Exhibit 99.1

ADVANCED - 2 TRIAL INTERIM RESULTS December 2024

FORWARD LOOKING STATEMENTS Statements contained in this presentation regarding matters that are not historical facts are "forward looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995 . Protara may, in some cases, use terms such as “predicts,” “believes,” “potential,” “proposed,” “continue,” “designed,” “estimates,” “anticipates,” “expects,” “plans,” “intends,” “may,” “could,” “might,” “will,” “should” or other words or expressions referencing future events, conditions or circumstances that convey uncertainty of future events or outcomes to identify these forward - looking statements . Such forward - looking statements include but are not limited to, statements regarding Protara’s intentions, beliefs, projections, outlook, analyses or current expectations concerning, among other things : Protara’s business strategy, including its development plans for its product candidates and plans regarding the timing or outcome of existing or future clinical trials (including reporting initial data from 12 - month evaluable patients in mid - 2025 ) ; statements related to expectations regarding interactions with the FDA , Protara’s financial footing ; statements regarding the anticipated safety or efficacy of Protara’s product candidates ; and Protara’s outlook for the remainder of the year and future periods . Because such statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward - looking statements . Factors that contribute to the uncertain nature of the forward - looking statements include : risks that Protara’s financial guidance may not be as expected, as well as risks and uncertainties associated with : Protara’s development programs, including the initiation and completion of non - clinical studies and clinical trials and the timing of required filings with the FDA and other regulatory agencies ; general market conditions ; changes in the competitive landscape ; changes in Protara’s strategic and commercial plans ; Protara’s ability to obtain sufficient financing to fund its strategic plans and development and commercialization efforts ; having to use cash in ways or on timing other than expected ; the impact of market volatility on cash reserves ; the loss of key members of management ; the impact of general U . S . and foreign, economic, industry, market, regulatory, political or public health conditions ; and the risks and uncertainties associated with Protara’s business and financial condition in general, including the risks and uncertainties described more fully under the caption “Risk Factors” and elsewhere in Protara's filings and reports with the United States Securities and Exchange Commission . All forward - looking statements contained in this presentation speak only as of the date on which they were made and are based on management's assumptions and estimates as of such date . Protara undertakes no obligation to update any forward - looking statements, whether as a result of the receipt of new information, the occurrence of future events or otherwise, except as required by law . 2 © 2024 Protara Therapeutics. All Rights Reserved – Do Not Copy or Distribute

Promising NMIBC therapy C de - risked rare disease programs 3 TARA - 002 in NMIBC • Dosing underway in Phase 2 STARBORN - 1 trial • TARA - 002 predecessor is standard of care in Japan • U.S. FDA granted Rare Pediatric Disease Designation – PRV eligible • Positive interim results from ADVANCED - 2 trial in NMIBC • Unique product characteristics anticipated to drive significant adoption • Potential to expand clinical program into BCG - naïve, combinations, systemic dosing and intermediate risk Oncology Rare Disease TARA - 002 in LMs 1. Data on file © 2024 Protara Therapeutics. All Rights Reserved – Do Not Copy or Distribute IV Choline for Parenteral Support • Enrolling pivotal study with PK endpoint • 30K patient population in the US 1 • FDA Orphan Drug and Fast Track Designations

Anticipated low burden on physicians s patients Favorable safety s tolerability Encouraging interim ADAVNCED - 2 data • No additional administration procedures or safety protocols required • Fast administration typically performed by nurse • Dedicated to ensuring access with minimal burden • To date, no Grade 2 or greater treatment - related adverse events • To date, majority of adverse events are grade 1 and transient • Compelling response rates in BCG - UN and BCG - naïve • 100% durability observed from 3 - to 6 - months and 80% reinduction salvage rate seen across all patients Unique product characteristics anticipated to drive significant adoption 4 © 2024 Protara Therapeutics. All Rights Reserved – Do Not Copy or Distribute

Activates Th1 Immune Cascade (1)(2)(3) BROAD IMMUNOPOTENTIATION = POTENTIAL FOR DURABLE RESPONSE Mechanism similar to BCG, unique to other agents in development 1. Fujimoto T., et al. J Immunol. 1997: 5619. | 2. Ryoma Y, et al. Anticancer Res. 2004; 3295 - 3298. | 3. Zhao H, et al. Microbiol. Immunol. 1994; 183 - 190. 5 IFN - IL - 1b NK - Cells IL - 6 IL - 12 TNF - α GM - CSF © 2024 Protara Therapeutics. All Rights Reserved – Do Not Copy or Distribute

TARA - 002 demonstrated 72% six - month CRR and 70% CRR at any time across BCG exposures Abbreviations: BCG = Bacillus Calmette - Guérin; CR = complete response; CIS = carcinoma in situ; Dx = diagnosis; NMIBC = non - muscle invasive bladder cancer Notes: At the time of data cutoff, 20 subjects were evaluated for high - grade CR at Month 3 and later. Eighteen subjects were evaluated for high - grade CR at Month 6 and 3 subjects at Month 9; Evaluable subjects include those who had at least one dose of study drug before the response assessment of time point and were discontinued due to dx progression or treatment failure. Subjects who have not yet completed week 12 visit as of study cut off date are not included; Central urine cytology is pending for 3 subjects at Month 6 and 1 subject at Month 9. 6 © 2024 Protara Therapeutics. All Rights Reserved – Do Not Copy or Distribute BCG Unresponsive BCG Naïve Combined Combined BCG Naïve BCG Un - responsive High - grade CR at Month 6 100 100% 80 72% 60 64% 40 20 0 N = 18 High - grade CR at any time 100 80 80% 70% 60 67% 40 20 0 N = 20 N = 20 N = 15 N = 5 (%) (%) (%) 14/20 (70.0) 10/15 (66.7) 4/5 (80.0) High - grade CR at any time High - grade CR at Timepoint 13/18 (72.2) 9/14 (64.3) 4/4 (100.0) Month 6 2/3 (66.7) 2/3 (66.7) N/A Month 9 High - grade CR at Month 6 by Baseline Dx 9/10 (90.0) 5/6 (83.3) 4/4 (100.0) CIS only 4/8 (50.0) 4/8 (50.0) N/A CIS + Ta/T1 Data cut - off date: 19 November 2024
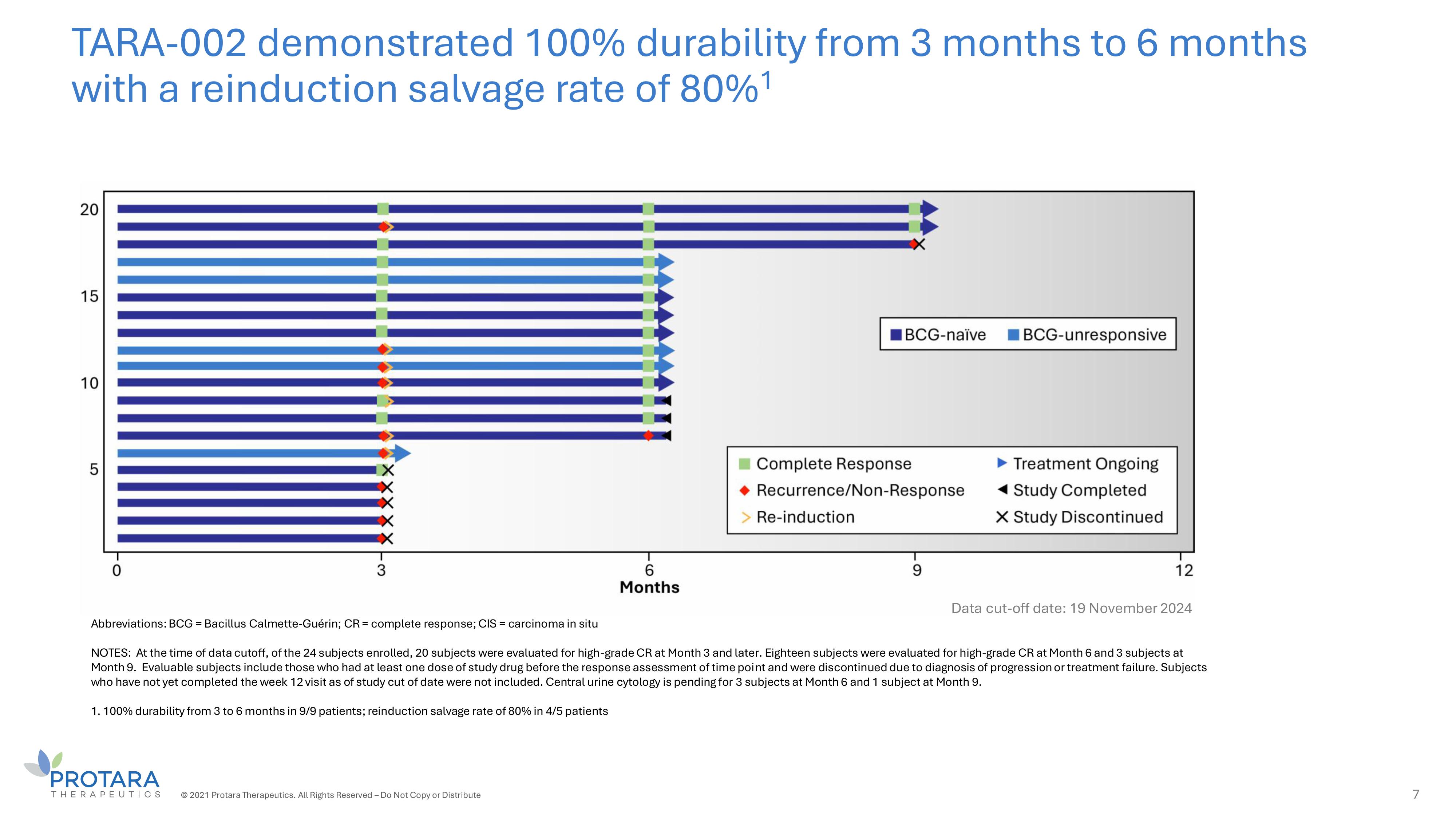
TARA - 002 demonstrated 100% durability from 3 months to 6 months with a reinduction salvage rate of 80% 1 © 2021 Protara Therapeutics. All Rights Reserved – Do Not Copy or Distribute 7 Abbreviations: BCG = Bacillus Calmette - Guérin; CR = complete response; CIS = carcinoma in situ NOTES: At the time of data cutoff, of the 24 subjects enrolled, 20 subjects were evaluated for high - grade CR at Month 3 and later. Eighteen subjects were evaluated for high - grade CR at Month 6 and 3 subjects at Month 9. Evaluable subjects include those who had at least one dose of study drug before the response assessment of time point and were discontinued due to diagnosis of progression or treatment failure. Subjects who have not yet completed the week 12 visit as of study cut of date were not included. Central urine cytology is pending for 3 subjects at Month 6 and 1 subject at Month 9. 1. 100% durability from 3 to 6 months in 9/9 patients; reinduction salvage rate of 80% in 4/5 patients Data cut - off date: 19 November 2024

8 TARA - 002 demonstrated favorable safety and tolerability in interim analysis of ADVANCED - 2 trial Data cut - off date: 19 November 2024 AEs reflect urinary tract instrumentation effects and known safety profile of an immune - potentiating drug Abbreviations: AE = adverse event; NMIBC = non - muscle invasive bladder cancer; TEAE = treatment emergent AE ^ Subjects may be counted in multiple categories + Non - drug related Serious TEAEs included urinary tract infection (UTI; N = 2) and urosepsis (N =1) Note: the safety population includes any patients who have had at least 1 dose of TARA - 002. The 24 patients in safety analysis include 3 patients who have not reached their week 12 assessment, and 1 patient withdrew consent prior to their week 12 assessment © 2024 Protara Therapeutics. All Rights Reserved – Do Not Copy or Distribute Grade 4/5 Grade 3 Grade 2 Grade 1 Any Grade N=24 0 3 (13) 7 (29) 11 (46) 16 (67) Number of Subjects with TEAEs , n^ (%) 0 0 0 6 (25) 6 (25) Number of Subjects with Related TEAEs ^, n (%) 0 0 0 3 (13) 3 (13) Dysuria 0 0 0 1 (4) 1 (4) Bladder Discomfort 0 0 0 1 (4) 1 (4) Bladder Spasm 0 0 0 1 (4) 1 (4) Chills 0 0 0 1 (4) 1 (4) Fatigue 0 0 0 1 (4) 1 (4) Hematuria 0 0 0 1 (4) 1 (4) Micturition Urgency 0 0 0 1 (4) 1 (4) Urinary Incontinence 0 2 (8) 1 (4) 0 3 (13) Number of Subjects with Serious TEAEs + , n (%) 0 0 0 0 0 Number of Subjects with TEAEs leading to Study Drug Withdrawal, n (%)

9 UNIQUE MOA • Only broad immunopotentiator in the industry pipeline • Non - clinical data points to encouraging durability • No overlapping toxicities with other novel therapeutic in NMIBC POTENTIAL EASE ON PROVIDERS s PATIENTS • To date, no Grade 2 or greater treatment - related adverse events • Simple, fast administration via catheter OPPORTUNITIES TO EXPAND • First to publish efficacy in BCG - naïve patients; assessing potential next steps • Only novel agent with the ability to dose systemically – potentially replacing intravesical administration TARA - 002 HAS A DIFFERENTIATED PROFILE IN NMIBC WITH ENCOURAGING INTERIM DATA PROMISING CLINICAL DATA • Positive interim results across BCG exposures RELIABLE MANUFACTURING • Advanced, FDA - inspected, cGMP manufacturing with 20mm vial annual capacity • Doubling time (2 hrs) vs. BCG (16 hrs) adds to TARA - 002’s benefit over BCG in the non - refractory setting • Dedicated to ensuring access with minimal burden © 2024 Protara Therapeutics. All Rights Reserved – Do Not Copy or Distribute

QCA © 2024 Protara Therapeutics. All Rights Reserved – Do Not Copy or Distribute 10

APPENDIX

TARA - 002 in NMIBC: ADVANCED - 2 trial design 12 © 2024 Protara Therapeutics. All Rights Reserved – Do Not Copy or Distribute Protara Confidential Information Data cut - off date: 19 November 2024 RE - induction (if eligible*) Maintenance (months 6 - 18) Month 3 Month 6 Month 18 Month 60 Maintenance (months 6 - 18) Month 18 Month 60 Month 3 Month 6 Abbreviations: CR = complete response; CIS = carcinoma in situ *Aligned with the FDA’s 2024 BCG Unresponsive NMIBC: Developing Drugs and Biologics for Treatment Guidance for Industry. †Residual CIS and/or recurrence of HGTa Primary endpoint of high - grade complete response (CR) at any time at 6mos; Key secondary of 12 - month DOR
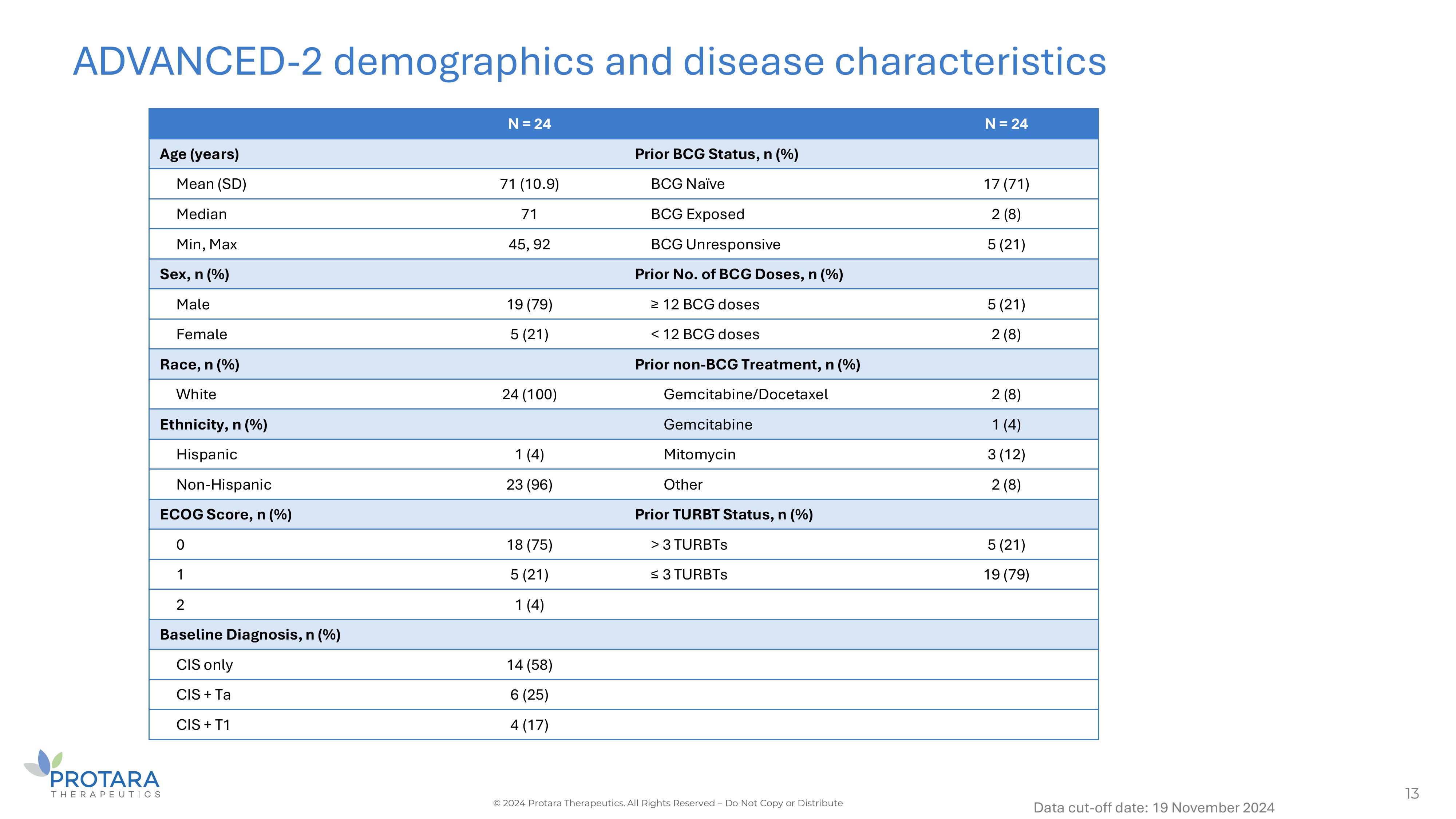
ADVANCED - 2 demographics and disease characteristics 13 © 2024 Protara Therapeutics. All Rights Reserved – Do Not Copy or Distribute Data cut - off date: 19 November 2024 N = 24 N = 24 Prior BCG Status, n (%) Age (years) 17 (71) BCG Naïve 71 (10.9) Mean (SD) 2 (8) BCG Exposed 71 Median 5 (21) BCG Unresponsive 45, 92 Min, Max Prior No. of BCG Doses, n (%) Sex, n (%) 5 (21) ≥ 12 BCG doses 19 (79) Male 2 (8) < 12 BCG doses 5 (21) Female Prior non - BCG Treatment, n (%) Race, n (%) 2 (8) Gemcitabine/Docetaxel 24 (100) White 1 (4) Gemcitabine Ethnicity, n (%) 3 (12) Mitomycin 1 (4) Hispanic 2 (8) Other 23 (96) Non - Hispanic Prior TURBT Status, n (%) ECOG Score, n (%) 5 (21) > 3 TURBTs 18 (75) 0 19 (79) ≤ 3 TURBTs 5 (21) 1 1 (4) 2 Baseline Diagnosis, n (%) 14 (58) CIS only 6 (25) CIS + Ta 4 (17) CIS + T1












