulting from exchange rate fluctuation on transactions denominated in a currency other than the functional currency are reported in earnings. Assets and liabilities of the foreign operations denominated in local currencies are translated at the rate of exchange at the balance sheet date. Revenues and expenses are translated at the weighted average rate of exchange during the period. Translations of intercompany loans of a long-term investment nature are included as a component of translation adjustment in other comprehensive gain (loss).
Income Taxes—The Company utilizes the asset and liability method of accounting for income taxes as set forth in ASC 740. Under the liability method, deferred taxes are determined based on the temporary differences between the financial statement and tax basis of assets and liabilities using tax rates expected to be in effect during the years in which the basis differences reverse. A valuation allowance is recorded when it is more likely than not that some of the deferred tax assets will not be realized. In determining the need for valuation allowances, the Company considers projected future taxable income and the availability of tax planning strategies. If in the future the Company determines that it will not be able to realize its recorded deferred tax assets, an increase in the valuation allowance would be recorded, decreasing earnings in the period in which such determination is made.
The Company assesses its income tax positions and records tax benefits for all years subject to examination based upon the Company’s evaluation of the facts, circumstances, and information available at the reporting date. For those tax positions where there is greater than 50% likelihood that a tax benefit will be sustained, the Company has recorded the largest amount of tax benefit that may potentially be realized upon ultimate settlement with a taxing authority that has full knowledge of all relevant information. For those income tax positions where there is less than 50% likelihood that a tax benefit will be sustained, no tax benefit has been recognized in the consolidated financial statements.
Per Share Information—Basic earnings per share (“EPS”) is computed as net income divided by the weighted average number of shares of common stock outstanding during the period. Diluted EPS reflects potential dilution to EPS that could occur if securities or other contracts, which, for the Company, consists of restricted stock grants and options to purchase shares of the Company’s common stock, are exercised as calculated using the treasury stock method.
The components of basic and diluted earnings per share were as follows:
| | | | | | | | | | | | |
| | 2023 | | | 2022 | | | 2021 | |
Numerator: | | | | | | | | | |
Net income | | $ | 7,519 | | | $ | 27,404 | | | $ | 18,587 | |
Denominator: | | | | | | | | | |
Weighted average shares outstanding—basic | | | 28,128 | | | | 29,234 | | | | 29,811 | |
Dilutive effect of stock options and grants | | | 405 | | | | 638 | | | | 599 | |
Weighted average shares outstanding—diluted | | | 28,533 | | | | 29,872 | | | | 30,410 | |
For the years ended December 31, 2023, 2022, and 2021, no options or grants were excluded from the computation.
Use of Estimates—The preparation of consolidated financial statements in conformity with accounting principles generally accepted in the United States of America (“U.S. GAAP”) requires management to make estimates and assumptions that affect the reported amounts of assets, liabilities, and revenues, at the date that the consolidated financial statements are prepared. Significant estimates relate to the allowance for expected credit losses, inventory reserves, impairment of long-lived assets, investments and goodwill, assets acquired, and liabilities assumed in connections with business combinations and asset acquisitions, accrued program costs, stock-based compensation and income taxes. Actual results could materially differ from those estimates.
Total comprehensive income—In addition to net income, total comprehensive income includes changes in equity that are excluded from the consolidated statements of operations and are recorded directly into a separate section of stockholders’ equity on the consolidated balance sheets. For the years ended December 31, 2023, 2022, and 2021, total comprehensive income consisted of net income and foreign currency translation adjustments.
Stock-Based Compensation—The Company estimates the fair value of share-based payment awards on the date of grant. The value of the portion of the award that is ultimately expected to vest is recognized as expense over the requisite service periods in the Company’s consolidated statements of operations. Compensation expense on awards subject to performance conditions is based on the quantity of awards that is probable of vesting.
Stock-based compensation expense recognized is reduced for estimated forfeitures. Estimated forfeitures recognized in the Company’s consolidated statements of operations reduced compensation expense by $322, $370, and $320 for the years ended December 31, 2023, 2022, and 2021, respectively. The Company estimates that 17.4% of both restricted stock grants and performance-based restricted shares that are currently subject to vesting will be forfeited. These estimates are reviewed quarterly and revised as necessary.
The Company values restricted stock grants using the Company’s traded stock price at closing on the date of grant. The weighted average grant-date fair values of restricted stock grants during 2023, 2022, and 2021 were $20.31, $23.53, and $20.00, respectively.
Recently Issued Accounting Guidance—In November 2023, the FASB issued ASU No. 2023-07, “Segment Reporting (Topic 280): Improvements to Reportable Segment Disclosure.” The ASU updates reportable segment disclosure requirements, primarily through requiring enhanced disclosures about significant segment expenses and information used to assess segment performance. The ASU is effective for fiscal years beginning after December 15, 2023, with early adoption permitted. The Company is currently evaluating the impact of adopting this ASU on its disclosures.
In December 2023, the FASB issued ASU No. 2023-09, “Income Taxes (Topic 740): Improvements to Income Tax Disclosures.” The ASU includes amendments requiring enhanced income tax disclosures, primarily related to standardization and disaggregation of rate reconciliation categories and income taxes paid by jurisdiction. The guidance is effective for fiscal years beginning after December 15, 2024, with early adoption permitted, and should be applied either prospectively or retrospectively. The Company is currently evaluating the impact of adopting this ASU on its disclosures.
The Company reviewed all other recently issued accounting pronouncements and concluded that they were either not applicable or not expected to have a significant impact on its consolidated financial statements.
(2) Property, Plant and Equipment
Property, plant and equipment at December 31, 2023 and 2022 consist of the following:
| | | | | | | | | | |
| | 2023 | | | 2022 | | | Estimated
useful lives |
Land | | $ | 2,765 | | | $ | 2,757 | | | |
Buildings and improvements | | | 21,088 | | | | 20,794 | | | 10 to 40 years |
Machinery and equipment | | | 148,912 | | | | 142,980 | | | 3 to 25 years |
Office furniture, fixtures and equipment | | | 10,622 | | | | 13,231 | | | 3 to 10 years |
Automotive equipment | | | 1,247 | | | | 1,584 | | | 5 to 20 years |
Construction in progress | | | 10,553 | | | | 5,897 | | | |
Total gross value | | | 195,187 | | | | 187,243 | | | |
Less accumulated depreciation | | | (120,627 | ) | | | (116,331 | ) | | |
Total net value | | $ | 74,560 | | | $ | 70,912 | | | |
| | | | | | | | |
Domestic | | $ | 69,615 | | | $ | 66,268 | | | |
International | | | 4,945 | | | | 4,644 | | | |
Total net value | | $ | 74,560 | | | $ | 70,912 | | | |
For the years ended December 31, 2023, 2022, and 2021, the Company’s aggregate depreciation expense related to property, plant and equipment was $8,352, $7,974, and $8,530, respectively. For the years ended December 31, 2023, 2022, and 2021, the Company disposed fully depreciated assets in the mount of $4,056, $416 and $658, respectively. Interest capitalized amounted to $567 and $317 for the years ended December 31, 2023 and 2022, respectively.
(3) Long-Term Debt
Long-term debt of the Company at December 31, 2023 and 2022 is summarized as follows:
| | | | | | | | |
| | 2023 | | | 2022 | |
Senior credit facility | | $ | 138,900 | | | $ | 52,300 | |
Less deferred loan fees | | | (1,218 | ) | | | (823 | ) |
| | $ | 137,682 | | | $ | 51,477 | |
Principal payments on long-term debt at December 31, 2023 of $138,900 are due in August 2026. The deferred loan fees are included in other assets on the consolidated balance sheet at December 31, 2023.
The Company’s main bank is BMO Bank, N.A. (formerly Bank of the West), a wholly owned subsidiary of BMO Financial Group. BMO is the syndication manager for the Company’s loans.
The Company and certain of its affiliates are parties to a revolving line of senior credit facility agreement entitled the “Third Amended and Restated Loan and Security Agreement” dated as of August 5, 2021 (the “Credit Agreement”), which is a senior secured lending facility among AMVAC, the Company’s principal operating subsidiary, as Agent (including the Company and AMVAC BV), as Borrowers, on the one hand, and a group of commercial lenders led by BMO Bank, N.A. as administrative agent, documentation agent, syndication agent, collateral agent and sole lead arranger, on the other hand. The Credit Agreement consists of a line of credit of up to $275,000, an accordion feature of up to $150,000, a letter of credit and swingline sub-facility (each having limits of $25,000) and has a maturity date of August 5, 2026. The Credit Agreement amended and restated the previous credit facility, which had a maturity date of June 30, 2022. With respect to key financial covenants, the Credit Agreement contains two: namely, borrowers are required to maintain a Total Leverage (“TL”) Ratio of no more than 3.5-to-1, during the first three years, stepping down to 3.25-to-1 as of December 31, 2024, and a Fixed Charge Coverage Ratio ("FCCR") of at least 1.25-to-1. In addition, to the extent that it completes acquisitions totaling $15 million or more in any 90-day period, AMVAC may step-up the TL Ratio by 0.5-to-1, not to exceed 4.00-to-1, for the next three full consecutive quarters. Acquisitions below $50 million do not require Agent consent.
The Company’s borrowing capacity varies with its financial performance, measured in terms of Consolidated EBITDA as defined in the Credit Agreement, for the trailing twelve-month period. Under the Credit Agreement, revolving loans bear interest at a variable rate based, at borrower’s election with proper notice, on either (i) LIBOR plus the “Applicable Margin” which is based upon the Total Leverage (“TL”) Ratio (“LIBOR Revolver Loan”) or (ii) the greater of (x) the Prime Rate, (y) the Federal Funds Rate plus 0.5%, and (z) the Daily One-Month LIBOR Rate plus 1.00%, plus, in the case of (x), (y) or (z) the Applicable Margin (“Adjusted Base Rate Revolver Loan”). The Company and the Lenders entered into an amendment to the Credit Agreement, effective March 9, 2023, whereby LIBOR was replaced by SOFR with a credit spread adjustment of 10.0 bps for all SOFR periods. The revolving loans now bear interest at a variable rate based at our election with proper notice, on either (i) SOFR plus 0.1% per annum and the “Applicable Margin” or (ii) the greater of (x) the Prime Rate, (y) the Federal Funds Rate plus 0.5%, and (z) the Daily One-Month SOFR Rate plus 1.10%, plus, in the case of (x), (y) or (z) the Applicable Margin (“Adjusted Base Rate Revolver Loan”). Interest payments for SOFR Revolver Loans are payable on the last day of each interest period (either one-, three- or six- month periods, as selected by the Company) and the maturity date, while interest payments for Adjusted Base Rate Revolver Loans are payable on the last business day of each month and the maturity date.
On November 7, 2023, the Company entered into Amendment Number Six to the Third Amended Loan and Security Agreement that provided relief in respect of both financial covenants. Specifically, with respect to the Maximum Total Leverage Ratio, the existing ratio of 3.5 through September 30, 2024 and 3.25 through December 31, 2024 and thereafter was changed to 5.5 through September 30, 2023, 4.5 for the periods ending December 31, 2023 and March 31, 2024, 4.0 for the period ending June 30, 2024, 3.5 through September 30, 2024 and returning to 3.25 from December 31, 2024 and thereafter. In addition, the Minimum Fixed Charge Coverage Ratio was changed from 1.25 to 1.0 for the periods ending September 30, 2023, December 31, 2023 and March 31, 2024 and returning to 1.25 for the period ending June 30, 2024 and thereafter. Further, after the delivery of financial statements and a covenant compliance certificate for the period ending December 31, 2023, the Borrowers may elect to terminate the covenant modification period (“CMP”) and revert to the terms of the existing Credit Agreement, if Total Leverage is less than 2.75. Further, for the duration of the CMP, the Company is restricted from making share repurchases. Finally, the Applicable Margin (SOFR and Adjusted Base Rate) and Letter of Credit fees increase by 0.50 basis points for each tier of interest during CMP. As of December 31, 2023, the Company is in compliance with the terms of the CMP and is considering terminating the related covenant modification. The interest rate on December 31, 2023, was 8.33%. Interest incurred, including amortization of deferred loan fees, was $12,391, $4,238, and $3,781 for the years ended December 31, 2023, 2022 and 2021, respectively.
According to the terms of the Credit Agreement, as amended, and based on our performance against the most restrictive covenant listed above, the Company had the capacity to increase its borrowings by up to $115,002 and $200,372 as of December 31, 2023 and 2022, respectively. As of December 31, 2023, the Company was deemed to be in compliance with its financial covenants. Furthermore, the Company’s leverage at December 31, 2023, as defined in the Credit Agreement, was 2.46.
Substantially all the Company’s assets are pledged as collateral under the Credit Agreement, as amended.
(4) Income Taxes
The provisions for income taxes are:
| | | | | | | | | | | | |
| | 2023 | | | 2022 | | | 2021 | |
Current: | | | | | | | | | |
Federal | | $ | 8,038 | | | $ | 7,439 | | | $ | 6,684 | |
State | | | 1,211 | | | | 2,173 | | | | 2,149 | |
Foreign | | | 3,238 | | | | 3,943 | | | | 1,106 | |
| | | 12,487 | | | | 13,555 | | | | 9,939 | |
Deferred: | | | — | | | | | | | |
Federal | | | (6,263 | ) | | | (2,763 | ) | | | (2,369 | ) |
State | | | (1,029 | ) | | | (1,243 | ) | | | (1,039 | ) |
Foreign | | | (2,417 | ) | | | (988 | ) | | | 1,635 | |
| | | (9,709 | ) | | | (4,994 | ) | | | (1,773 | ) |
Total | | $ | 2,778 | | | $ | 8,561 | | | $ | 8,166 | |
Total income tax expense differed from the amounts computed by applying the U.S. Federal income tax rate of 21.0% to income before income tax expense, as a result of the following:
| | | | | | | | | | | | |
| | 2023 | | | 2022 | | | 2021 | |
Computed tax expense at statutory federal rates | | $ | 2,162 | | | $ | 7,553 | | | $ | 5,619 | |
Increase (decrease) in taxes resulting from: | | | | | | | | | |
State taxes, net of federal income tax benefit | | | 756 | | | | 1,493 | | | | 1,485 | |
Unrecognized tax benefits | | | (585 | ) | | | (1,441 | ) | | | (1,783 | ) |
Bargain purchase gain on business acquisition | | | — | | | | — | | | | (35 | ) |
Income tax credits | | | (720 | ) | | | (1,342 | ) | | | (1,206 | ) |
Foreign tax rate differential | | | 1,025 | | | | 785 | | | | 262 | |
Stock based compensation | | | 219 | | | | 55 | | | | 208 | |
Global intangible low-taxed income | | | 685 | | | | — | | | | 162 | |
Change in valuation allowance | | | 1,376 | | | | 379 | | | | 3,304 | |
Return to provision | | | 158 | | | | (693 | ) | | | (651 | ) |
Nondeductible expenses / (tax deductions) | | | (327 | ) | | | 989 | | | | (103 | ) |
Gross receipts taxes | | | 425 | | | | 602 | | | | 567 | |
IP migration | | | (2,455 | ) | | | — | | | | — | |
Other | | | 59 | | | | 181 | | | | 337 | |
Total | | $ | 2,778 | | | $ | 8,561 | | | $ | 8,166 | |
Income before provision for income taxes and losses on equity investments are:
| | | | | | | | | | | | |
| | 2023 | | | 2022 | | | 2021 | |
Domestic | | $ | 6,672 | | | $ | 28,739 | | | $ | 21,212 | |
International | | | 3,625 | | | | 7,226 | | | | 5,929 | |
Total | | $ | 10,297 | | | $ | 35,965 | | | $ | 27,141 | |
Temporary differences between the consolidated financial statements’ carrying amounts and tax bases of assets and liabilities that give rise to significant portions of the net deferred tax liability at December 31, 2023 and 2022 relate to the following:
| | | | | | | | |
| | 2023 | | | 2022 | |
Deferred tax assets | | | | | | |
Inventories | | $ | 2,764 | | | $ | 2,401 | |
Program accrual | | | 9,742 | | | | 8,277 | |
Vacation pay accrual | | | 864 | | | | 772 | |
Accrued bonuses | | | 37 | | | | 1,707 | |
Bad debt expense | | | 2,143 | | | | 1,450 | |
Stock compensation | | | 1,536 | | | | 1,414 | |
Domestic NOL carryforward | | | 543 | | | | 609 | |
Foreign NOL carryforward | | | 6,322 | | | | 2,554 | |
Tax credits | | | 1,582 | | | | 842 | |
Lease liability | | | 5,812 | | | | 6,209 | |
Accrued expenses | | | 696 | | | | 570 | |
Unrealized foreign exchange loss | | | (1,182 | ) | | | 3,220 | |
Capitalized R&D costs | | | 7,140 | | | | 4,600 | |
Deferred tax assets | | | 37,999 | | | | 34,625 | |
Less valuation allowance | | | (3,317 | ) | | | (3,853 | ) |
Deferred tax assets, net | | $ | 34,682 | | | $ | 30,772 | |
Deferred tax liabilities | | | | | | |
Plant and equipment, principally due to differences in depreciation and capitalized interest | | $ | 32,336 | | | $ | 36,158 | |
Lease assets | | | 5,617 | | | | 6,079 | |
Prepaid expenses | | | 1,406 | | | | 1,685 | |
Deferred revenue | | | — | | | | 777 | |
Other | | | 366 | | | | 529 | |
Deferred tax liabilities | | $ | 39,725 | | | $ | 45,228 | |
| | | | | | |
Total net deferred tax liabilities | | $ | 5,043 | | | $ | 14,456 | |
As of December 31, 2023, the Company maintained a full valuation allowance against its net deferred income tax assets related to the Company’s operations in Brazil, Spain, Singapore, and Ukraine totaling $3,317. The valuation allowance decreased by $536 for the year ended December 31, 2023, of which $1,912 relates to unrealized foreign exchange gains and foreign currency translation included in other comprehensive income for 2023, and $1,376 included in the provision for income taxes for 2023. As of December 31, 2022, the Company recorded a full valuation allowance against the net deferred income tax assets related to the Company’s operations in Brazil, Spain, and Ukraine totaling $3,853, of which $379 is included in the provision for income taxes for 2022 and $788 related to unrealized foreign exchange gains included in other comprehensive income for 2022.
Gross foreign NOLs related to the Company's foreign operations were $19,699 and $8,342, for the years ended December 31, 2023 and 2022, respectively. Substantially all of the Company’s foreign NOLs can be carried forward indefinitely.
Gross domestic federal and state NOLs available across all jurisdictions in which we operate were $3,598 and $3,622 as of December 31, 2023 and 2022, respectively. The Company’s federal and state NOLs expire over varying intervals in the future and are subject to annual limitation in accordance with IRC Section 382.
The following is a roll-forward of the Company’s total gross unrecognized tax benefits, not including interest and penalties, for the years ended December 31, 2023 and 2022 included in other liabilities on the Company’s consolidated balance sheets:
| | | | | | | | |
| | 2023 | | | 2022 | |
Balance at beginning of year | | $ | 2,006 | | | $ | 2,426 | |
Additions for tax positions related to the current year | | | 230 | | | | 225 | |
Additions for tax positions related to the prior years | | | 302 | | | | 5 | |
Reduction for tax positions related to the prior years | | | (799 | ) | | | (745 | ) |
Effect of exchange rate changes | | | 57 | | | | 95 | |
Balance at end of year | | $ | 1,796 | | | $ | 2,006 | |
The Company recognizes accrued interest and penalties related to unrecognized tax benefits in the provision for income taxes in the Company’s consolidated financial statements. As of December 31, 2023, 2022, and 2021 the Company incurred $1,342, $2,161, and $2,909, respectively in interest and penalties related to unrecognized tax benefits on its consolidated balance sheets.
It is expected that the amount of unrecognized tax benefits will change and $304 of unrecognized tax benefits is expected to be released within the next twelve months due to expiration of the statute of limitations.
The Company believes it is more likely than not that the deferred assets detailed in the table above, exclusive of those in Brazil, Spain, Singapore and Ukraine with the previously mentioned full valuation allowances, will be realized in the normal course of business. It is the intent of the Company that undistributed earnings of foreign subsidiaries that amounted to $78,600 at December 31, 2023, are permanently reinvested. Determination of the unrecognized deferred tax liability is not practical due to the complexities of a hypothetical calculation.
The Company is subject to U.S. federal income tax as well as to income tax in multiple state jurisdictions. Federal income tax returns of the Company are subject to Internal Revenue Service (“IRS”) examination for the 2020 through 2022 tax years. State income tax returns are subject to examination for the 2019 through 2022 tax years. The Company has foreign income tax returns subject to examination.
Beginning in 2022, The Tax Cuts and Jobs Act of 2017 ("TCJA"), requires taxpayers to capitalize and amortize research and development expenditures pursuant to Internal Revenue Code, or IRC, Section 174, which resulted in increases in the Company’s deferred tax asset balance of $7,140 as of December 31, 2023. There was an increase in cash tax payments in the amount of $3,344 and $6,180 for the years ended December 31, 2023 and December 31, 2022, respectively.
(5) Litigation and Environmental
The Company records a liability on its consolidated financial statements for loss contingencies when a loss is known or considered probable, and the amount can be reasonably estimated. When determining the estimated loss or range of loss, significant judgment is required to estimate the amount and timing of a loss to be recorded. The Company recognizes legal expenses in connection with loss contingencies as incurred.
DBCP Cases
Over the course of the past 30 years, AMVAC and/or the Company have been named or otherwise implicated in a number of lawsuits concerning injuries allegedly arising from either contamination of water supplies or personal exposure to 1, 2-dibromo-3-chloropropane (“DBCP”). DBCP was manufactured by several chemical companies, including Dow Chemical Company, Shell Oil Company and AMVAC (which ceased manufacture in about 1980) and was approved by the USEPA to control nematodes. DBCP was also applied on banana farms in Latin America. The USEPA suspended registrations of DBCP in October 1979, except for use on pineapples in Hawaii. That suspension was partially based on 1977 studies by other manufacturers that indicated a possible link between male fertility and exposure to DBCP among their factory production workers involved with producing the product. Four inactive cases (two pending in Nicaragua and two in Hawai’i) remain pending, while one active case is described below.
Chavez & Marquinez. Two cases were filed independently in 2012 by the same law firm (HendlerLaw, P.C.) in Louisiana and Delaware involving claims on behalf of banana workers for personal injury allegedly arising from exposure to DBCP. Through several years of law and motion practice, the number of plaintiffs in the actions has been reduced from about 2,750 to 290 banana workers from Costa Rica, Ecuador, Guatemala and Panama, and both cases have been consolidated before the United States District Court for the District of Delaware (USDC DE No. 1:12-CV-00695 & 00697). Discovery commenced in 2018 and has consisted largely of seeking medical examinations from the remaining plaintiffs. In December 2022, defendants in this matter filed a motion for summary judgment against the Ecuadorian plaintiffs under the theory that the statute of limitations for negligence barred the action. The trial court granted the motion. Plaintiffs appealed that ruling. However, in January 2024, the court rejected defendants’ motion for summary judgment on the basis of “the most analogous case” doctrine and remanded the matter to the trial court for further proceedings. At this stage in the proceedings, the Company does not believe that a loss is probable or reasonably estimable and has not recorded a loss contingency for these matters.
Other Matters
Pitre etc. v. Agrocentre Ladauniere et al. On February 11, 2022, a strawberry grower named Les Enterprises Pitre, Inc. filed a complaint in the Superior Court, District of Labelle, Province of Quebec, Canada, entitled Pitre, etc. v. Agrocentre Ladauniere, Inc. etal, including Amvac Chemical Corporation, seeking damages in the amount of approximately $5 million arising from stunted growth of, and reduced yield from, its strawberry crop allegedly from the application of Amvac’s soil fumigant, Vapam, in spring of 2021. Examinations of plaintiff were held in mid-August 2022, during which plaintiff in effect confirmed that he had planted his seedlings before expiration of the full time interval following product application (as per the product label), that he had failed to follow the practice of planting a few test seedlings before planting an entire farm, and that he had placed his blind trust in his application adviser on all manner of timing and rate. An examination of the Company’s most knowledgeable witness is scheduled to take place in 2024. The Company believes that the claims have no merit and intends to defend the matter. At this stage in the proceedings, there is not sufficient information to form a judgment as to either the probability or amount of loss; thus, the company has not set aside a reserve in connection with this matter.
Catalano v. AMVAC Chemical Corp. On June 6, 2022, AMVAC was served with a summons and complaint for a matter entitled Andrew Catalano and Ruth Catalano v. AMVAC in the Superior Court of the State of California, County of Orange (30-2022-01263987-CU-PL-CXC) in which plaintiff, who worked as a professional applicator of pesticides, including Orthene (for which AMVAC is registrant) seeks damages for an injury (specifically, cardiomyopathy) allegedly arising from his exposure to this product. AMVAC is unaware of any link between cardiovascular disease and Orthene (which has been commercially available for over 30 years) and believes that this case has no merit and intends to defend it vigorously. The Company filed an answer, including multiple affirmative defenses. Further, the parties are exchanging document requests, and plaintiffs have been unable to supply any data establishing a causal link between use of this product and the heart condition that plaintiff alleges. At this stage, there is not sufficient information to form a judgment as to either the probability or amount of any loss; thus, the company has not set aside a reserve in connection with this matter.
Reyes v. AMVAC. On September 28, 2023, the Company received correspondence from counsel for ex-employee Jorge Reyes Jr. addressed to the California Department of Industrial Relations alleging a host of wage and hour violations under California law. This is a precursor to a civil filing under applicable state law. Subsequently, plaintiff, putatively on behalf of the class of similarly situated, non-exempt California-based employees, served a summons and complaint on the Company’s registered agent that had been electronically filed as Case No. 238TCV23665, encaptioned Jorge Reyes v. AMVAC etc., etal., with the Superior Court for the County of Los Angeles, Central District. As is typical of this sort of action, plaintiff alleges wages and hours violations of all imaginable types, including overtime, minimum wage, sick leave, rest periods and so on. The Company intends to defend the matter vigorously. At this stage of the proceedings, it is too early to tell whether any of the claims have merit. Accordingly, the company has not recorded a loss contingency in connection therewith.
Department of Justice and Environmental Protection Agency Investigation. On November 10, 2016, AMVAC was served with a grand jury subpoena from the United States Attorney’s Office for the Southern District of Alabama, seeking documents regarding the importation, transportation, and management of a specific pesticide. The Company retained defense counsel to assist in responding to the subpoena and otherwise in defending the Company’s interests. AMVAC is cooperating in the investigation. After interviewing multiple witnesses (including three employees before a grand jury in February 2022) and making multiple document requests, the Department of Justice (“DOJ”) identified the Company and a manager-level employee as targets of the government’s investigation. DOJ’s investigation focused on potential violations of two environmental statutes, the Federal Insecticide, Fungicide, and Rodenticide Act (“FIFRA”) and the Resource Conservation and Recovery Act (“RCRA”), as well as obstruction of an agency proceeding and false statement statutes. In March 2022, the individual target entered into a plea agreement relating to provision of false information in a government proceeding. In July 2022, the DoJ sent correspondence to the Company’s counsel to the effect that it was focusing on potential RCRA violations relating to the reimportation of Australian containers in 2015. Our defense counsel conferred with DoJ from time to time over the past 18 months in the interest in resolving the matter. In January 2024, the Company and DoJ reached an agreement in principle, which is subject to approval by the cognizant court and with respect to which the Company recorded a loss contingency.
The governmental agencies involved in this investigation have a range of civil and criminal penalties they may seek to impose against corporations and individuals for violations of FIFRA, RCRA and other federal statutes including, but not limited to, injunctive relief, fines, penalties and modifications to business practices and compliance programs, including the appointment of a monitor. If violations are established, the amount of any fines or monetary penalties which could be assessed and the scope of possible non-monetary relief would depend on, among other factors, findings regarding the amount, timing, nature and scope of the violations, and the level of cooperation provided to the governmental authorities during the investigation. Based upon the content of agreement in principle, the Company does not believe that the investigation will have a material adverse effect on our business prospects, operations, financial condition or cash flow.
(6) U.S. Employee Deferred Compensation and Stock Purchase Plans
The Company maintains a deferred compensation plan (“the Plan”) for all eligible employees. The Plan calls for each eligible employee, at the employee’s election, to participate in an income deferral arrangement under Internal Revenue Code Section 401(k). The plan allows eligible employees to make contributions, which cannot exceed 100% of compensation, or the annual dollar limit set by the Internal Revenue Code. The Company matches the first 5% of employee contributions. The Company’s contributions to the Plan amounted to $2,507, $2,409 and $2,273 in 2023, 2022 and 2021, respectively.
During 2001, the Company’s Board of Directors adopted the AVD Employee Stock Purchase Plan (the “ESPP Plan”). The Plan allows eligible employees to purchase shares of common stock through payroll deductions at a discounted price. An original aggregate number of approximately 1,000,000 shares of the Company’s Common Stock, par value $0.10 per share (subject to adjustment for any stock dividend, stock split or other relevant changes in the Company’s capitalization) were allowed to be sold pursuant to the Plan, which is intended to qualify under Section 423 of the Internal Revenue Code. The Plan allows for purchases in a series of offering periods, each six months in duration, with new offering periods (other than the initial offering period) commencing on January 1 and July 1 of each year. The initial offering period commenced on July 1, 2001. Pursuant to action taken by the Company’s Board of Directors on December 10, 2010, the expiration of the Plan was extended to December 31, 2013. The Plan was amended and restated on June 30, 2011, following stockholders’ ratification of the extended expiration date. The Plan was amended as of June 6, 2018, following stockholders’ ratification of a ten-year extension to the expiration date (which now stands at December 31, 2028). Under the Plan, as amended as of June 6, 2018, 995,000 shares of the Company’s common stock were authorized. As of December 31, 2023, 2022, and 2021, 441,915, 491,940, and 543,180 shares, respectively, remained available under the plan. The expense recognized under the Plan was immaterial during the years ended December 31, 2023, 2022 and 2021, respectively.
Shares of common stock purchased through the Plan in 2023, 2022 and 2021 were 50,025, 51,240 and 50,782, respectively.
(7) Major Customers
In 2023, there were three customers that accounted for 15%, 14% and 8%, respectively, of the Company’s consolidated sales. In 2022, there were three customers that accounted for 18%, 13%, and 8% of the Company’s consolidated sales. In 2021, there were three customers that accounted for 17%, 14% and 8% of the Company’s consolidated sales.
The Company primarily sells its products to distributors, buying cooperatives, other co-operative groups and, in certain territories, end users, and extends credit based on an evaluation of the customer’s financial condition. The Company had three significant customers who each accounted for approximately 6%, 5% and 3% of the Company’s receivables as of December 31, 2023. The Company had three significant customers who each accounted for approximately 15%, 3% and 3% of the Company’s receivables as of December 31, 2022. The Company has long-standing relationships with its customers and considers its credit risk associated with its domestic business for accounts receivable to be insignificant.
The Company’s receivables, excluding allowances for expected credit losses, by geography as of December 31, 2023 and 2022 are summarized as follows:
| | | | | | | | |
| | 2023 | | | 2022 | |
Domestic receivables | | $ | 89,315 | | | $ | 65,825 | |
International receivables | | | 108,761 | | | | 105,619 | |
Total receivables | | $ | 198,076 | | | $ | 171,444 | |
International sales by territory based on customer location for 2023, 2022 and 2021 were as follows:
| | | | | | | | | | | | |
| | 2023 | | | 2022 | | | 2021 | |
South and Central America | | $ | 117,727 | | | $ | 124,525 | | | $ | 108,975 | |
Mexico | | | 49,800 | | | | 45,995 | | | | 40,724 | |
Asia | | | 28,071 | | | | 26,588 | | | | 26,234 | |
Australia | | | 19,712 | | | | 19,674 | | | | 21,061 | |
Canada | | | 12,268 | | | | 14,860 | | | | 10,377 | |
Africa | | | 3,715 | | | | 8,840 | | | | 3,468 | |
Middle East | | | 1,354 | | | | 1,836 | | | | 2,357 | |
Europe | | | 2,208 | | | | 1,964 | | | | 2,243 | |
Total international net sales | | $ | 234,855 | | | $ | 244,282 | | | $ | 215,439 | |
(8) Product and Business Acquisitions
On October 5, 2023, the Company completed the acquisition of all outstanding stock of Punto Verde S.A. Punversa (Punto Verde), a well-established distributor in Guayaquil, Ecuador, to strengthen its product portfolio and market access in the Latin American region. The Company paid cash consideration of $4,492, which was net of cash acquired of $233. The acquisition was accounted for as a business combination and the purchase consideration was allocated as follows:
| | | | |
| | Amount | |
Trade receivables | | $ | 1,883 | |
Inventory and other current assets | | | 1,330 | |
Property, plant, and equipment | | | 45 | |
Product registrations and product rights | | | 104 | |
Goodwill | | | 2,949 | |
Liabilities assumed | | | (1,819 | ) |
Total | | $ | 4,492 | |
The purchase price allocation is preliminary with respect to the valuation of intangible assets, goodwill, and income taxes. The operating results of Punto Verde have been included in the Company's consolidated statements of operations from the date of acquisition. Goodwill is not expected to be deductible for income tax purposes. Pro-forma financial information is not included herein as the pro-forma impact of the acquisition is not material.
(9) Intangible Assets and Goodwill
The following schedule represents intangible assets recognized in connection with product acquisitions (See Note 1 for the Company’s accounting policy regarding intangible assets):
| | | | |
| | Amount | |
Intangible assets at December 31, 2020 | | $ | 197,514 | |
Additions during fiscal 2021 | | | 10,524 | |
Measurement period adjustment | | | 4,226 | |
Impact of movement in exchange rates | | | (710 | ) |
Amortization expense | | | (13,713 | ) |
Intangible assets at December 31, 2021 | | | 197,841 | |
Additions during fiscal 2022 | | | 1,292 | |
Impact of movement in exchange rates | | | (516 | ) |
Amortization expense | | | (13,953 | ) |
Intangible assets at December 31, 2022 | | | 184,664 | |
Additions during fiscal 2023 | | | 941 | |
Impact of movement in exchange rates | | | 177 | |
Amortization expense | | | (13,274 | ) |
Intangible assets at December 31, 2023 | | $ | 172,508 | |
| | | |
Goodwill at December 31, 2020 | | $ | 52,108 | |
Measurement period adjustment | | | (4,054 | ) |
Impact of movement in exchange rates | | | (1,794 | ) |
Goodwill at December 31, 2021 | | | 46,260 | |
Impact of movement in exchange rates | | | 750 | |
Goodwill at December 31, 2022 | | | 47,010 | |
Additions during fiscal 2023 | | | 2,949 | |
Impact of movement in exchange rates | | | 1,240 | |
Goodwill at December 31, 2023 | | $ | 51,199 | |
| | | |
Intangible assets and goodwill at December 31, 2023 | | $ | 223,707 | |
The following schedule represents the gross carrying amount and accumulated amortization of intangible assets and goodwill as of December 31, 2023 and 2022. Product rights and trademarks are amortized over the lesser of the useful life ranging from 15 to 32 years, or the patent life. Customer lists are amortized over their expected useful lives of nine to ten years. The amortization expense is included in operating expenses on the consolidated statements of operations.
| | | | | | | | | | | | | | | | | | | | | | | | |
| | 2023 | | | 2022 | |
| | Gross | | | Accumulated
Amortization | | | Net Book
Value | | | Gross | | | Accumulated
Amortization | | | Net Book
Value | |
Product rights and patents | | $ | 272,879 | | | $ | 131,778 | | | $ | 141,101 | | | $ | 272,339 | | | $ | 121,209 | | | $ | 151,130 | |
Trademarks | | | 40,896 | | | | 13,290 | | | | 27,606 | | | | 40,459 | | | | 11,615 | | | | 28,844 | |
Customer lists | | | 11,549 | | | | 7,748 | | | | 3,801 | | | | 11,204 | | | | 6,514 | | | | 4,690 | |
Total intangibles assets | | | 325,324 | | | | 152,816 | | | | 172,508 | | | | 324,002 | | | | 139,338 | | | | 184,664 | |
Goodwill | | | 51,199 | | | | — | | | | 51,199 | | | | 47,010 | | | | — | | | | 47,010 | |
Total intangibles and goodwill | | $ | 376,523 | | | $ | 152,816 | | | $ | 223,707 | | | $ | 371,012 | | | $ | 139,338 | | | $ | 231,674 | |
| | | | | | | | | | | | | | | | | | |
Domestic | | $ | 195,265 | | | $ | 99,598 | | | $ | 95,667 | | | $ | 194,395 | | | $ | 92,352 | | | $ | 102,043 | |
International | | | 181,258 | | | | 53,218 | | | | 128,040 | | | | 176,617 | | | | 46,986 | | | | 129,631 | |
Total intangibles and goodwill | | $ | 376,523 | | | $ | 152,816 | | | $ | 223,707 | | | $ | 371,012 | | | $ | 139,338 | | | $ | 231,674 | |
The following schedule represents future amortization charges related to intangible assets:
| | | | |
Year ending December 31, | | Amount | |
2024 | | $ | 12,887 | |
2025 | | | 12,704 | |
2026 | | | 12,644 | |
2027 | | | 12,501 | |
2028 | | | 11,633 | |
Thereafter | | | 110,139 | |
| | $ | 172,508 | |
(10) Contingent Consideration
The following table illustrates the Company’s contingent earn-out movements related to its business acquisitions for the years ended December 31, 2023, 2022 and 2021:
| | | | |
| | Amount | |
Obligations at December 31, 2020 | | $ | 2,468 | |
Purchase price adjustment | | | (955 | ) |
Fair value adjustment | | | 758 | |
Accretion of discounted liabilities | | | (8 | ) |
Payments on existing obligations | | | (1,301 | ) |
Foreign exchange effect | | | (176 | ) |
Obligations at December 31, 2021 | | | 786 | |
Fair value adjustment | | | 610 | |
Accretion of discounted liabilities | | | 27 | |
Payments on existing obligations | | | (1,389 | ) |
Foreign exchange effect | | | (34 | ) |
Obligations at December 31, 2022 | | $ | — | |
The Company did not have any contingent earn-out obligations as of December 31, 2023.
(11) Commitments
We enter into various obligations in the ordinary course of business, generally of a short-term nature. They primarily relate to purchase commitments for inventory and orders submitted for equipment for our production plants as well as service agreements.
(12) Research and Development
Research and development expenses which are included in operating expenses were $12,347, $10,829 and $10,354 for the years ended December 31, 2023, 2022 and 2021, respectively.
(13) Equity Plan Awards
Under the Company’s Equity Incentive Plan of 1993, as amended (“the Plan”), all employees are eligible to receive non-assignable and non-transferable restricted stock, options to purchase common stock, and other forms of equity. As of December 31, 2023, the number of securities remaining available for future issuance under the Plan is 1,376,610.
The below tables illustrate the Company’s stock-based compensation, unamortized stock-based compensation, and remaining weighted average period for the years ended December 31, 2023, 2022 and 2021. This projected expense will change if any stock options and restricted stock are granted or cancelled prior to the respective reporting periods, or if there are any changes required to be made for estimated forfeitures.
| | | | | | | | | | | | |
| | Stock-Based
Compensation | | | Unamortized
Stock-Based
Compensation | | | Remaining
Weighted
Average
Period (years) | |
December 31, 2023 | | | | | | | | | |
Restricted Stock | | $ | 4,830 | | | $ | 6,593 | | | | 1.8 | |
Unrestricted Stock | | | 520 | | | | 217 | | | | 0.4 | |
Performance-Based Restricted Stock | | | 788 | | | | 2,500 | | | | 1.8 | |
Total | | $ | 6,138 | | | $ | 9,310 | | | | |
December 31, 2022 | | | | | | | | | |
Restricted Stock | | $ | 4,407 | | | $ | 6,585 | | | | 1.8 | |
Unrestricted Stock | | | 499 | | | | 217 | | | | 0.4 | |
Performance-Based Restricted Stock | | | 778 | | | | 2,441 | | | | 1.8 | |
Total | | $ | 5,684 | | | $ | 9,243 | | | | |
December 31, 2021 | | | | | | | | | |
Restricted Stock | | $ | 5,682 | | | $ | 6,804 | | | | 1.8 | |
Unrestricted Stock | | | 421 | | | | 187 | | | | 0.4 | |
Performance-Based Restricted Stock | | | 777 | | | | 2,888 | | | | 1.8 | |
Total | | $ | 6,880 | | | $ | 9,879 | | | | |
The Company also granted stock options in past periods. All outstanding stock options are fully vested and exercisable and no expense was recorded during the years ended December 31, 2023, 2022 and 2021.
Restricted and Unrestricted Stock
A summary of nonvested restricted and unrestricted stock is presented below:
| | | | | | | | | | | | | | | | | | | | | | | | |
| | 2023 | | | 2022 | | | 2021 | |
| | Number
of Shares | | | Weighted
Average
Grant
Date Fair
Value | | | Number
of Shares | | | Weighted
Average
Grant
Date Fair
Value | | | Number
of Shares | | | Weighted
Average
Grant
Date Fair
Value | |
Nonvested shares at January 1st | | | 742,050 | | | $ | 18.86 | | | | 817,290 | | | $ | 17.04 | | | | 820,624 | | | $ | 16.64 | |
Granted | | | 306,515 | | | | 20.31 | | | | 256,417 | | | | 23.53 | | | | 295,619 | | | | 20.00 | |
Vested | | | (319,751 | ) | | | 14.90 | | | | (262,521 | ) | | | 17.84 | | | | (244,651 | ) | | | 19.23 | |
Forfeited | | | (42,629 | ) | | | 20.61 | | | | (69,136 | ) | | | 18.58 | | | | (54,302 | ) | | | 17.11 | |
Nonvested shares at December 31st | | | 686,185 | | | $ | 21.24 | | | | 742,050 | | | $ | 18.86 | | | | 817,290 | | | $ | 17.04 | |
Performance-Based Restricted Stock
A summary of nonvested performance-based stock is presented below:
| | | | | | | | | | | | | | | | | | | | | | | | |
| | December 31, 2023 | | | December 31, 2022 | | | December 31, 2021 | |
| | Number
of Shares | | | Weighted
Average
Grant
Date Fair
Value | | | Number
of Shares | | | Weighted
Average
Grant
Date Fair
Value | | | Number
of Shares | | | Weighted
Average
Grant
Date Fair
Value | |
Nonvested shares at January 1st | | | 318,699 | | | $ | 18.05 | | | | 379,061 | | | $ | 16.43 | | | | 391,771 | | | $ | 16.26 | |
Granted | | | 94,028 | | | | 21.51 | | | | 83,190 | | | | 23.63 | | | | 102,043 | | | | 20.03 | |
Change based on performance achievement | | | (58,827 | ) | | | 14.73 | | | | (68,484 | ) | | | 16.87 | | | | 71,180 | | | | 20.53 | |
Vested | | | (86,188 | ) | | | 13.99 | | | | (51,308 | ) | | | 17.09 | | | | (175,087 | ) | | | 19.78 | |
Forfeited | | | (4,387 | ) | | | 17.67 | | | | (23,760 | ) | | | 17.21 | | | | (10,846 | ) | | | 16.89 | |
Nonvested shares at December 31st | | | 263,325 | | | $ | 21.37 | | | | 318,699 | | | $ | 18.05 | | | | 379,061 | | | $ | 16.43 | |
Performance Based Restricted Stock Granted in 2023— During the year ended December 31, 2023, the Company issued a total of 94,028 performance-based shares to employees. The shares granted during 2023 have an average fair value of $21.51. The fair value was determined by using the publicly traded share price as of the market close on the date of grant. The Company will recognize as expense the value of the performance-based shares over the required service period from grant date. The shares will cliff vest on April 20, 2026, with a measurement period commencing January 1, 2023, and ending December 31, 2025. Eighty percent of these performance-based shares are based upon the financial performance of the Company, specifically, earnings before interest and tax (“EBIT”) goal weighted at 50% and a net sales goal weighted at 30%. The remaining 20% of performance-based shares are based upon AVD stock price appreciation over the same performance measurement period. The EBIT and net sales goals measure the relative growth of the Company’s EBIT and net sales for the performance measurement period, as compared to the median growth of EBIT and net sales for an identified peer group. The stockholder return goal measures the relative growth of the fair market value of the Company’s stock price over the performance measurement period, as compared to that of the Russell 2000 Index and the median fair market value of the common stock of the comparator companies, identified in the Company’s 2022 Proxy Statement. All parts of these awards vest in three years but are subject to reduction to a minimum (or even zero) for recording less than the targeted performance and to increase to a maximum of 200% for achieving in excess of the targeted performance.
Performance Based Restricted Stock Granted in 2022— During the year ended December 31, 2022, the Company issued a total of 83,190 performance-based shares to employees. The shares granted during 2022 have an average fair value of $23.63. The fair value was determined by using the publicly traded share price as of the market close on the date of grant. The Company will recognize as expense the value of the performance-based shares over the required service period from grant date. The shares will cliff vest on April 20, 2025, with a measurement period commencing January 1, 2022, and ending December 31, 2024. Eighty percent of these performance-based shares are based upon the financial performance of the Company, specifically, earnings before interest and tax (“EBIT”) goal weighted at 50% and a net sales goal weighted at 30%. The remaining 20% of performance-based shares are based upon AVD stock price appreciation over the same performance measurement period. The EBIT and net sales goals measure the relative growth of the Company’s EBIT and net sales for the performance measurement period, as compared to the median growth of EBIT and net sales for an identified peer group. The stockholder return goal measures the relative growth of the fair market value of the Company’s stock price over the performance measurement period, as compared to that of the Russell 2000 Index and the median fair market value of the common stock of the comparator companies, identified in the Company’s 2021 Proxy Statement. All parts of these awards vest in three years but are subject to reduction to a minimum (or even zero) for recording less than the targeted performance and to increase to a maximum of 200% for achieving in excess of the targeted performance.
Performance Based Restricted Stock Granted in 2021— During the year ended December 31, 2021, the Company issued a total of 102,043 performance-based shares to employees. The shares granted during 2021 have an average fair value of $20.03. The fair value was determined by using the publicly traded share price as of the market close on the date of grant. The Company will recognize as expense the value of the performance-based shares over the required service period from grant date. The shares will cliff vest on April 16, 2024, with a measurement period commencing January 1, 2021, and ending December 31, 2023. Eighty percent of these performance-based shares are based upon the financial performance of the Company, specifically, an EBIT goal weighted at 50% and a net sales goal weighted at 30%. The remaining 20% of performance-based shares are based upon AVD stock price appreciation over the same performance measurement period. The EBIT and net sales goals measure the relative growth of the Company’s EBIT and net sales for the performance measurement period, as compared to the median growth of EBIT and net sales for an identified peer group. The stockholder return goal measures the relative growth of the fair market value of the Company’s stock price over the performance measurement period, as compared to that of the Russell 2000 Index and the median fair market value of the common stock of the comparator companies, identified in the Company’s 2020 Proxy Statement. All parts of these awards vest in three years but are subject to reduction to a minimum (or even zero) for recording less than the targeted performance and to increase to a maximum of 200% for achieving in excess of the targeted performance.
During 2023, the Company concluded that the performance measure based on EBIT and net sales for the performance-based shares granted in 2020, when compared to the peer group, was met at 0% for EBIT and 88.26% for net sales of targeted performance and all related additional expenses were recorded as of December 31, 2023. The 2020 performance shares based on market price was met at 164.9%.
Stock Options
Under the terms of the Company’s ISOP, under which options to purchase common stock can be issued, all employees are eligible to receive non-assignable and non-transferable options to purchase shares. The exercise price of any option may not be less than the fair market value of the shares on the date of grant; provided, however, that the exercise price of any option granted to an eligible employee owning more than 10% of the outstanding common stock may not be less than 110% of the fair market value of the shares underlying such option on the date of grant. No options granted may be exercisable more than ten years after the date of grant.
In 2023, 2022 and 2021, no options were granted.
Incentive Stock Option Plans
Activity of the incentive stock option plans:
| | | | | | | | |
| | Number of
Shares | | | Weighted Average
Exercise Price Per
Share | |
Balance outstanding, December 31, 2020 | | | 123,087 | | | | 11.48 | |
Options exercised | | | (15,051 | ) | | | 11.41 | |
Balance outstanding, December 31, 2021 | | | 108,036 | | | | 11.49 | |
Options exercised | | | (39,140 | ) | | | 11.49 | |
Balance outstanding, December 31, 2022 | | | 68,896 | | | | 11.49 | |
Options exercised | | | (4,024 | ) | | | 11.49 | |
Balance outstanding, December 31, 2023 | | | 64,872 | | | | 11.49 | |
Outstanding stock options at December 31, 2023, summarized by exercise price:
| | | | | | | | | | |
| | Outstanding Weighted Average | |
Exercise Price Per Share | | Number of
Shares | | | Remaining
Life
(Months) | | Exercise
Price | |
Outstanding stock options, December 31, 2023 | | | 64,872 | | | 12 | | $ | 11.49 | |
Performance Incentive Stock Option Plan
Activity of the performance incentive stock option plan:
| | | | | | | | | |
| | Number of
Shares | | | Weighted
Average
Price Per
Share | | |
Balance outstanding, December 31, 2021 and 2020 | | | 114,658 | | | $ | 11.49 | | |
Options exercised | | | (32,850 | ) | | | 11.49 | | |
Balance outstanding, December 31, 2022 | | | 81,808 | | | $ | 11.49 | | |
Options exercised | | | — | | | | 11.49 | | |
Balance outstanding, December 31, 2023 | | | 81,808 | | | $ | 11.49 | | |
All the performance incentive stock options outstanding as of December 31, 2023, have an exercise price per share of $11.49 and a remaining life of 12 months.
The total intrinsic value of options exercised during 2023, 2022, and 2021 was $35, $877, and $119, respectively. Cash received from stock options exercised during 2023, 2022 and 2021 was $46, $827, and $172, respectively. All outstanding options are fully vested and the intrinsic value amounted to $0 as of December 31, 2023.
(14) Accumulated Other Comprehensive Loss
The following table lists the beginning balance, annual activity and ending balance of foreign currency translation adjustment included as a component of accumulated other comprehensive loss:
| | | | |
Balance, December 31, 2020 | | $ | (9,322 | ) |
Foreign currency translation adjustment, net of tax effects of $76 | | | (4,462 | ) |
Balance, December 31, 2021 | | | (13,784 | ) |
Foreign currency translation adjustment, net of tax effects of ($245) | | | 1,602 | |
Balance, December 31, 2022 | | | (12,182 | ) |
Foreign currency translation adjustment, net of tax effects of ($277) | | | 6,219 | |
Balance, December 31, 2023 | | $ | (5,963 | ) |
(15) Equity Method Investment
On August 2, 2016, AMVAC BV entered into a joint venture with Huifeng (Hong Kong) Ltd, which is a wholly owned subsidiary of the Huifeng Group. The resulting entity, Hong Kong JV, was intended to focus on activities such as market access and technology transfer between the two members. AMVAC BV is a 50% owner of the entity.
On June 27, 2017, both AMVAC BV and Huifeng (Hong Kong) Ltd. made individual capital contributions of $950 to the Hong Kong JV. As of December 31, 2023, 2022 and 2021, the Company’s ownership position in the Hong Kong JV was 50%. The Company utilizes the equity method of accounting with respect to this investment.
On July 7, 2017, the Hong Kong JV purchased the shares of Profeng Australia, Pty Ltd. (“Profeng”), for a total consideration of $1,900. The purchase consists of Profeng Australia, Pty Ltd Trustee and Profeng Australia Unit Trust. Both Trust and Trustee were previously owned by Huifeng (via its wholly owned subsidiary Huifeng (Hong Kong) Ltd). For the years ended December 31, 2023, 2022, and 2021, the Company recognized losses of $0 for both 2023 and 2022 and $388 (including a full impairment charge of $288 of the Company’s remaining book value of its Hong Kong JV investment) for 2021, as a result of the Company’s ownership position in the Hong Kong JV. The Hong Kong JV is an inactive entity.
(16) Equity Investments
In February 2016, AMVAC BV made an equity investment of $3,283 in Biological Products for Agriculture (“Bi-PA”). Bi-PA develops biological plant protection products that can be used for the control of pests and disease of agricultural crops. As of December 31, 2023, 2022 and 2021, the Company’s ownership position in Bi-PA was 15%. Since this investment does not have readily determinable fair value, the Company has elected to measure the investment at cost less impairment, if any, and to record an increase or decrease for changes resulting from observable price changes in orderly transactions for the identical or a similar investment of Bi-PA. The Company periodically reviews the investment for possible impairment. The Company recorded an impairment charge in the amount of $399 during the year ended December 31, 2021. There were no impairment or observable price changes on the investment during the years ended December 31, 2023 and 2022. The investment is recorded within other assets on the consolidated balance sheets and amounted to $2,884 as of December 31, 2023 and 2022.
On April 1, 2020, AMVAC purchased 6.25 million shares, an ownership of approximately 8%, of common stock of Clean Seed Capital Group Ltd. (TSX Venture Exchange: “CSX”) for $1,190. The shares are publicly traded, have a readily determinable fair value, and are considered a Level 1 investment. The fair value of the stock amounted to $425 and $784 as of December 31, 2023 and 2022, respectively, and the Company recorded losses of $359, $732 and $391 for the years ended December 31, 2023, 2022 and 2021, respectively. The investment is recorded within other assets on the consolidated balance sheets and the associated gains and losses are included in the change in fair value of equity investments.
(17) Share Repurchase Programs
The Company periodically repurchases shares of its common stock under a board-authorized repurchase program through a combination of open market transactions and accelerated share repurchase ("ASR") arrangements.
On May 25, 2023, pursuant to a Board of Directors resolution, the Company announced its intention to repurchase up to $15,000 of its common stock under a 10b5-1 plan, par value $0.10 per share, in the open market over the succeeding one year, subject to limitations and restrictions under applicable securities laws. During the year ended December 31, 2023, the Company purchased 857,455 shares of its common stock for a total of $14,982 at an average price of $17.55 per share under this plan.
On August 22, 2022, pursuant to a Board of Directors resolution, the Company entered into an ASR to repurchase $20,000 of its common stock. Under the ASR agreement, the Company paid $20,000 and immediately received an initial delivery of 802,810 shares in the amount of $16,000, based on a price of $19.93 per share, which represented 80% of the notional amount of the ASR based on the closing price of the Company’s common stock on the New York Stock Exchange ("NYSE") on August 22, 2022. On December 14, 2022, the ASR was completed, and pursuant to the settlement terms of the ASR, the Company received an additional 131,892 shares of its common stock. The average price paid for all of the shares delivered under the ASR was $21.40 per share.
On March 8, 2022, pursuant to a Board of Directors resolution, the Company announced its intention to repurchase an aggregate number of up to 1,000,000 shares of its common stock under a 10b5-1 plan, par value $0.10 per share, in the open market over the succeeding one year, subject to limitations and restrictions under applicable securities laws. The plan terminated on March 8, 2023. During 2023, the Company purchased 27,835 shares of its common stock for a total of $557 at an average price of $19.96 per share under this plan. During 2022, the Company purchased 734,150 shares of its common stock for a total of $14,002 at an average price of $19.07 per share under this plan.
On August 30, 2021, pursuant to a Board of Directors resolution, the Company announced its intention to repurchase an aggregate number of 300,000 shares of its common stock, par value $0.10 per share, in the open market over the succeeding six months. During 2021, the Company purchased 300,000 shares of its common stock for a total of $4,579 at an average price of $15.26 per share.
The shares and respective amount are recorded as treasury shares on the Company’s consolidated balance sheets. The table below summarized the number of shares of the Company's common stock that were repurchased during the years ended December 31, 2023, 2022 and 2021.
| | | | | | | | | | | | |
Year ended | | Total number of
shares purchased | | | Average price paid
per share | | | Total amount paid | |
December 31, 2023 | | | 885,290 | | | $ | 17.55 | | | $ | 15,539 | |
December 31, 2022 | | | 1,668,852 | | | $ | 20.37 | | | $ | 34,002 | |
December 31, 2021 | | | 300,000 | | | $ | 15.26 | | | $ | 4,579 | |
Pursuant to Amendment Number Six to the Third Amended Loan and Security Agreement, effective November 7, 2023, the Company is currently prevented from making stock repurchases.
(18) Supplemental Cash Flows Information
| | | | | | | | | | | | |
| | 2023 | | | 2022 | | | 2021 | |
Supplemental cash flow information: | | | | | | | | | |
Cash paid during the year for: | | | | | | | | | |
Interest | | $ | 11,902 | | | $ | 3,834 | | | $ | 3,520 | |
Income taxes, net | | $ | 9,428 | | | $ | 19,960 | | | $ | 5,796 | |
Non-cash transactions: | | | | | | | | | |
Cash dividends declared and included in accrued expenses | | $ | 834 | | | $ | 851 | | | $ | 594 | |
ITEM 9 CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
None.
ITEM 9A CONTROLS AND PROCEDURES
Disclosure Controls and Procedures
Management, under the supervision of the Company’s Chief Executive Officer and Chief Financial Officer, periodically evaluate the effectiveness of the design and operation of the Company’s disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)). Based upon this evaluation, as of December 31, 2023, the Chief Executive Officer and the Chief Financial Officer have concluded that these disclosure controls and procedures are effective in ensuring that the information required to be disclosed in reports filed under the Securities Exchange Act of 1934 is (i) recorded, processed, summarized and reported on a timely basis, and (ii) accumulated and communicated to the Company’s management, including the Chief Executive Officer and Chief Financial Officer, as appropriate to allow timely decisions regarding required disclosure.
Management’s Report on Internal Control over Financial Reporting
Management of the Company is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rules 13a-15(f) and 15d-15(f) of the Securities Exchange Act of 1934 for the Company. The Company’s internal control system over financial reporting is designed to provide reasonable assurance to management and the Board of Directors as to the fair, reliable and timely preparation and presentation of consolidated financial statements in accordance with accounting principles generally accepted in the United States of America filed with the SEC.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Therefore, even processes determined to be effective can provide only reasonable assurance with respect to the financial statement preparation and presentation.
Management conducted an evaluation of the Company’s internal controls over financial reporting based on a framework set forth by the Committee of Sponsoring Organizations of the Treadway Commission in Internal Control—Integrated Framework (2013). This evaluation included review of the documentation of controls, evaluation of the design effectiveness of controls, testing of the effectiveness of controls and a conclusion on the evaluation. Based on this evaluation, management believes that as of December 31, 2023, the Company’s internal control over financial reporting is effective.
Deloitte & Touche, LLP, the independent registered public accounting firm that audited the consolidated financial statements as of and for the year ended December 31, 2023, included in the Annual Report on Form 10-K, was engaged to attest to and report on the effectiveness of AVD’s internal control over financial reporting as of December 31, 2023. Their report is included herein.
Changes in Internal Control over Financial Reporting
There were no changes in internal control over financial reporting during the fourth quarter of the year ended December 31, 2023, that have materially affected, or are reasonably likely to materially affect, the Company’s internal control over financial reporting.
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Stockholders and the Board of Directors of
American Vanguard Corporation
Newport Beach, California
Opinion on Internal Control over Financial Reporting
We have audited the internal control over financial reporting of American Vanguard Corporation and subsidiaries (the “Company”) as of December 31, 2023, based on criteria established in Internal Control — Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). In our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2023, based on criteria established in Internal Control — Integrated Framework (2013) issued by COSO.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the consolidated financial statements as of and for the year ended December 31, 2023, of the Company and our report dated March 27, 2024, expressed an unqualified opinion on those financial statements and included an explanatory paragraph regarding the Company’s change in accounting principle.
Basis for Opinion
The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management’s Report on Internal Control Over Financial Reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
Definition and Limitations of Internal Control over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/ Deloitte & Touche LLP
Costa Mesa, California
March 27, 2024
AMERICAN VANGUARD CORPORATION
ITEM 9B OTHER INFORMATION
None.
ITEM 9C DISCLOSURE REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS
Not applicable.
AMERICAN VANGUARD CORPORATION
PART III
ITEM 10 DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
The information set forth under the captions “Executive Officers of the Company,” “Election of Directors,” “Information about the Board of Directors and Committees of the Board” and “Transactions with Management and Others—Section 16(a) Beneficial Ownership Reporting Compliance” in our definitive proxy statement (the “Proxy Statement”) for our Annual Meeting of Stockholders to be held on June 5, 2024, which will be filed with the SEC within 120 days of the end of our fiscal year ended December 31, 2023, is incorporated herein by reference.
ITEM 11 EXECUTIVE COMPENSATION
Except as specifically provided, the information set forth under the captions “Compensation of Executive Officers” and “Information about the Board of Directors and Committees of the Board—Compensation of Directors” in the Proxy Statement is incorporated herein by reference.
ITEM 12 SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The disclosure contained in Part II, Item 5 under “Equity Compensation Plan Information” is incorporated herein by reference. Information regarding security ownership of certain beneficial owners and management is incorporated by reference to the information set forth under the caption “Security Ownership of Certain Beneficial Owners and Management” in the Proxy Statement.
ITEM 13 CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
The information set forth under the captions “Transactions with Management and Others” and “Information about the Board of Directors and Committees of the Board” in the Proxy Statement is incorporated herein by reference.
ITEM 14 PRINCIPAL ACCOUNTANT FEES AND SERVICES
Information regarding principal accountant fees and services is incorporated herein by reference to the information set forth under the caption “Ratification of the Selection of Independent Registered Public Accounting Firm—Relationship of the Company with Independent Registered Public Accounting Firm” in the Proxy Statement.
AMERICAN VANGUARD CORPORATION
PART IV
ITEM 15 FINANCIAL STATEMENTS, SCHEDULES, AND EXHIBITS
1.Financial Statements. The consolidated financial statements as set forth under Item 8 of this Annual Report on Form 10-K are incorporated herein.
2.Financial Statement Schedules: Schedule II-A
AMERICAN VANGUARD CORPORATION
Schedule II-A—Valuation and Qualifying Accounts
Allowance for Current Expected Credit Losses (in thousands)
| | | | | | | | | | | | | | | | |
| | Balance at | | | | | | Foreign | | | Balance at | |
Fiscal Year Ended | | Beginning of
Period | | | Additions | | | exchange
impact | | | End of
Period | |
December 31, 2023 | | $ | 5,136 | | | | 1,935 | | | | 36 | | | $ | 7,107 | |
December 31, 2022 | | $ | 3,938 | | | | 1,171 | | | | 27 | | | $ | 5,136 | |
December 31, 2021 | | $ | 3,297 | | | | 649 | | | | (8 | ) | | $ | 3,938 | |
Deferred Tax Assets Valuation Allowance (in thousands)
| | | | | | | | | | | | | | | | |
| | Balance at | | | Additions
Charged to | | | Balance at | |
Fiscal Year Ended | | Beginning of
Period | | | Net Income (Loss) | | | Other Comprehensive (Gain) Loss | | | End of
Period | |
December 31, 2023 | | $ | 3,853 | | | $ | 1,376 | | | $ | (1,912 | ) | | $ | 3,317 | |
December 31, 2022 | | $ | 4,262 | | | $ | 379 | | | $ | (788 | ) | | $ | 3,853 | |
December 31, 2021 | | $ | — | | | $ | 3,304 | | | $ | 958 | | | $ | 4,262 | |
See accompanying report of independent registered public accounting firms on page 28 and 31of this annual report.
AMERICAN VANGUARD CORPORATION
EXHIBIT INDEX
| | |
Exhibit Number | | Description of Exhibit |
| | |
3.1 | | Amended and Restated Certificate of Incorporation of American Vanguard Corporation (filed as Exhibit 3.1 to the Company’s Form 10-K for the year ended December 31, 2003, which was filed on March 30, 2004, with the Securities Exchange Commission and incorporated herein by reference). |
| | |
3.2 | | Certificate of Amendment of Amended and Restated Certificate of Incorporation of American Vanguard Corporation (filed as Exhibit 3.2 to the Company’s Form 10-Q/A for the period ended June 30, 2004, which was filed with the Securities Exchange Commission on February 23, 2005, and incorporated herein by reference). |
| | |
3.3 | | Amended and Restated Bylaws of American Vanguard Corporation dated as of June 5, 2014 (filed as Exhibit 99.1 to the Company’s Form 8-K, which was filed with the Securities Exchange Commission on June 7, 2014, and incorporated herein by reference.) |
| | |
4.1 | | Form of Indenture (filed as Exhibit 4.4 to the Company’s Registration Statement on Form S-3 (File No. 333-122981) and incorporated herein by reference). |
| | |
4.2 | | Description of Registrant's Capital Stock as of December 31, 2023* |
| | |
10.1 | | American Vanguard Corporation Employee Stock Purchase Plan (filed as Appendix A to the Company’s Proxy Statement filed with the Securities and Exchange Commission on April 23, 2018, and incorporated herein by reference). |
| | |
10.2 | | American Vanguard Corporation Amended and Restated Stock Incentive Plan as of June 8, 2016 (filed as Appendix A to the Company’s Proxy Statement filed with the Securities and Exchange Commission on April 25, 2016, and incorporated herein by reference). |
| | |
10.3 | | Form of Incentive Stock Option Agreement under the American Vanguard Corporation Fourth Amended and Restated Stock Incentive Plan, (filed as Exhibit 10.3 with the Company’s Annual Report on Form 10-K for the period ended December 31, 2004, which was filed with the Securities and Exchange Commission on March 16, 2005, and incorporated herein by reference). |
| | |
10.4 | | Form of Non-Qualified Stock Option Agreement under the American Vanguard Corporation Fourth Amended and Restated Stock Incentive Plan, (filed as Exhibit 10.4 with the Company’s Annual Report on Form 10-K for the period ended December 31, 2004, which was filed with the Securities and Exchange Commission on March 16, 2005, and incorporated herein by reference). |
| | |
10.5 | | Employment Agreement between American Vanguard Corporation and Eric G. Wintemute dated January 15, 2008 (filed as Exhibit 10.1 to the Company’s Form 8-K, which was filed with the Securities Exchange Commission on April 7, 2022, and incorporated herein by reference). |
| | |
10.8 | | Form of Change of Control Severance Agreement, dated effective as of January 1, 2004, between American Vanguard Corporation and its executive and senior officers (filed as Exhibit 10.2 to the Company’s Form 10-Q for the period ended March 31, 2004, which was filed with the Securities Exchange Commission on May 17, 2004, and incorporated herein by reference.) |
| | |
10.9 | | Form of Amendment of Change of Control Severance Agreement, dated effective as of July 11, 2008, between American Vanguard Corporation and named executive officers and senior officers (filed as Exhibit 99.1 to the Company’s Form 8-K, which was filed on July 11, 2008, with the Securities and Exchange Commission and incorporated herein by reference). |
| | |
10.10 | | Form of Indemnification Agreement between American Vanguard Corporation and its Directors (as filed as Exhibit 10.7 to the Company’s Annual Report on Form 10-K for the period ended December 31, 2004, which was filed with the Securities and Exchange Commission on March 16, 2005, and incorporated herein by reference). |
| | |
10.11 | | Description of Compensatory Arrangements Applicable to Non-Employee Directors (as set forth on page 34 of the Company’s Proxy Statement which was filed with the Securities and Exchange Commission on April 22, 2019, and incorporated herein by reference). |
AMERICAN VANGUARD CORPORATION
| | |
Exhibit Number | | Description of Exhibit |
| | |
| | |
10.12 | | Form of Restricted Stock Agreement between American Vanguard Corporation and named executive officers dated as of November 13, 2020. Filed as exhibit 10.12 to the Company’s Form 10-K for the period ended December 31, 2020. |
| | |
10.14 | | Form of Performance-Based Restricted Stock Units Award Agreement between American Vanguard Corporation and named executive officer dated as of November 13, 2020. Filed as exhibit 10.14 to the Company’s Form 10-K for the period ended December 31, 2020. |
| | |
10.15 | | Form of American Vanguard Corporation Amended and Restated Stock Incentive Plan TSR-Based Restricted Stock Units Award Agreement dated June 6, 2013 (filed as Exhibit 10.15 to the Company’s 10-K, which was filed with the Securities Exchange Commission on February 28, 2014, and incorporated herein by reference). |
| | |
10.16 | | Form of American Vanguard Corporation Amended and Restated Stock Incentive Plan Performance-Based Restricted Stock Units Award Agreement dated June 6, 2013 (filed as Exhibit 10.16 to the Company’s 10-K, which was filed with the Securities Exchange Commission on February 28, 2014, and incorporated herein by reference). |
| | |
10.17 | | Third Amendment to Second Amended and Restated Credit Agreement dated as of June 30, 2017 among AMVAC and certain affiliates on the other hand, and a group of commercial lenders led by Bank of the West as agent, swing line lender, and letter of credit issuer, on the other hand (filed as Exhibit 10.1 to the Company’s Form 8-K, which was filed with the Securities Exchange Commission on July 6, 2017 and is incorporated herein by reference). |
| | |
10.18 | | Fourth Amendment to Second Amended and Restated Credit Agreement dated as of November 27, 2019, among AMVAC and certain affiliates, on the one hand, and a group of commercial lenders led by Bank of the West as agent, swing line lender, and letter of credit issuer, on the other hand (filed with the Company’s Form 10-K for the period ended December 31, 2019). Third Amended and Restated Loan and Security Agreement dated August 5, 2021 by and among American Vanguard Corporation et al. and Bank of the West et al. a form of which was filed with the SEC as an exhibit to the Company’s Form 8-K on or about August 10, 2021. |
| | |
10.19 | | Amendment Number Five to the Third Amended Loan and Security Agreement dated as of May 8, 2023, among AMVAC and certain affiliates, on the one hand, and a group of commercial lenders led by BMO Harris Bank, N.A., on the other hand (filed with the SEC as an exhibit to the Company’s Form 80K on May 10, 2023). |
| | |
10.20 | | Amendment Number Six to the Third Amended Loan and Security Agreement dated as of November 7, 2023, among AMVAC and certain affiliates on the one hand, and a group of commercial lenders led by BMO Harris Bank, N.A., on the other hand (filed with the SEC as an exhibit to the Company’s Form 8-K on November 9, 2023). |
| | |
10.21 | | American Vanguard Corporation Clawback Policy dated as of September 6, 2023.* |
| | |
10.22 | | 2022 Stock Incentive Plan, Incentive Stock Option agreement dated as of January 22, 2024* |
| | |
10.23 | | 2022 Stock Incentive Plan, Incentive Stock Option FMV Agreement dated as of January 22, 2024* |
| | |
21 | | List of Subsidiaries of the Company.* |
| | |
23.1 | | Consent of Deloitte & Touche LLP, Independent Registered Public Accounting Firm* |
| | |
23.2 | | Consent of BDO USA, P.C., Independent Registered Public Accounting Firm.* |
| | |
31.1 | | Certification of Chief Executive Officer Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002.* |
| | |
31.2 | | Certification of Chief Financial Officer Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002.* |
| | |
AMERICAN VANGUARD CORPORATION
* Filed herewith.
(c)Valuation and Qualifying Accounts
ITEM 16 FORM 10-K SUMMARY
None.
AMERICAN VANGUARD CORPORATION
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, American Vanguard Corporation has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
AMERICAN VANGUARD CORPORATION
(Registrant)
| | | | |
By: | /s/ ERIC G. WINTEMUTE | | By: | /s/ DAVID T. JOHNSON |
| Eric G. Wintemute Chief Executive Officer and Chairman of the Board |
|
| David T. Johnson Chief Financial Officer and Principal Accounting Officer |
| | | | |
| March 27, 2024 | | | March 27, 2024 |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the Registrant and in the capacities indicated.
| | | | |
By: | /s/ ERIC G. WINTEMUTE | | By: | /s/ DAVID T. JOHNSON |
| Eric G. Wintemute Principal Executive Officer and Chairman of the Board | | | David T. Johnson Principal Financial Officer and Principal Accounting Officer |
| | | | |
| March 27, 2024 | | | March 27, 2024 |
| | | | |
By: | /s/ DEBRA EDWARDS | | By: | /s/ MORTON D. ERLICH |
| Debra Edwards Director | | | Morton D. Erlich Director |
| | | | |
| March 27, 2024 | | | March 27, 2024 |
| | | | |
By: | /s/ MARISOL ANGELINI | | By: | /s/ SCOTT D. BASKIN |
| Marisol Angelini Director | | | Scott D. Baskin Director |
| | | | |
| March 27, 2024 | | | March 27, 2024 |
| | | | |
By: | /s/ ÉMER GUNTER | | By: | /s/ PATRICK E. GOTTSCHALK |
| Émer Gunter Director | | | Patrick E. Gottschalk Director |
| | | | |
| March 27, 2024 | | | March 27, 2024 |
| | | | |
By: | /s/ MARK R. BASSETT | | By: | /s/ KEITH M. ROSENBLOOM |
| Mark R. Bassett Director | | | Keith M. Rosenbloom Director |
| | | | |
| March 27, 2024 | | | March 27, 2024 |
