UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, DC 20549
FORM 10-Q | | | | | | | | |
| ☒ | QUARTERLY REPORT UNDER SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
| For the quarterly period ended | October 25, 2024 |
| | |
| ☐ | Transition report pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934.
For the transition period from __________ to __________ |
Commission File Number 001-36820
 ®
® | | | | | |
| Medtronic plc |
| (Exact name of registrant as specified in its charter) |
| | |
| Ireland | 98-1183488 |
| (State of incorporation) | (I.R.S. Employer
Identification No.) |
Building Two, Parkmore Business Park West
Galway, Ireland
(Address of principal executive offices) (Zip Code)
+353 1 438-1700
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act: | | | | | | | | |
| Title of each class | Trading Symbol | Name of each exchange on which registered |
| Ordinary shares, par value $0.0001 per share | MDT | New York Stock Exchange |
| 0.250% Senior Notes due 2025 | MDT/25 | New York Stock Exchange |
| 0.000% Senior Notes due 2025 | MDT/25A | New York Stock Exchange |
| 2.625% Senior Notes due 2025 | MDT/25B | New York Stock Exchange |
| 1.125% Senior Notes due 2027 | MDT/27 | New York Stock Exchange |
| 0.375% Senior Notes due 2028 | MDT/28 | New York Stock Exchange |
| 3.000% Senior Notes due 2028 | MDT/28A | New York Stock Exchange |
| 3.650% Senior Notes due 2029 | MDT/29 | New York Stock Exchange |
| 1.625% Senior Notes due 2031 | MDT/31 | New York Stock Exchange |
| 1.000% Senior Notes due 2031 | MDT/31A | New York Stock Exchange |
| 3.125% Senior Notes due 2031 | MDT/31B | New York Stock Exchange |
| 0.750% Senior Notes due 2032 | MDT/32 | New York Stock Exchange |
| 3.375% Senior Notes due 2034 | MDT/34 | New York Stock Exchange |
| 3.875% Senior Notes due 2036 | MDT/36 | New York Stock Exchange |
| 2.250% Senior Notes due 2039 | MDT/39A | New York Stock Exchange |
| 1.500% Senior Notes due 2039 | MDT/39B | New York Stock Exchange |
| 1.375% Senior Notes due 2040 | MDT/40A | New York Stock Exchange |
| 4.150% Senior Notes due 2043 | MDT/43A | New York Stock Exchange |
| 1.750% Senior Notes due 2049 | MDT/49 | New York Stock Exchange |
| 1.625% Senior Notes due 2050 | MDT/50 | New York Stock Exchange |
| 4.150% Senior Notes due 2053 | MDT/53 | New York Stock Exchange |
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the Registrant was required to file such reports) and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer”, “smaller reporting company,” and "emerging growth company" in Rule 12b-2 of the Exchange Act.
| | | | | | | | | | | | | | | | | |
| Large accelerated filer | ☒ | Accelerated filer | ☐ | Emerging growth company | ☐ |
| Non-accelerated filer | ☐ | Smaller Reporting Company | ☐ | | |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 1(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act).
Yes ☐ No ☒
As of November 20, 2024, 1,282,285,681 ordinary shares, par value $0.0001, of the registrant were outstanding.
TABLE OF CONTENTS | | | | | | | | | | | | | | |
| Item | | Description | | Page |
| | | | | |
| | | | | |
| 1. | | | | |
| 2. | | | | |
| 3. | | | | |
| 4. | | | | |
| | | | | |
| 1. | | | | |
| 2. | | | | |
| 5. | | | | |
| 6. | | | | |
| | | | |
PART I — FINANCIAL INFORMATION
Item 1. Financial Statements
Medtronic plc
Consolidated Statements of Income
(Unaudited) | | | | | | | | | | | | | | | | | | | | | | | |
| | Three months ended | | Six months ended |
| (in millions, except per share data) | October 25, 2024 | | October 27, 2023 | | October 25, 2024 | | October 27, 2023 |
| Net sales | $ | 8,403 | | | $ | 7,984 | | | $ | 16,318 | | | $ | 15,686 | |
| Costs and expenses: | | | | | | | |
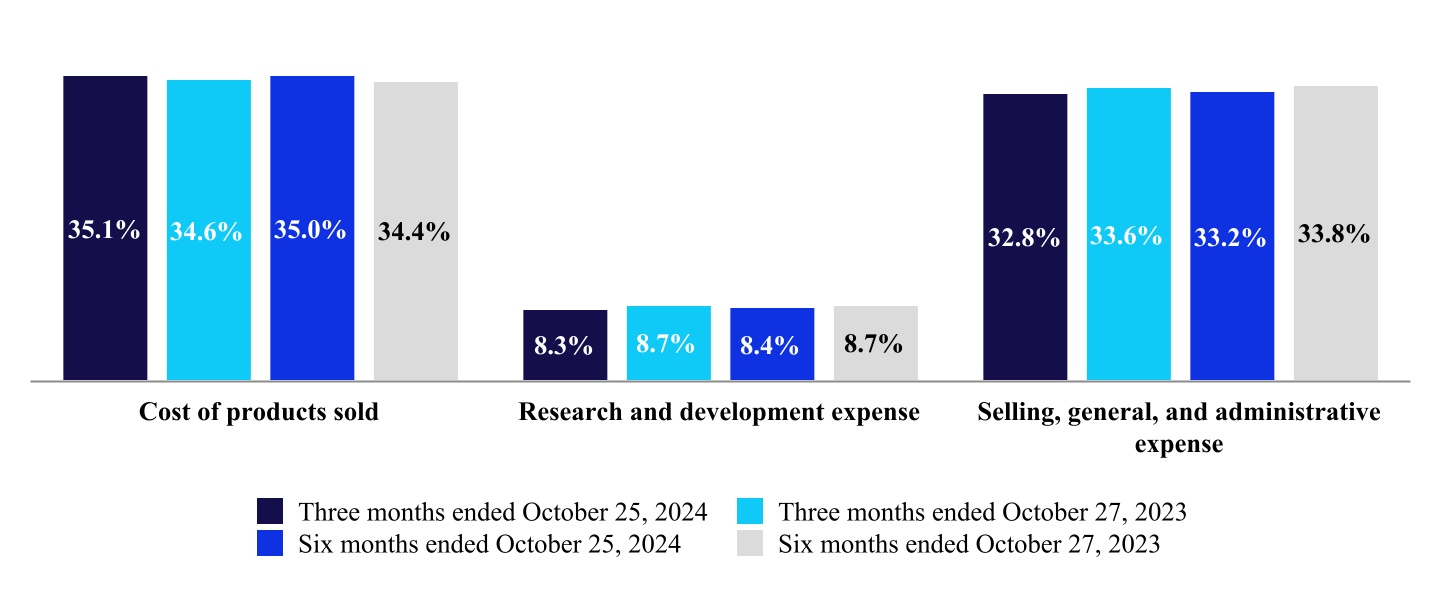
| Cost of products sold, excluding amortization of intangible assets | 2,946 | | | 2,761 | | | 5,707 | | | 5,390 | |
| Research and development expense | 697 | | | 698 | | | 1,373 | | | 1,365 | |
| Selling, general, and administrative expense | 2,757 | | | 2,686 | | | 5,412 | | | 5,299 | |
| Amortization of intangible assets | 413 | | | 425 | | | 827 | | | 855 | |
| Restructuring charges, net | 30 | | | 40 | | | 77 | | | 94 | |
| Certain litigation charges, net | — | | | 65 | | | 81 | | | 105 | |
| Other operating income, net | (34) | | | (31) | | | (33) | | | (30) | |
| Operating profit | 1,595 | | | 1,340 | | | 2,873 | | | 2,608 | |
| Other non-operating income, net | (173) | | | (154) | | | (330) | | | (230) | |
| Interest expense, net | 209 | | | 180 | | | 376 | | | 329 | |
| Income before income taxes | 1,559 | | | 1,313 | | | 2,827 | | | 2,510 | |
| Income tax provision | 281 | | | 402 | | | 500 | | | 802 | |
| Net income | 1,278 | | | 911 | | | 2,327 | | | 1,708 | |
| Net income attributable to noncontrolling interests | (9) | | | (2) | | | (15) | | | (8) | |
| Net income attributable to Medtronic | $ | 1,270 | | | $ | 909 | | | $ | 2,312 | | | $ | 1,700 | |
| Basic earnings per share | $ | 0.99 | | | $ | 0.68 | | | $ | 1.79 | | | $ | 1.28 | |
| Diluted earnings per share | $ | 0.99 | | | $ | 0.68 | | | $ | 1.79 | | | $ | 1.28 | |
| Basic weighted average shares outstanding | 1,282.4 | | | 1,330.2 | | | 1,288.6 | | | 1,330.3 | |
| Diluted weighted average shares outstanding | 1,286.9 | | | 1,331.9 | | | 1,292.5 | | | 1,332.8 | |
The accompanying notes are an integral part of these consolidated financial statements.
Medtronic plc
Consolidated Statements of Comprehensive Income
(Unaudited) | | | | | | | | | | | | | | | | | | | | | | | |
| | Three months ended | | Six months ended |
| (in millions) | October 25, 2024 | | October 27, 2023 | | October 25, 2024 | | October 27, 2023 |
| Net income | $ | 1,278 | | | $ | 911 | | | $ | 2,327 | | | $ | 1,708 | |
| Other comprehensive income (loss), net of tax: | | | | | | | |
| Unrealized gain (loss) on investment securities | 35 | | | (19) | | | 111 | | | (38) | |
| Translation adjustment | 116 | | | (926) | | | 218 | | | (911) | |
| Net investment hedge | 35 | | | 915 | | | (170) | | | 771 | |
| Net change in retirement obligations | — | | | 1 | | | 1 | | | 4 | |
| Unrealized (loss) gain on cash flow hedges | (27) | | | 324 | | | (93) | | | 294 | |
| Other comprehensive income | 161 | | | 295 | | | 69 | | | 120 | |
| Comprehensive income including noncontrolling interests | 1,439 | | | 1,206 | | | 2,396 | | | 1,828 | |
| Comprehensive income attributable to noncontrolling interests | (9) | | | — | | | (15) | | | (6) | |
| Comprehensive income attributable to Medtronic | $ | 1,431 | | | $ | 1,206 | | | $ | 2,381 | | | $ | 1,822 | |
The accompanying notes are an integral part of these consolidated financial statements.
Medtronic plc
Consolidated Balance Sheets
(Unaudited) | | | | | | | | | | | |
| (in millions) | October 25, 2024 | | April 26, 2024 |
| ASSETS | | | |
| Current assets: | | | |
| Cash and cash equivalents | $ | 1,394 | | | $ | 1,284 | |
| Investments | 6,595 | | | 6,721 | |
| Accounts receivable, less allowances and credit losses of $195 and $173, respectively | 6,260 | | | 6,128 | |
| Inventories | 5,479 | | | 5,217 | |
| Other current assets | 2,710 | | | 2,584 | |
| Total current assets | 22,438 | | | 21,935 | |
| Property, plant, and equipment, net | 6,438 | | | 6,131 | |
| Goodwill | 41,161 | | | 40,986 | |
| Other intangible assets, net | 12,423 | | | 13,225 | |
| Tax assets | 3,572 | | | 3,657 | |
| Other assets | 4,009 | | | 4,047 | |
| Total assets | $ | 90,042 | | | $ | 89,981 | |
| LIABILITIES AND EQUITY | | | |
| Current liabilities: | | | |
| Current debt obligations | $ | 3,719 | | | $ | 1,092 | |
| Accounts payable | 2,376 | | | 2,410 | |
| Accrued compensation | 1,893 | | | 2,375 | |
| Accrued income taxes | 947 | | | 1,330 | |
| Other accrued expenses | 3,260 | | | 3,582 | |
| Total current liabilities | 12,195 | | | 10,789 | |
| Long-term debt | 24,607 | | | 23,932 | |
| Accrued compensation and retirement benefits | 1,084 | | | 1,101 | |
| Accrued income taxes | 1,432 | | | 1,859 | |
| Deferred tax liabilities | 473 | | | 515 | |
| Other liabilities | 1,534 | | | 1,365 | |
| Total liabilities | 41,326 | | | 39,561 | |
| Commitments and contingencies (Note 16) | | | |
| Shareholders’ equity: | | | |
| Ordinary shares— par value $0.0001, 2.6 billion shares authorized, 1,282,553,150 and 1,311,337,531 shares issued and outstanding, respectively | — | | | — | |
| Additional paid-in capital | 20,824 | | | 23,129 | |
| Retained earnings | 30,919 | | | 30,403 | |
| Accumulated other comprehensive loss | (3,250) | | | (3,318) | |
| Total shareholders’ equity | 48,494 | | | 50,214 | |
| Noncontrolling interests | 222 | | | 206 | |
| Total equity | 48,716 | | | 50,420 | |
| Total liabilities and equity | $ | 90,042 | | | $ | 89,981 | |
The accompanying notes are an integral part of these consolidated financial statements.
Medtronic plc
Consolidated Statements of Equity
(Unaudited) | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Ordinary Shares | | Additional Paid-in Capital | | Retained
Earnings | | Accumulated
Other
Comprehensive
Loss | | Total
Shareholders’
Equity | | Noncontrolling Interests | | Total Equity |
| (in millions) | | Number | | Par Value | | | | | | |
| April 26, 2024 | | 1,311 | | | $ | — | | | $ | 23,129 | | | $ | 30,403 | | | $ | (3,318) | | | $ | 50,214 | | | $ | 206 | | | $ | 50,420 | |
| Net income | | — | | | — | | | — | | | 1,042 | | | — | | | 1,042 | | | 6 | | | 1,049 | |
| Other comprehensive loss | | — | | | — | | | — | | | — | | | (92) | | | (92) | | | — | | | (92) | |
| Dividends to shareholders ($0.70 per ordinary share) | | — | | | — | | | — | | | (898) | | | — | | | (898) | | | — | | | (898) | |
| Issuance of shares under stock purchase and award plans | | 1 | | | — | | | 87 | | | — | | | — | | | 87 | | | — | | | 87 | |
| Repurchase of ordinary shares | | (30) | | | — | | | (2,489) | | | — | | | — | | | (2,489) | | | — | | | (2,489) | |
| Stock-based compensation | | — | | | — | | | 83 | | | — | | | — | | | 83 | | | — | | | 83 | |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
| July 26, 2024 | | 1,282 | | | $ | — | | | $ | 20,810 | | | $ | 30,547 | | | $ | (3,410) | | | $ | 47,947 | | | $ | 213 | | | $ | 48,160 | |
| Net income | | — | | | — | | | — | | | 1,270 | | | — | | | 1,270 | | | 9 | | | 1,278 | |
| Other comprehensive income | | — | | | — | | | — | | | — | | | 161 | | | 161 | | | — | | | 161 | |
| Dividends to shareholders ($0.70 per ordinary share) | | — | | | — | | | — | | | (897) | | | — | | | (897) | | | — | | | (897) | |
| Issuance of shares under stock purchase and award plans | | 3 | | | — | | | 103 | | | — | | | — | | | 103 | | | — | | | 103 | |
| Repurchase of ordinary shares | | (3) | | | — | | | (248) | | | — | | | — | | | (248) | | | — | | | (248) | |
| Stock-based compensation | | — | | | — | | | 159 | | | — | | | — | | | 159 | | | — | | | 159 | |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
| October 25, 2024 | | 1,283 | | | $ | — | | | $ | 20,824 | | | $ | 30,919 | | | $ | (3,250) | | | $ | 48,494 | | | $ | 222 | | | $ | 48,716 | |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Ordinary Shares | | Additional Paid-in Capital | | Retained
Earnings | | Accumulated
Other
Comprehensive
Loss | | Total
Shareholders’
Equity | | Noncontrolling Interests | | Total Equity |
| (in millions) | | Number | | Par Value | | | | | | |
| April 28, 2023 | | 1,331 | | | $ | — | | | $ | 24,590 | | | $ | 30,392 | | | $ | (3,499) | | | $ | 51,483 | | | $ | 182 | | | $ | 51,665 | |
| Net income | | — | | | — | | | — | | | 791 | | | — | | | 791 | | | 6 | | | 797 | |
| Other comprehensive loss | | — | | | — | | | — | | | — | | | (175) | | | (175) | | | — | | | (175) | |
| Dividends to shareholders ($0.69 per ordinary share) | | — | | | — | | | — | | | (918) | | | — | | | (918) | | | — | | | (918) | |
| Issuance of shares under stock purchase and award plans | | 1 | | | — | | | 73 | | | — | | | — | | | 73 | | | — | | | 73 | |
| Repurchase of ordinary shares | | (2) | | | — | | | (148) | | | — | | | — | | | (148) | | | — | | | (148) | |
| Stock-based compensation | | — | | | — | | | 73 | | | — | | | — | | | 73 | | | — | | | 73 | |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
| July 28, 2023 | | 1,330 | | | $ | — | | | $ | 24,587 | | | $ | 30,265 | | | $ | (3,674) | | | $ | 51,178 | | | $ | 188 | | | $ | 51,366 | |
| Net income | | — | | | — | | | — | | | 909 | | | — | | | 909 | | | 2 | | | 911 | |
| Other comprehensive income (loss) | | — | | | — | | | — | | | — | | | 297 | | | 297 | | | (2) | | | 295 | |
| Dividends to shareholders ($0.69 per ordinary share) | | — | | | — | | | — | | | (918) | | | — | | | (918) | | | — | | | (918) | |
| Issuance of shares under stock purchase and award plans | | 2 | | | — | | | 35 | | | — | | | — | | | 35 | | | — | | | 35 | |
| Repurchase of ordinary shares | | (2) | | | — | | | (189) | | | — | | | — | | | (189) | | | — | | | (189) | |
| Stock-based compensation | | — | | | — | | | 146 | | | — | | | — | | | 146 | | | — | | | 146 | |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
| October 27, 2023 | | 1,330 | | | $ | — | | | $ | 24,580 | | | $ | 30,256 | | | $ | (3,377) | | | $ | 51,460 | | | $ | 187 | | | $ | 51,647 | |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
The accompanying notes are an integral part of these consolidated financial statements.
Medtronic plc
Consolidated Statements of Cash Flows
(Unaudited) | | | | | | | | | | | |
| | Six months ended |
| (in millions) | October 25, 2024 | | October 27, 2023 |
| Operating Activities: | | | |
| Net income | $ | 2,327 | | | $ | 1,708 | |
| Adjustments to reconcile net income to net cash provided by operating activities: | | | |
| Depreciation and amortization | 1,337 | | | 1,344 | |
| Provision for credit losses | 45 | | | 37 | |
| Deferred income taxes | 57 | | | (36) | |
| Stock-based compensation | 242 | | | 219 | |
| Other, net | (98) | | | 182 | |
| Change in operating assets and liabilities, net of acquisitions and divestitures: | | | |
| Accounts receivable, net | (181) | | | (117) | |
| Inventories | (278) | | | (616) | |
| Accounts payable and accrued liabilities | (707) | | | (699) | |
| Other operating assets and liabilities | (800) | | | (486) | |
| | | |
| | | |
| Net cash provided by operating activities | 1,944 | | | 1,536 | |
| Investing Activities: | | | |
| Acquisitions, net of cash acquired | — | | | (22) | |
| | | |
| Additions to property, plant, and equipment | (924) | | | (815) | |
| Purchases of investments | (4,019) | | | (3,403) | |
| Sales and maturities of investments | 4,338 | | | 3,336 | |
| Other investing activities, net | 1 | | | (59) | |
| Net cash used in investing activities | (604) | | | (963) | |
| Financing Activities: | | | |
| Change in current debt obligations, net | (67) | | | 1,321 | |
| | | |
| | | |
| Issuance of long-term debt | 3,209 | | | — | |
| | | |
| Dividends to shareholders | (1,795) | | | (1,836) | |
| Issuance of ordinary shares | 232 | | | 149 | |
| Repurchase of ordinary shares | (2,780) | | | (378) | |
| Other financing activities, net | (64) | | | 153 | |
| Net cash used in financing activities | (1,265) | | | (591) | |
| Effect of exchange rate changes on cash and cash equivalents | 35 | | | (214) | |
| Net change in cash and cash equivalents | 110 | | | (232) | |
| Cash and cash equivalents at beginning of period | 1,284 | | | 1,543 | |
| Cash and cash equivalents at end of period | $ | 1,394 | | | $ | 1,311 | |
| | | |
| Supplemental Cash Flow Information | | | |
| Cash paid for: | | | |
| Income taxes | $ | 1,335 | | | $ | 1,110 | |
| Interest | 513 | | | 476 | |
|
The accompanying notes are an integral part of these consolidated financial statements.
Medtronic plc
Notes to Consolidated Financial Statements
(Unaudited)
1. Basis of Presentation
The accompanying unaudited consolidated financial statements of Medtronic plc and its subsidiaries (Medtronic plc, Medtronic, or the Company) have been prepared in accordance with accounting principles generally accepted in the United States of America (U.S.) (U.S. GAAP) for interim financial information and with the instructions to Form 10-Q and Article 10 of Regulation S-X. In the opinion of management, the consolidated financial statements include all the adjustments necessary for a fair statement in conformity with U.S. GAAP. Certain reclassifications have been made to prior year financial statements to conform to classifications used in the current year.
Operating results for interim periods are not necessarily indicative of results that may be expected for the fiscal year as a whole. The preparation of the financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets, liabilities, revenues, expenses, and the related disclosures at the date of the financial statements and during the reporting period. Actual results could materially differ from these estimates.
The accompanying unaudited consolidated financial statements include the accounts of Medtronic plc, its wholly-owned subsidiaries, entities for which the Company has a controlling financial interest, and variable interest entities for which the Company is the primary beneficiary. Intercompany transactions and balances have been eliminated in consolidation. Amounts reported in millions within this quarterly report are computed based on the amounts in thousands, and therefore, the sum of the components may not equal the total amount reported in millions due to rounding. Additionally, certain columns and rows within tables may not sum due to rounding.
The accompanying unaudited consolidated financial statements and related notes should be read in conjunction with the audited consolidated financial statements of the Company and related notes included in the Company’s Annual Report on Form 10-K for the fiscal year ended April 26, 2024. The Company’s fiscal years 2025, 2024, and 2023 will end or ended on April 25, 2025, April 26, 2024, and April 28, 2023, respectively.
2. New Accounting Pronouncements
Recently Adopted Accounting Standards
As of October 25, 2024, there have been no newly adopted accounting pronouncements that materially impact our consolidated financial statements. Refer to the Company's Annual Report on Form 10-K for the fiscal year ended April 26, 2024 for pronouncements recently adopted.
Not Yet Adopted Accounting Standards
Segment Reporting
In November 2023, the FASB issued ASU 2023-07, Improvements to Segment Reporting (Topic 280), which requires incremental disclosures on reportable segments, primarily through enhanced disclosures on significant segment expenses. The Company will adopt this guidance beginning in the fourth quarter of fiscal year 2025 for our annual report and for interim periods starting in fiscal year 2026. We are currently evaluating the potential effect that the updated standard will have on our financial statement disclosures.
Income Taxes
In December 2023, the FASB issued ASU 2023-09, Improvements to Income Tax Disclosures (Topic 740), which requires incremental annual disclosures on income taxes, including rate reconciliations, income taxes paid, and other disclosures. The Company will adopt this guidance beginning in the fourth quarter of fiscal year 2026 for our annual report. We are currently evaluating the potential effect that the updated standard will have on our financial statement disclosures.
3. Revenue
The Company's revenues are principally derived from device-based medical therapies and services related to cardiac rhythm disorders, cardiovascular disease, neurological disorders and diseases, spinal conditions and musculoskeletal trauma, chronic pain, urological and digestive disorders, ear, nose, and throat conditions, and diabetes conditions as well as advanced and general surgical care products, respiratory and monitoring solutions, and neurological surgery technologies. The Company's primary customers include healthcare systems, clinics, third-party healthcare providers, distributors, and other institutions, including governmental healthcare programs and group purchasing organizations. Certain prior period net sales has been recast to conform to the new operating segment structure in the fourth quarter of fiscal year 2024. Refer to Note 17 to the consolidated financial statements for additional information regarding the Company's reporting structure. In addition, starting in the first quarter of fiscal year 2025, the Company combined the non-U.S. developed markets and the emerging markets into an international market geography. Prior period net sales has been recast to conform to the new presentation.
Medtronic plc
Notes to Consolidated Financial Statements
(Unaudited)
The table below illustrates net sales by segment and division and by market geography for the three and six months ended October 25, 2024 and October 27, 2023. The U.S. revenue includes United States and U.S. territories, and the international revenue includes all other non-U.S. countries.
| | | | | | | | | | | | | | | | | | | | | | | |
| World wide |
| | Three months ended | | Six months ended |
| (in millions) | October 25, 2024 | | October 27, 2023 | | October 25, 2024 | | October 27, 2023 |
| Cardiac Rhythm & Heart Failure | $ | 1,578 | | | $ | 1,492 | | | $ | 3,114 | | | $ | 2,938 | |
| Structural Heart & Aortic | 881 | | | 819 | | | 1,736 | | | 1,633 | |
| Coronary & Peripheral Vascular | 643 | | | 613 | | | 1,259 | | | 1,202 | |
| Cardiovascular | 3,102 | | | 2,923 | | | 6,108 | | | 5,773 | |
| Cranial & Spinal Technologies | 1,234 | | | 1,157 | | | 2,382 | | | 2,260 | |
| Specialty Therapies | 737 | | | 705 | | | 1,450 | | | 1,400 | |
| Neuromodulation | 480 | | | 426 | | | 937 | | | 846 | |
| Neuroscience | 2,451 | | | 2,288 | | | 4,768 | | | 4,506 | |
| Surgical & Endoscopy | 1,649 | | | 1,641 | | | 3,193 | | | 3,187 | |
| Acute Care & Monitoring | 478 | | | 462 | | | 930 | | | 921 | |
| Medical Surgical | 2,128 | | | 2,103 | | | 4,123 | | | 4,107 | |
| Diabetes | 686 | | | 610 | | | 1,333 | | | 1,189 | |
| Reportable segment net sales | 8,366 | | | 7,923 | | | 16,333 | | | 15,575 | |
Other operating segment(1) | 37 | | | 61 | | | 75 | | | 111 | |
Other adjustments(2) | — | | | — | | | (90) | | | — | |
| | | | | | | |
| Total net sales | $ | 8,403 | | | $ | 7,984 | | | $ | 16,318 | | | $ | 15,686 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | U.S. | | International | | | | U.S. | | International | | | | |
| Three months ended | | Six months ended | | |
| (in millions) | October 25, 2024 | | October 27, 2023 | | October 25, 2024 | | October 27, 2023 | | | | October 25, 2024 | | October 27, 2023 | | October 25, 2024 | | October 27, 2023 | | | | | | | | |
| Cardiovascular | $ | 1,434 | | | $ | 1,427 | | | $ | 1,668 | | | $ | 1,496 | | | | | $ | 2,836 | | | $ | 2,776 | | | $ | 3,272 | | | $ | 2,996 | | | | | | | | | |
| Neuroscience | 1,677 | | | 1,560 | | | 774 | | | 728 | | | | | 3,242 | | | 3,057 | | | 1,526 | | | 1,449 | | | | | | | | | |
| Medical Surgical | 944 | | | 948 | | | 1,183 | | | 1,155 | | | | | 1,825 | | | 1,815 | | | 2,298 | | | 2,292 | | | | | | | | | |
| Diabetes | 232 | | | 217 | | | 455 | | | 394 | | | | | 447 | | | 405 | | | 886 | | | 784 | | | | | | | | | |
| Reportable segment net sales | 4,286 | | | 4,151 | | | 4,080 | | | 3,772 | | | | | 8,350 | | | 8,054 | | | 7,983 | | | 7,521 | | | | | | | | | |
Other operating segment(1) | 18 | | | 23 | | | 19 | | | 37 | | | | | 37 | | | 45 | | | 38 | | | 66 | | | | | | | | | |
Other adjustments(2) | — | | | — | | | — | | | — | | | | | — | | | — | | | (90) | | | — | | | | | | | | | |
| Total net sales | $ | 4,304 | | | $ | 4,175 | | | $ | 4,099 | | | $ | 3,809 | | | | | $ | 8,387 | | | $ | 8,099 | | | $ | 7,931 | | | $ | 7,587 | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | |
| | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
(1)Includes the historical operations and ongoing transition agreements from businesses the Company has exited or divested.
(2)Incremental Italian payback accruals resulting from the two July 22, 2024 rulings by the Constitutional Court of Italy relating to certain prior years since 2015.
The amount of revenue recognized is reduced by sales rebates and returns. Adjustments to rebates and returns reserves are recorded as increases or decreases to revenue. At October 25, 2024, $1.0 billion of rebates were classified as other accrued expenses, and $639 million of rebates were classified as a reduction of accounts receivable in the consolidated balance sheet. At April 26, 2024, $1.0 billion of rebates were classified as other accrued expenses, and $574 million of rebates were classified as a reduction of accounts receivable in the consolidated balance sheet.
Medtronic plc
Notes to Consolidated Financial Statements
(Unaudited)
Deferred Revenue and Remaining Performance Obligations
Deferred revenue at October 25, 2024 and April 26, 2024 was $467 million and $453 million, respectively. At October 25, 2024 and April 26, 2024, $369 million and $352 million was included in other accrued expenses, respectively, and $98 million and $101 million was included in other liabilities, respectively. During the six months ended October 25, 2024, the Company recognized $196 million of revenue that was included in deferred revenue as of April 26, 2024. During the six months ended October 27, 2023, the Company recognized $216 million of revenue that was included in deferred revenue as of April 28, 2023.
Remaining performance obligations include goods and services that have not yet been delivered or provided under existing, noncancellable contracts with minimum purchase commitments. At October 25, 2024, the estimated revenue expected to be recognized in future periods related to unsatisfied performance obligations for executed contracts with an original duration of one year or more was approximately $0.4 billion. The Company expects to recognize revenue on the majority of these remaining performance obligations over the next three years.
4. Acquisitions and Dispositions
Acquisition Activity
During the three and six months ended October 25, 2024, the Company had no acquisitions that were accounted for as business combinations. During the fiscal year ended April 26, 2024, the Company had acquisitions that were accounted for as business combinations. For the three and six months ended October 25, 2024 and the fiscal year ended April 26, 2024, purchase price allocation adjustments were not significant.
Fiscal year 2024
The acquisition date fair value of net assets acquired during the fiscal year ended April 26, 2024 was $335 million. Based on preliminary valuations, assets acquired were primarily comprised of $131 million of goodwill, $150 million of IPR&D, and $29 million of technology-based intangible assets with estimated useful lives of 10 years. For tax purposes, $51 million of goodwill is deductible while $80 million is not deductible. The IPR&D was placed into service as a definite-lived intangible asset during the second quarter of fiscal year 2025. The Company recognized $30 million of non-cash contingent consideration liabilities in connection with these business combinations during the fiscal year ended April 26, 2024, which are comprised of revenue and product development milestone-based payments.
Contingent Consideration
Certain of the Company’s business combinations involve potential payment of future consideration that is contingent upon the achievement of certain product development milestones and/or contingent on the acquired business reaching certain performance milestones. A liability is recorded for the estimated fair value of the contingent consideration on the acquisition date. The fair value of the contingent consideration is remeasured at each reporting period, and the change in fair value is recognized within other operating income, net in the consolidated statements of income.
The fair value of contingent consideration liabilities at October 25, 2024 and April 26, 2024 was $124 million and $149 million, respectively. At October 25, 2024, $77 million was recorded in other accrued expenses, and $47 million was recorded in other liabilities in the consolidated balance sheet. At April 26, 2024, $96 million was recorded in other accrued expenses, and $53 million was recorded in other liabilities in the consolidated balance sheet.
The following table provides a reconciliation of the beginning and ending balances of contingent consideration liabilities:
| | | | | | | | | | | | | | | | | | | | | | | |
| | Three months ended | | Six months ended |
| (in millions) | October 25, 2024 | | October 27, 2023 | | October 25, 2024 | | October 27, 2023 |
| Beginning balance | $ | 128 | | | $ | 206 | | | $ | 149 | | | $ | 206 | |
| Purchase price contingent consideration | — | | | 25 | | | — | | | 25 | |
| | | | | | | |
| Payments | (8) | | | — | | | (14) | | | (3) | |
| Change in fair value | 4 | | | (10) | | | (11) | | | (7) | |
| Ending balance | $ | 124 | | | $ | 220 | | | $ | 124 | | | $ | 220 | |
Medtronic plc
Notes to Consolidated Financial Statements
(Unaudited)
The recurring Level 3 fair value measurements of contingent consideration for which a liability is recorded include the following significant unobservable inputs:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Fair Value at | | | | | | | | |
| (in millions) | | October 25, 2024 | | | | Unobservable Input | | Range | | Weighted Average (1) |
| Revenue and other performance-based payments | | $58 | | | | Discount rate | | 16.5% - 28.2% | | 22.1% |
| | | | Projected fiscal year of payment | | 2025 - 2029 | | 2027 |
| Product development and other milestone-based payments | | $66 | | | | Discount rate | | 5.5% | | 5.5% |
| | | | Projected fiscal year of payment | | 2025 - 2027 | | 2025 |
(1) Unobservable inputs were weighted by the relative fair value of the contingent consideration liability. For projected fiscal year of payment, the amount represents the median of the inputs and is not a weighted average.
On April 1, 2023, the Company and DaVita Inc. (“DaVita”) completed the transaction for the Company to sell half of its Renal Care Solutions (RCS) business. In connection with the sale, the Company may be entitled to receive additional consideration based on the achievement of certain revenue, regulatory, and profitability milestones, with potential payouts starting in fiscal year 2026 through 2029. The fair value of the contingent consideration receivable at October 25, 2024 and April 26, 2024 was $61 million and $58 million, and was recorded in other assets in the consolidated balance sheet.
The following table provides a reconciliation of the beginning and ending balances of the Level 3 measurement of contingent consideration receivable:
| | | | | | | | | | | | | | | | | | | | | | | |
| Three months ended | | Six months ended |
| (in millions) | October 25, 2024 | | October 27, 2023 | | October 25, 2024 | | October 27, 2023 |
| Beginning balance | $ | 58 | | | $ | 152 | | | $ | 58 | | | $ | 195 | |
| Change in fair value | 3 | | | — | | | 3 | | | (43) | |
| Ending balance | $ | 61 | | | $ | 152 | | | $ | 61 | | | $ | 152 | |
5. Restructuring and Other Costs
Restructuring and associated costs for the three and six months ended October 25, 2024 were $46 million and $108 million, respectively, as compared to $91 million and $182 million for the three and six months ended October 27, 2023, respectively. Restructuring and associated costs primarily related to employee termination benefits and facility consolidations to support cost reduction initiatives.
Employee-related costs primarily consist of termination benefits provided to employees who have been involuntarily terminated. Associated and other costs primarily include salaries and wages of employees that are fully-dedicated to restructuring activities, consulting expenses, and asset write-offs.
The following table presents the classification of restructuring and associated costs in the consolidated statements of income:
| | | | | | | | | | | | | | | | | | | | | | | |
| Three months ended | | Six months ended |
| (in millions) | October 25, 2024 | | October 27, 2023 | | October 25, 2024 | | October 27, 2023 |
| Cost of products sold | $ | 11 | | | $ | 15 | | | $ | 20 | | | $ | 30 | |
| Selling, general, and administrative expenses | 6 | | | 36 | | | 11 | | | 57 | |
| Restructuring charges, net | 30 | | | 40 | | | 77 | | | 94 | |
| Total restructuring and associated costs | $ | 46 | | | $ | 91 | | | $ | 108 | | | $ | 182 | |
Medtronic plc
Notes to Consolidated Financial Statements
(Unaudited)
The following table summarizes the activity for the six months ended October 25, 2024:
| | | | | | | | | | | | | | | | | | | | | |
| (in millions) | Employee Termination Benefits | | Associated and Other Costs | | | | | | Total |
| April 26, 2024 | $ | 136 | | | $ | 11 | | | | | | | $ | 147 | |
| Charges | 87 | | | 31 | | | | | | | 118 | |
| Cash payments | (156) | | | (25) | | | | | | | (181) | |
| Settled non-cash | — | | | (4) | | | | | | | (4) | |
Accrual adjustments(1) | (10) | | | — | | | | | | | (10) | |
| October 25, 2024 | $ | 58 | | | $ | 13 | | | | | | | $ | 70 | |
| | | | | | | | | |
(1)Accrual adjustments primarily relate to certain employees identified for termination, finding other positions within the Company.
Medtronic plc
Notes to Consolidated Financial Statements
(Unaudited)
6. Financial Instruments
Debt Securities
The Company holds investments in marketable debt securities that are classified and accounted for as available-for-sale and are remeasured on a recurring basis. The following tables summarize the Company's investments in available-for-sale debt securities by significant investment category and the related consolidated balance sheet classification at October 25, 2024 and April 26, 2024:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| October 25, 2024 |
| Valuation | | Balance Sheet Classification |
| (in millions) | Cost | | Unrealized
Gains | | Unrealized
Losses | | Fair Value | | Investments | | Other Assets |
| Level 1: | | | | | | | | | | | |
| U.S. government and agency securities | $ | 538 | | | $ | — | | | $ | (11) | | | $ | 527 | | | $ | 527 | | | $ | — | |
| Level 2: | | | | | | | | | | | |
| Corporate debt securities | 3,429 | | | 13 | | | (59) | | | 3,384 | | | 3,384 | | | — | |
| U.S. government and agency securities | 874 | | | — | | | (28) | | | 845 | | | 845 | | | — | |
| Mortgage-backed securities | 772 | | | 3 | | | (33) | | | 742 | | | 742 | | | — | |
| Non-U.S. government and agency securities | 9 | | | — | | | — | | | 9 | | | 9 | | | — | |
| | | | | | | | | | | |
| Other asset-backed securities | 1,051 | | | 5 | | | (3) | | | 1,053 | | | 1,053 | | | — | |
| Total Level 2 | 6,134 | | | 22 | | | (124) | | | 6,032 | | | 6,032 | | | — | |
| Level 3: | | | | | | | | | | | |
| Auction rate securities | 36 | | | — | | | (3) | | | 33 | | | — | | | 33 | |
| Total available-for-sale debt securities | $ | 6,708 | | | $ | 22 | | | $ | (138) | | | $ | 6,592 | | | $ | 6,559 | | | $ | 33 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| April 26, 2024 |
| Valuation | | Balance Sheet Classification |
| (in millions) | Cost | | Unrealized
Gains | | Unrealized
Losses | | Fair Value | | Investments | | Other Assets |
| Level 1: | | | | | | | | | | | |
| U.S. government and agency securities | $ | 494 | | | $ | — | | | $ | (22) | | | $ | 472 | | | $ | 472 | | | $ | — | |
| Level 2: | | | | | | | | | | | |
| Corporate debt securities | 3,953 | | | 4 | | | (125) | | | 3,832 | | | 3,832 | | | — | |
| U.S. government and agency securities | 847 | | | — | | | (43) | | | 804 | | | 804 | | | — | |
| Mortgage-backed securities | 692 | | | 1 | | | (50) | | | 643 | | | 643 | | | — | |
| Non-U.S. government and agency securities | 5 | | | — | | | — | | | 5 | | | 5 | | | — | |
| | | | | | | | | | | |
| Other asset-backed securities | 941 | | | 2 | | | (9) | | | 934 | | | 934 | | | — | |
| Total Level 2 | 6,438 | | | 7 | | | (227) | | | 6,218 | | | 6,218 | | | — | |
| Level 3: | | | | | | | | | | | |
| Auction rate securities | 36 | | | — | | | (3) | | | 33 | | | — | | | 33 | |
| Total available-for-sale debt securities | $ | 6,968 | | | $ | 7 | | | $ | (252) | | | $ | 6,723 | | | $ | 6,690 | | | $ | 33 | |
The amortized cost of debt securities excludes accrued interest, which is reported in other current assets in the consolidated balance sheets.
Medtronic plc
Notes to Consolidated Financial Statements
(Unaudited)
The following tables present the gross unrealized losses and fair values of the Company’s available-for-sale debt securities that have been in a continuous unrealized loss position deemed to be temporary, aggregated by investment category at October 25, 2024 and April 26, 2024: | | | | | | | | | | | | | | | | | | | | | | | |
| | October 25, 2024 |
| | Less than 12 months | | More than 12 months |
| (in millions) | Fair Value | | Unrealized
Losses | | Fair Value | | Unrealized
Losses |
| Corporate debt securities | $ | 565 | | | $ | (7) | | | $ | 1,645 | | | $ | (51) | |
| U.S. government and agency securities | 216 | | | (2) | | | 713 | | | (37) | |
| Mortgage-backed securities | — | | | — | | | 535 | | | (33) | |
| Other asset-backed securities | — | | | — | | | 212 | | | (3) | |
| Auction rate securities | — | | | — | | | 33 | | | (3) | |
| Total | $ | 782 | | | $ | (10) | | | $ | 3,137 | | | $ | (128) | |
| | | | | | | | | | | | | | | | | | | | | | | |
| | April 26, 2024 |
| | Less than 12 months | | More than 12 months |
| (in millions) | Fair Value | | Unrealized
Losses | | Fair Value | | Unrealized
Losses |
| Corporate debt securities | $ | 661 | | | $ | (10) | | | $ | 2,448 | | | $ | (116) | |
| U.S. government and agency securities | 177 | | | (4) | | | 730 | | | (61) | |
| Mortgage-backed securities | — | | | — | | | 582 | | | (50) | |
| Other asset-backed securities | — | | | — | | | 502 | | | (9) | |
| Auction rate securities | — | | | — | | | 33 | | | (3) | |
| Total | $ | 838 | | | $ | (14) | | | $ | 4,296 | | | $ | (238) | |
The Company reviews the fair value hierarchy classification on a quarterly basis. Changes in the ability to observe valuation inputs may result in a reclassification of levels for certain securities within the fair value hierarchy. There were no transfers into or out of Level 3 during the three and six months ended October 25, 2024 and October 27, 2023. When a determination is made to classify an asset or liability within Level 3, the determination is based upon the significance of the unobservable inputs to the overall fair value measurement.
Activity related to the Company’s available-for-sale debt securities portfolio is as follows: | | | | | | | | | | | | | | | | | | | | | | | |
| | Three months ended | | Six months ended |
| (in millions) | October 25, 2024 | | October 27, 2023 | | October 25, 2024 | | October 27, 2023 |
| Proceeds from sales | $ | 2,087 | | | $ | 1,573 | | | $ | 4,244 | | | $ | 3,320 | |
| Gross realized gains | 7 | | | 6 | | | 14 | | | 11 | |
| Gross realized losses | (6) | | | (3) | | | (13) | | | (15) | |
| | | | | | | |
The contractual maturities of available-for-sale debt securities at October 25, 2024 is shown in the following table. Within the table, maturities of mortgage-backed securities have been allocated based upon timing of estimated cash flows assuming no change in the current interest rate environment. Actual maturities may differ from contractual maturities because the issuers of the securities may have the right to prepay obligations without prepayment penalties.
| | | | | | | | | | | |
| (in millions) | Amortized Cost | | Fair Value |
| Due in one year or less | $ | 1,488 | | | $ | 1,478 | |
| Due after one year through five years | 3,322 | | | 3,248 | |
| Due after five years through ten years | 750 | | | 749 | |
| Due after ten years | 1,148 | | | 1,117 | |
| Total | $ | 6,708 | | | $ | 6,592 | |
Interest income is recognized in other non-operating income, net, in the consolidated statements of income. During the three and six months ended October 25, 2024, there were $137 million and $249 million of interest income, respectively. During the three and six months ended October 27, 2023, there was $148 million and $259 million of interest income, respectively.
Medtronic plc
Notes to Consolidated Financial Statements
(Unaudited)
Equity Securities, Equity Method Investments, and Other Investments
The Company holds investments in equity securities with readily determinable fair values, equity method investments for which the Company has elected the fair value option, equity investments without readily determinable fair values, investments accounted for under the equity method, and other investments. Equity securities with readily determinable fair values are included in Level 1 of the fair value hierarchy, as they are measured using quoted market prices. Equity method investments for which the Company has elected the fair value option are included within Level 3 of the fair value hierarchy due to the use of significant unobservable inputs to determine fair value. To determine the fair value of these investments, the Company uses a discounted cash flow methodology, taking into consideration various assumptions including discount rate, and all pertinent financial information available related to the investees, including the timing of anticipated product launches, historical financial results, and projections of future cash flows. Equity investments that do not have readily determinable fair values, and that are not accounted for via the fair value option, are included within Level 3 of the fair value hierarchy, as they are measured using the measurement alternative at cost minus impairment, if any, plus or minus changes resulting from observable price changes in orderly transactions for an identical or similar investment of the same issuer.
The following table summarizes the Company's equity and other investments at October 25, 2024 and April 26, 2024, which are classified as primarily other assets in the consolidated balance sheets:
| | | | | | | | | | | | | | |
| (in millions) | | October 25, 2024 | | April 26, 2024 |
| Investments with readily determinable fair value (marketable equity securities) | | $ | 34 | | | $ | 28 | |
| Investments for which the fair value option has been elected | | 311 | | 311 |
| Investments without readily determinable fair values | | 826 | | | 859 | |
| Equity method and other investments | | 92 | | | 84 | |
| Total equity and other investments | | $ | 1,265 | | | $ | 1,282 | |
Gains and losses on the Company's portfolio of equity and other investments are recognized in other non-operating income, net in the consolidated statements of income. During the three and six months ended October 25, 2024, there were $10 million of net unrealized losses and $7 million of net unrealized gains, respectively, on equity securities and other investments still held at October 25, 2024. During the three and six months ended October 27, 2023, there were $7 million and $70 million, respectively, of net unrealized losses on equity securities and other investments still held at October 27, 2023.
Mozarc Medical Investment
As further described in Note 4, on April 1, 2023, the Company sold half of its RCS business to Mozarc, and as a result of the transaction the Company retained a 50 percent equity interest in Mozarc. Although the equity investment provides the Company with the ability to exercise significant influence over Mozarc, the Company has elected the fair value option to account for this equity investment. The Company believes the fair value option best reflects the economics of the underlying transaction.
Under the fair value option, changes in the fair value of the investment are recognized through earnings each reporting period in other non-operating income, net in the consolidated statements of income. During the three and six months ended October 25, 2024 and October 27, 2023, the change in fair value was not significant.
7. Financing Arrangements
Commercial Paper
The Company maintains commercial paper programs that allow the Company to issue U.S. dollar or Euro-denominated unsecured commercial paper notes. The aggregate amount outstanding at any time under the commercial paper programs may not exceed the equivalent of $3.5 billion. Commercial paper outstanding at October 25, 2024 was $899 million. During the three and six months ended October 25, 2024, the commercial paper outstanding had a weighted average original maturity of 13 days and 14 days, respectively and a weighted average interest rate of 5.23 percent and 5.34 percent, respectively. Commercial paper outstanding at April 26, 2024 was $1.1 billion. During fiscal year 2024, the weighted average original maturity of the commercial paper outstanding was approximately 20 days and the weighted average interest rate was 5.45 percent. The issuance of commercial paper reduces the amount of credit available under the Company’s existing Credit Facility, as defined below.
Line of Credit
The Company has a $3.5 billion five-year unsecured revolving credit facility (Credit Facility), which provides back-up funding for the commercial paper programs described above. The Credit Facility includes a multi-currency borrowing feature for certain specified foreign currencies. At October 25, 2024 and April 26, 2024, no amounts were outstanding under the Credit Facility.
Medtronic plc
Notes to Consolidated Financial Statements
(Unaudited)
Interest rates on advances on the Credit Facility are determined by a pricing matrix, based on the Company’s long-term debt ratings, assigned by Standard & Poor’s Ratings Services and Moody’s Investors Service. Facility fees are payable on the Credit Facility and are determined in the same manner as the interest rates. The Company is in compliance with the covenants under the Credit Facility.
Debt Obligations
The Company's debt obligations consisted of the following: | | | | | | | | | | | | | | | | | | | | |
| (in millions) | | Maturity by
Fiscal Year | | October 25, 2024 | | April 26, 2024 |
| Current debt obligations | | 2025 - 2026 | | $ | 3,719 | | | $ | 1,092 | |
| | | | | | |
| Long-term debt | | | | | | |
0.250 percent six-year 2019 senior notes | | 2026 | | — | | | 1,070 | |
2.625 percent three-year 2022 senior notes | | 2026 | | — | | | 535 | |
0.000 percent five-year 2020 senior notes | | 2026 | | — | | | 1,070 | |
1.125 percent eight-year 2019 senior notes | | 2027 | | 1,620 | | | 1,606 | |
4.250 percent five-year 2023 senior notes | | 2028 | | 1,000 | | | 1,000 | |
3.000 percent six-year 2022 senior notes | | 2029 | | 1,080 | | | 1,070 | |
0.375 percent eight-year 2020 senior notes | | 2029 | | 1,080 | | | 1,070 | |
3.650 percent five-year 2024 senior notes | | 2030 | | 918 | | | — | |
1.625 percent twelve-year 2019 senior notes | | 2031 | | 1,080 | | | 1,070 | |
1.000 percent twelve-year 2019 senior notes | | 2032 | | 1,080 | | | 1,070 | |
3.125 percent nine-year 2022 senior notes | | 2032 | | 1,080 | | | 1,070 | |
0.750 percent twelve-year 2020 senior notes | | 2033 | | 1,080 | | | 1,070 | |
4.500 percent ten-year 2023 senior notes | | 2033 | | 1,000 | | | 1,000 | |
3.375 percent twelve-year 2022 senior notes | | 2035 | | 1,080 | | | 1,070 | |
4.375 percent twenty-year 2015 senior notes | | 2035 | | 1,932 | | | 1,932 | |
3.875 percent twelve-year 2024 senior notes | | 2037 | | 918 | | | — | |
6.550 percent thirty-year 2007 CIFSA senior notes | | 2038 | | 253 | | | 253 | |
2.250 percent twenty-year 2019 senior notes | | 2039 | | 1,080 | | | 1,070 | |
6.500 percent thirty-year 2009 senior notes | | 2039 | | 158 | | | 158 | |
1.500 percent twenty-year 2019 senior notes | | 2040 | | 1,080 | | | 1,070 | |
5.550 percent thirty-year 2010 senior notes | | 2040 | | 224 | | | 224 | |
1.375 percent twenty-year 2020 senior notes | | 2041 | | 1,080 | | | 1,070 | |
4.500 percent thirty-year 2012 senior notes | | 2042 | | 105 | | | 105 | |
4.000 percent thirty-year 2013 senior notes | | 2043 | | 305 | | | 305 | |
4.150 percent nineteen-year 2024 senior notes | | 2044 | | 648 | | | — | |
4.625 percent thirty-year 2014 senior notes | | 2044 | | 127 | | | 127 | |
4.625 percent thirty-year 2015 senior notes | | 2045 | | 1,813 | | | 1,813 | |
1.750 percent thirty-year 2019 senior notes | | 2050 | | 1,080 | | | 1,070 | |
1.625 percent thirty-year 2020 senior notes | | 2051 | | 1,080 | | | 1,070 | |
| | | | | | |
4.150 percent twenty-nine-year 2024 senior notes | | 2054 | | 756 | | | — | |
| Finance lease obligations | | 2026 - 2036 | | 50 | | | 55 | |
| Deferred financing costs | | 2027 - 2054 | | (125) | | | (110) | |
| Debt discount, net | | 2027 - 2054 | | (56) | | | (55) | |
| Total long-term debt | | | | $ | 24,607 | | | $ | 23,932 | |
Interest expense on outstanding borrowings, including amortization of debt issuance costs and debt discounts and premiums is recognized in interest expense, net in the consolidated statements of income. During the three and six months ended October 25, 2024, there was $252
Medtronic plc
Notes to Consolidated Financial Statements
(Unaudited)
million and $469 million, respectively, of interest expense on outstanding borrowings, including amortization of debt issuance costs and debt discounts and premiums. During the three and six months ended October 27, 2023, there was $230 million and $427 million, respectively, of interest expense on outstanding borrowings, including amortization of debt issuance costs and debt discounts and premiums.
Senior Notes
The Company has outstanding unsecured senior obligations, described as senior notes in the tables above (collectively, the Senior Notes). The Senior Notes rank equally with all other unsecured and unsubordinated indebtedness of the Company. The Company is in compliance with all covenants related to the Senior Notes.
On June 3, 2024, Medtronic Inc. issued four tranches of EUR-denominated Senior Notes with an aggregate principal of €3.0 billion, with maturities ranging from fiscal year 2030 to 2054, resulting in cash proceeds of approximately $3.2 billion, net of discounts and issuance costs. In anticipation of the Euro-denominated debt issuance, the Company entered into forward currency exchange rate contracts to manage the exposure to exchange rate movements. These contracts were settled in conjunction with the issuance of the June 2024 Notes.
Financial Instruments Not Measured at Fair Value
At October 25, 2024, the estimated fair value of the Company’s Senior Notes was $25.3 billion compared to a principal value of $27.4 billion. At April 26, 2024, the estimated fair value was $21.2 billion compared to a principal value of $24.0 billion. The fair value was estimated using quoted market prices for the publicly registered Senior Notes, which are classified as Level 2 within the fair value hierarchy. The fair values and principal values consider the terms of the related debt and exclude the impacts of debt discounts and hedging activity.
8. Derivatives and Currency Exchange Risk Management
The Company uses derivative instruments and foreign currency denominated debt to manage the impact that currency exchange rate and interest rate changes have on reported financial statements. The Company does not enter into derivative contracts for speculative purposes.
Fair Value Hedges
Beginning in the first quarter of fiscal year 2025, the Company began using foreign currency forward contracts designated as fair value hedges to manage its exposure to changes in the fair value of its fixed-rate debt obligation.
At inception, foreign currency forward contracts are designated as fair value hedges. Changes in the fair value of these derivatives are reported as a component of other operating income, net. Amounts excluded from the assessment of effectiveness are recognized in interest expense, net on a straight-line basis over the term of the hedge. During the three and six months ended October 25, 2024, after-tax unrealized losses related to included components in other operating income, net were not significant. During the three and six months ended October 25, 2024, amounts related to excluded components that are amortized in interest expense, net over the life of the hedging instrument were not significant. Cash flows related to the Company's derivative instruments designated as fair value hedges are reported as financing activities in the consolidated statements of cash flows. Cash flows attributed to amounts excluded from the assessment of effectiveness are reported as operating activities in the consolidated statements of cash flows.
Cash Flow Hedges
The Company uses foreign currency forward and option contracts designated as cash flow hedges to manage its exposure to the variability of future cash flows that are denominated in a foreign currency.
At inception, foreign currency forward and option contracts are designated as cash flow hedges. Changes in the fair value of these derivatives are reported as a component of accumulated other comprehensive loss until the hedged transaction affects earnings. When the hedged transaction affects earnings, the gain or loss on the derivative is reclassified to earnings. Amounts excluded from the measurement of hedge effectiveness are recognized in earnings on a straight-line basis over the term of the hedge. Cash flows are reported as operating activities in the consolidated statements of cash flows.
The Company's cash flow hedges will mature within the subsequent three-year period. At October 25, 2024 and April 26, 2024, the Company had $139 million and $229 million in after-tax unrealized gains, respectively, associated with cash flow hedging instruments recorded in accumulated other comprehensive loss. The Company expects that $108 million of after-tax net unrealized gains at October 25, 2024 will be recognized in the consolidated statements of income over the next 12 months.
Net Investment Hedges
The Company uses derivative instruments and foreign currency denominated debt to manage foreign currency risk associated with its net investment in foreign operations. The derivative instruments that the Company uses for this purpose may include foreign currency forward exchange contracts used on a standalone basis or in combination with option collars and standalone cross currency interest rate contracts.
Medtronic plc
Notes to Consolidated Financial Statements
(Unaudited)
For instruments that are designated as net investment hedges, the gains or losses are reported as a component of accumulated other comprehensive loss. The gains or losses are reclassified into earnings upon a liquidation event or deconsolidation of the foreign subsidiary. Amounts excluded from the assessment of effectiveness are recognized in interest expense, net on a straight-line basis over the term of the hedge. During the three and six months ended October 25, 2024, the Company recognized $47 million and $98 million, respectively, in after-tax unrealized gains representing excluded components in interest expense, net. During the three and six months ended October 27, 2023, the Company recognized $50 million and $99 million, respectively, in after-tax unrealized gains representing excluded components in interest expense, net. The cash flows related to the Company's derivative instruments designated as net investment hedges are reported as investing activities in the consolidated statements of cash flows. Cash flows attributable to amounts excluded from the assessment of effectiveness are reported as operating activities in the consolidated statements of cash flows.
Undesignated Derivatives
The Company uses foreign currency forward exchange contracts to offset the Company’s exposure to the change in the value of non-functional currency denominated assets, liabilities, and cash flows.
These foreign currency forward exchange rate contracts are not designated as hedges at inception, and therefore, changes in the fair value of these contracts are recognized in the consolidated statements of income. Cash flows related to the Company’s undesignated derivative contracts are reported in the consolidated statements of cash flows based on the nature of the derivative instrument.
Outstanding Instruments
The following table presents the contractual amounts of the Company's outstanding instruments:
| | | | | | | | | | | | | | | | | |
| | | As of |
| (in billions) | Designation | | October 25, 2024 | | April 26, 2024 |
Currency exchange rate contracts(1) | Fair value hedge | | $ | 1.1 | | | $ | — | |
| Currency exchange rate contracts | Cash flow hedge | | 11.3 | | | 10.4 | |
Currency exchange rate contracts(2) | Net investment hedge | | 7.5 | | | 7.4 | |
Foreign currency-denominated debt(3) | Net investment hedge | | 19.4 | | | 17.1 | |
| Currency exchange rate contracts | Undesignated | | 4.7 | | | 5.9 | |
(1)At October 25, 2024, includes derivative contracts with a notional value of €1.0 billion, or $1.1 billion, designated as hedges of a portion of our fixed-rate debt obligations.
(2)At October 25, 2024, includes derivative contracts with a notional value of €5.0 billion, or $5.4 billion, designated as hedges of a portion of our net investment in certain European operations and derivative contracts with a notional value of ¥322.2 billion, or $2.1 billion, designated as hedges of a portion of our net investment in certain Japanese operations. These derivative contracts mature in fiscal years 2025 through 2033.
(3)At October 25, 2024, includes €18.0 billion, or $19.4 billion, of outstanding Euro-denominated debt designated as hedges of a portion of our net investment in foreign operations. This debt matures in fiscal years 2026 through 2054.
Medtronic plc
Notes to Consolidated Financial Statements
(Unaudited)
Gains and Losses on Hedging Instruments and Derivatives not Designated as Hedging Instruments
The amount of the gains and losses on hedging instruments and the classification of those gains and losses within our consolidated financial statements for the three and six months ended October 25, 2024 and October 27, 2023 were as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| (Gain) Loss Recognized in Accumulated Other Comprehensive Loss | | (Gain) Loss Reclassified into Income | | |
| | | |
| Three months ended | | Six months ended | | Three months ended | | Six months ended | | Location of (Gain) Loss in Income Statement |
| (in millions) | October 25, 2024 | | October 27, 2023 | | October 25, 2024 | | October 27, 2023 | | October 25, 2024 | | October 27, 2023 | | October 25, 2024 | | October 27, 2023 | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| Cash flow hedges | | | | | | | | | | | | | | | | | |
| Currency exchange rate contracts | $ | — | | | $ | (394) | | | $ | 42 | | | $ | (398) | | | $ | (3) | | | $ | (75) | | | $ | (39) | | | $ | (125) | | | Other operating income, net |
| Currency exchange rate contracts | 20 | | | (75) | | | — | | | (109) | | | (21) | | | (15) | | | (39) | | | (19) | | | Cost of products sold |
| Net investment hedges | | | | | | | | | | | | | | | | | |
| Foreign currency-denominated debt | (91) | | | (725) | | | 160 | | | (612) | | | — | | | — | | | — | | | — | | | N/A |
| Currency exchange rate contracts | 52 | | | (190) | | | 12 | | | (160) | | | — | | | — | | | — | | | — | | | N/A |
| Total | $ | (19) | | | $ | (1,384) | | | $ | 214 | | | $ | (1,278) | | | $ | (24) | | | $ | (89) | | | $ | (78) | | | $ | (145) | | | |
The amount of the gains and losses on our derivative instruments not designated as hedging instruments and the classification of those gains and losses within our consolidated financial statements during the three and six months ended October 25, 2024 and October 27, 2023 were as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| (Gain) Loss Recognized in Income | | |
| Three months ended | | Six months ended | | Location of (Gain) Loss in Income Statement |
| (in millions) | October 25, 2024 | | October 27, 2023 | | October 25, 2024 | | October 27, 2023 | |
| | | | | | | | | |
| Currency exchange rate contracts | $ | (35) | | | $ | 94 | | | $ | (45) | | | $ | 92 | | | Other operating income, net |
| | | | | | | | | |
| | | | | | | | | |
Balance Sheet Presentation
The following table summarizes the balance sheet classification and fair value of derivative instruments included in the consolidated balance sheets at October 25, 2024 and April 26, 2024. The fair value amounts are presented on a gross basis, and are segregated between derivatives that are designated and qualify as hedging instruments and those that are not designated and do not qualify as hedging instruments, and are further segregated by type of contract within those two categories.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Fair Value - Assets | | Fair Value - Liabilities |
| (in millions) | October 25, 2024 | | April 26, 2024 | | Balance Sheet Classification | | October 25, 2024 | | April 26, 2024 | | Balance Sheet Classification |
| Derivatives designated as hedging instruments | | | | | | | | | | | |
| Currency exchange rate contracts | $ | 328 | | | $ | 368 | | | Other current assets | | $ | 56 | | | $ | 37 | | | Other accrued expenses |
| | | | | | | | | | | |
| Currency exchange rate contracts | 227 | | | 276 | | | Other assets | | 22 | | | 17 | | | Other liabilities |
| Total derivatives designated as hedging instruments | 556 | | | 644 | | | | | 78 | | | 54 | | | |
| Derivatives not designated as hedging instruments | | | | | | | | | | | |
| Currency exchange rate contracts | 19 | | | 15 | | | Other current assets | | 32 | | | 12 | | | Other accrued expenses |
| Total return swaps | 15 | | | — | | | Other current assets | | — | | | — | | | Other accrued expenses |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| Total derivatives not designated as hedging instruments | 34 | | | 15 | | | | | 32 | | | 12 | | | |
| Total derivatives | $ | 589 | | | $ | 659 | | | | | $ | 110 | | | $ | 66 | | | |
Medtronic plc
Notes to Consolidated Financial Statements
(Unaudited)
The following table provides information by level for the derivative assets and liabilities that are measured at fair value on a recurring basis.
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
| October 25, 2024 | | April 26, 2024 |
| (in millions) | Derivative Assets | | Derivative Liabilities | | | | Derivative Assets | | Derivative Liabilities | | |
| Level 1 | $ | 574 | | | $ | 110 | | | | | $ | 659 | | | $ | 66 | | | |
| Level 2 | 15 | | | — | | | | | — | | | — | | | |
| Total | $ | 589 | | | $ | 110 | | | | | $ | 659 | | | $ | 66 | | | |
The Company has elected to present the fair value of derivative assets and liabilities within the consolidated balance sheets on a gross basis, even when derivative transactions are subject to master netting arrangements and may otherwise qualify for net presentation. The cash flows related to collateral posted and received are reported gross as investing and financing activities, respectively, in the consolidated statements of cash flows.
The following tables provide information as if the Company had elected to offset the asset and liability balances of derivative instruments, netted in accordance with various criteria as stipulated by the terms of the master netting arrangements with each of the counterparties. Derivatives not subject to master netting arrangements are not eligible for net presentation.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | October 25, 2024 |
| | | | Gross Amount Not Offset on the Balance Sheet | | | | |
| (in millions) | | Gross Amount of Recognized Assets (Liabilities) | | Financial Instruments | | Cash Collateral (Received) Posted | | | | Net Amount |
| Derivative assets: | | | | | | | | | | |
| Currency exchange rate contracts | | $ | 574 | | | $ | (107) | | | $ | (52) | | | | | $ | 417 | |
| | | | | | | | | | |
| | | | | | | | | | |
| Total return swaps | | 15 | | | — | | | — | | | | | 15 | |
| | 589 | | | (107) | | | (52) | | | | | 432 | |
| Derivative liabilities: | | | | | | | | | | |
| Currency exchange rate contracts | | (110) | | | 107 | | | — | | | | | (4) | |
| | | | | | | | | | |
| | | | | | | | | | |
| | | | | | | | | | |
| | | | | | | | | | |
| Total | | $ | 479 | | | $ | — | | | $ | (52) | | | | | $ | 428 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | April 26, 2024 | | |
| | | | Gross Amount Not Offset on the Balance Sheet | | | | | | |
| (in millions) | | Gross Amount of Recognized Assets (Liabilities) | | Financial Instruments | | Cash Collateral (Received) Posted | | | | Net Amount | | |
| Derivative assets: | | | | | | | | | | | | |
| Currency exchange rate contracts | | $ | 659 | | | $ | (66) | | | $ | (101) | | | | | $ | 492 | | | |
| | | | | | | | | | | | |
| | | | | | | | | | | | |
| | | | | | | | | | | | |
| | | | | | | | | | | | |
| Derivative liabilities: | | | | | | | | | | | | |
| Currency exchange rate contracts | | (66) | | | 66 | | | — | | | | | — | | | |
| | | | | | | | | | | | |
| | | | | | | | | | | | |
| | | | | | | | | | | | |
| | | | | | | | | | | | |
| Total | | $ | 593 | | | $ | — | | | $ | (101) | | | | | $ | 492 | | | |
9. Inventories
Inventory balances were as follows:
| | | | | | | | | | | |
| (in millions) | October 25, 2024 | | April 26, 2024 |
| Finished goods | $ | 3,729 | | | $ | 3,668 | |
| Work-in-process | 728 | | | 642 | |
| Raw materials | 1,022 | | | 907 | |
| Total | $ | 5,479 | | | $ | 5,217 | |
Medtronic plc
Notes to Consolidated Financial Statements
(Unaudited)
10. Goodwill and Other Intangible Assets
Goodwill
The following table presents the changes in the carrying amount of goodwill by segment:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| (in millions) | Cardiovascular | | Neuroscience | | Medical Surgical | | Diabetes | | Total |
| April 26, 2024 | $ | 7,966 | | | $ | 11,644 | | | $ | 19,121 | | | $ | 2,255 | | | $ | 40,986 | |
| | | | | | | | | |
| | | | | | | | | |
| Currency translation and other | 4 | | | 24 | | | 147 | | | — | | | 176 | |
| October 25, 2024 | $ | 7,970 | | | $ | 11,668 | | | $ | 19,268 | | | $ | 2,255 | | | $ | 41,161 | |
As further described in Note 17, the Company had changes to the operating segments and goodwill reporting units during the fourth quarter of fiscal year 2024. For further information on the reporting unit changes, refer to Note 9 to the consolidated financial statements included in the Company's Annual Report on Form 10-K for the fiscal year ended April 26, 2024. No goodwill impairment was recognized during the three and six months ended October 25, 2024 and October 27, 2023.
The following table presents the gross carrying amount and accumulated amortization of intangible assets:
| | | | | | | | | | | | | | | | | | | | | | | |
| October 25, 2024 | | April 26, 2024 |
| (in millions) | Gross Carrying Amount | | Accumulated Amortization | | Gross Carrying Amount | | Accumulated Amortization |
| Definite-lived: | | | | | | | |
| Customer-related | $ | 16,523 | | | $ | (9,164) | | | $ | 16,518 | | | $ | (8,689) | |
| Purchased technology and patents | 11,727 | | | (7,206) | | | 11,557 | | | (6,868) | |
| Trademarks and tradenames | 425 | | | (280) | | | 424 | | | (274) | |
| Other | 254 | | | (90) | | | 256 | | | (84) | |
| Total | $ | 28,929 | | | $ | (16,741) | | | $ | 28,755 | | | $ | (15,915) | |
| Indefinite-lived: | | | | | | | |
| IPR&D | $ | 235 | | | $ | — | | | $ | 385 | | | $ | — | |
| | | | | | | |
The Company did not recognize any definite-lived intangible asset impairment charges during the three and six months ended October 25, 2024 and October 27, 2023.
The Company did not recognize any indefinite-lived intangible asset impairment charges during the three and six months ended October 25, 2024. Indefinite-lived intangible asset impairment charges were not significant for the three and six months ended October 27, 2023. Due to the nature of IPR&D projects, the Company may experience future delays or failures to obtain regulatory approvals to conduct clinical trials, failures of clinical trials, delays or failures to obtain required market clearances, other failures to achieve a commercially viable product, or the discontinuation of certain projects, and as a result, may recognize impairment losses in the future.
Amortization Expense
Intangible asset amortization expense for the three months ended October 25, 2024 and October 27, 2023 was $413 million and $425 million, respectively. Intangible asset amortization expense for the six months ended October 25, 2024 and October 27, 2023 was $827 million and $855 million, respectively. Estimated aggregate amortization expense by fiscal year based on the carrying value of definite-lived intangible assets at October 25, 2024, excluding any possible future amortization associated with acquired IPR&D which has not yet met technological feasibility, is as follows:
| | | | | |
| (in millions) | Amortization Expense |
| Remaining 2025 | $ | 824 | |
| 2026 | 1,639 | |
| 2027 | 1,616 | |
| 2028 | 1,565 | |
| 2029 | 1,488 | |
| 2030 | 1,356 | |
| |
Medtronic plc
Notes to Consolidated Financial Statements
(Unaudited)
11. Income Taxes
The Organization for Economic Co-operation and Development (OECD) published Pillar Two Model Rules defining the global minimum tax, which calls for the taxation of large multinational corporations at a minimum rate of 15% in each jurisdiction in which the group operates. The OECD has since issued administrative guidance providing transition and safe harbor rules around the implementation of the Pillar Two global minimum tax. A number of countries, including Ireland, have enacted legislation to implement the core elements of Pillar Two, which are effective for the Company in fiscal year 2025. We will continue to monitor the impacts of further legislation, regulatory guidance, and regulations issued in the countries in which we do business.
The Israeli Central-Lod District Court issued its decision in the Medtronic Ventor Technologies Ltd (Ventor) v. Kfar Saba Assessing Office on June 1, 2023. The court determined that there was a deemed taxable transfer of intellectual property. As a result, the Company recorded a $187 million income tax charge during the first quarter of fiscal year 2024 and has filed an appeal with the Supreme Court of Israel.
The Company's effective tax rate for the three and six months ended October 25, 2024 was 18.0% and 17.7%, respectively, as compared to 30.6% and 32.0% for the three and six months ended October 27, 2023, respectively. The decrease in the effective tax rate for the three months ended October 25, 2024, primarily relates to the establishment of a valuation allowance on certain net operating losses recorded during the three months ended October 27, 2023, which was partially offset by the implementation of the Pillar Two global minimum tax. In addition to the items discussed in the current quarter, the decrease in the effective tax rate for the six months ended October 25, 2024 was also attributable to an income tax reserve adjustment made during the six months ended October 27, 2023 associated with the Ventor court decision noted above.
At October 25, 2024 and April 26, 2024, the Company's gross unrecognized tax benefits were $2.9 billion and $2.8 billion, respectively. In addition, the Company had accrued gross interest and penalties of $45 million at October 25, 2024. If all of the Company’s unrecognized tax benefits were recognized, approximately $2.7 billion would impact the Company’s effective tax rate. At October 25, 2024 and April 26, 2024, the amount of the Company's gross unrecognized tax benefits, net of cash advance, recorded as a noncurrent liability within accrued income taxes on the consolidated balance sheets was $1.9 billion and $1.8 billion, respectively. The Company recognizes interest and penalties related to income tax matters within income tax provision in the consolidated statements of income and records the liability within either current or noncurrent accrued income taxes on the consolidated balance sheets.
Refer to Note 16 to the consolidated financial statements for additional information regarding the status of current tax audits and proceedings.
12. Earnings Per Share
Basic earnings per share is computed based on the weighted average number of ordinary shares outstanding. Diluted earnings per share is computed based on the weighted number of ordinary shares outstanding, increased by the number of additional shares that would have been outstanding had the potentially dilutive ordinary shares been issued, and reduced by the number of shares the Company could have repurchased with the proceeds from issuance of the potentially dilutive shares. Potentially dilutive ordinary shares include stock-based awards granted under stock-based compensation plans and shares committed to be purchased under the employee stock purchase plan.
The table below sets forth the computation of basic and diluted earnings per share:
| | | | | | | | | | | | | | | | | | | | | | | |
| | Three months ended | | Six months ended |
| (in millions, except per share data) | October 25, 2024 | | October 27, 2023 | | October 25, 2024 | | October 27, 2023 |
| Numerator: | | | | | | | |
| Net income attributable to ordinary shareholders | $ | 1,270 | | | $ | 909 | | | $ | 2,312 | | | $ | 1,700 | |
| Denominator: | | | | | | | |
| Basic – weighted average shares outstanding | 1,282.4 | | | 1,330.2 | | | 1,288.6 | | | 1,330.3 | |
| Effect of dilutive securities: | | | | | | | |
| Employee stock options | 0.6 | | | 0.5 | | | 0.6 | | | 0.9 | |
| Employee restricted stock units | 2.1 | | | 0.9 | | | 2.0 | | | 1.3 | |
| Employee performance share units | 1.8 | | | 0.2 | | | 1.3 | | | 0.3 | |
| Diluted – weighted average shares outstanding | 1,286.9 | | | 1,331.9 | | | 1,292.5 | | | 1,332.8 | |
| Basic earnings per share | $ | 0.99 | | | $ | 0.68 | | | $ | 1.79 | | | $ | 1.28 | |
| Diluted earnings per share | $ | 0.99 | | | $ | 0.68 | | | $ | 1.79 | | | $ | 1.28 | |
Medtronic plc
Notes to Consolidated Financial Statements
(Unaudited)
The calculation of weighted average diluted shares outstanding excludes options to purchase approximately 28 million and 27 million ordinary shares for the three and six months ended October 25, 2024, respectively, and 31 million and 27 million ordinary shares for the three and six months ended October 27, 2023, respectively, because their effect would have been anti-dilutive on the Company’s earnings per share.
13. Stock-Based Compensation
The following table presents the components and classification of stock-based compensation expense for stock options, restricted stock, performance share units, and employee stock purchase plan shares recognized for the three and six months ended October 25, 2024 and October 27, 2023:
| | | | | | | | | | | | | | | | | | | | | | | |
| | Three months ended | | Six months ended |
| (in millions) | October 25, 2024 | | October 27, 2023 | | October 25, 2024 | | October 27, 2023 |
| Stock options | $ | 30 | | | $ | 35 | | | $ | 42 | | | $ | 46 | |
| Restricted stock | 68 | | | 55 | | | 111 | | | 93 | |
| Performance share units | 54 | | | 48 | | | 69 | | | 60 | |
| Employee stock purchase plan | 8 | | | 8 | | | 19 | | | 19 | |
| Total stock-based compensation expense | $ | 159 | | | $ | 146 | | | $ | 242 | | | $ | 219 | |
| | | | | | | |
| Cost of products sold | $ | 17 | | | $ | 13 | | | $ | 26 | | | $ | 20 | |
| Research and development expense | 19 | | | 18 | | | 29 | | | 27 | |
| Selling, general, and administrative expense | 123 | | | 115 | | | 187 | | | 172 | |
| Total stock-based compensation expense | 159 | | | 146 | | | 242 | | | 219 | |
| Income tax benefits | (25) | | | (25) | | | (38) | | | (36) | |
| Total stock-based compensation expense, net of tax | $ | 134 | | | $ | 121 | | | $ | 204 | | | $ | 183 | |
14. Retirement Benefit Plans
The Company sponsors various retirement benefit plans, including defined benefit pension plans, post-retirement medical plans, defined contribution savings plans, and termination indemnity plans, covering substantially all U.S. employees and many employees outside the U.S. The net periodic benefit cost of the defined benefit pension plans included the following components for the three and six months ended October 25, 2024 and October 27, 2023:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | U.S. | | Non-U.S. | | U.S. | | Non-U.S. |
| | Three months ended | | Six months ended |
| (in millions) | October 25, 2024 | | October 27, 2023 | | October 25, 2024 | | October 27, 2023 | | October 25, 2024 | | October 27, 2023 | | October 25, 2024 | | October 27, 2023 |
| | | | | | | | | | | | | | | |
| Service cost | $ | 13 | | | $ | 15 | | | $ | 11 | | | $ | 10 | | | $ | 26 | | | $ | 30 | | | $ | 22 | | | $ | 20 | |
| Interest cost | 43 | | | 40 | | | 13 | | | 12 | | | 86 | | | 80 | | | 26 | | | 24 | |
| Expected return on plan assets | (66) | | | (65) | | | (17) | | | (17) | | | (132) | | | (130) | | | (34) | | | (34) | |
| Amortization of prior service cost | (1) | | | (1) | | | — | | | — | | | (2) | | | (2) | | | — | | | — | |
| Amortization of net actuarial loss | 4 | | | 5 | | | — | | | — | | | 8 | | | 10 | | | 1 | | | — | |
| Net periodic (credit) benefit cost | $ | (7) | | | $ | (6) | | | $ | 7 | | | $ | 5 | | | $ | (14) | | | $ | (12) | | | $ | 14 | | | $ | 10 | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
Components of net periodic benefit (credit) cost other than the service component are recognized in other non-operating income, net in the consolidated statements of income.
Medtronic plc
Notes to Consolidated Financial Statements
(Unaudited)
15. Accumulated Other Comprehensive Loss
The following table provides changes in accumulated other comprehensive loss (AOCI), net of tax, and by component: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| (in millions) | Unrealized (Loss) Gain on Investment Securities | | Cumulative Translation Adjustments | | Net Investment Hedges | | Net Change in Retirement Obligations | | Unrealized Gain (Loss) on Cash Flow Hedges | | Total Accumulated Other Comprehensive (Loss) Income |
| April 26, 2024 | $ | (212) | | | $ | (3,686) | | | $ | 878 | | | $ | (529) | | | $ | 229 | | | $ | (3,318) | |
| Other comprehensive income (loss) before reclassifications | 102 | | | 218 | | | (170) | | | (1) | | | (37) | | | 111 | |
| Reclassifications | 9 | | | — | | | — | | | 3 | | | (55) | | | (43) | |
| Other comprehensive income (loss) | 111 | | | 218 | | | (170) | | | 1 | | | (93) | | | 69 | |
| October 25, 2024 | $ | (101) | | | $ | (3,468) | | | $ | 708 | | | $ | (529) | | | $ | 139 | | | $ | (3,250) | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| (in millions) | Unrealized (Loss) Gain on Investment Securities | | Cumulative Translation Adjustments | | Net Investment Hedges | | Net Change in Retirement Obligations | | Unrealized Gain (Loss) on Cash Flow Hedges | | Total Accumulated Other Comprehensive (Loss) Income |
| April 28, 2023 | $ | (258) | | | $ | (2,839) | | | $ | 245 | | | $ | (741) | | | $ | 93 | | | $ | (3,499) | |
| Other comprehensive income (loss) before reclassifications | (49) | | | (909) | | | 771 | | | — | | | 404 | | | 217 | |
| Reclassifications | 11 | | | — | | | — | | | 4 | | | (110) | | | (95) | |
| Other comprehensive income (loss) | (38) | | | (909) | | | 771 | | | 4 | | | 294 | | | 122 | |
| | | | | | | | | | | |
| October 27, 2023 | $ | (296) | | | $ | (3,748) | | | $ | 1,016 | | | $ | (737) | | | $ | 387 | | | $ | (3,377) | |
The income tax on gains and losses on investment securities in other comprehensive income before reclassifications during the six months ended October 25, 2024 and October 27, 2023, was an expense of $19 million and a benefit of $8 million, respectively. During the six months ended October 25, 2024 and October 27, 2023, realized gains and losses on investment securities reclassified from AOCI were reduced by income taxes of $2 million and $3 million, respectively. When realized, gains and losses on investment securities reclassified from AOCI are recognized within other non-operating income, net. Refer to Note 6 to the consolidated financial statements for additional information.
For the six months ended October 25, 2024 and October 27, 2023, there was no income tax on cumulative translation adjustments.
The income tax on net investment hedges in other comprehensive income before reclassifications during the six months ended October 25, 2024 was a benefit of $1 million. During the six months ended October 27, 2023, there were no tax impacts on net investment hedges.
The net change in retirement obligations in other comprehensive income includes amortization of net actuarial losses included in net periodic benefit cost. During the six months ended October 25, 2024 and October 27, 2023, there were no tax impacts on retirement obligations. During the six months ended October 25, 2024 and October 27, 2023, the gains and losses on defined benefit and pension items reclassified from AOCI were reduced by income taxes of $1 million and $2 million, respectively. When realized, net gains and losses on defined benefit and pension items reclassified from AOCI are recognized within other non-operating income, net. Refer to Note 14 to the consolidated financial statements for additional information.
The income tax on unrealized gains and losses on cash flow hedges in other comprehensive income before reclassifications during the six months ended October 25, 2024 and October 27, 2023, was a benefit of $5 million and expense of $103 million, respectively. During the six months ended October 25, 2024 and October 27, 2023, gains and losses on cash flow hedges reclassified from AOCI were reduced by income taxes of $22 million and $34 million, respectively. When realized, gains and losses on currency exchange rate contracts reclassified from AOCI are recognized within other operating income, net or cost of products sold. Refer to Note 8 to the consolidated financial statements for additional information.
16. Commitments and Contingencies
Legal Matters
The Company and its affiliates are involved in a number of legal actions from time to time involving product liability, employment, intellectual property and commercial disputes, shareholder related matters, environmental proceedings, tax disputes, and governmental proceedings and investigations, including those described below. With respect to governmental proceedings and investigations, like other companies in our industry, the Company is subject to extensive regulation by national, state, and local governmental agencies in the United
Medtronic plc
Notes to Consolidated Financial Statements
(Unaudited)
States and in other jurisdictions in which the Company and its affiliates operate. As a result, interaction with governmental agencies is ongoing. The Company’s standard practice is to cooperate with regulators and investigators in responding to inquiries. The outcomes of legal actions are not within the Company’s complete control and may not be known for prolonged periods of time. In some actions, the enforcement agencies or private claimants seek damages, as well as other civil or criminal remedies (including injunctions barring the sale of products that are the subject of the proceeding), that could require significant expenditures, result in lost revenues, or limit the Company's ability to conduct business in the applicable jurisdictions.
The Company records a liability in the consolidated financial statements on an undiscounted basis for loss contingencies related to legal actions when a loss is known or considered probable and the amount may be reasonably estimated. If the reasonable estimate of a known or probable loss is a range, and no amount within the range is a better estimate than any other, the minimum amount of the range is accrued. If a loss is reasonably possible but not known or probable, and may be reasonably estimated, the estimated loss or range of loss is disclosed. When determining the estimated loss or range of loss, significant judgment is required. Estimates of probable losses resulting from litigation and governmental proceedings involving the Company are inherently difficult to predict, particularly when the matters are in early procedural stages with incomplete scientific facts or legal discovery, involve unsubstantiated or indeterminate claims for damages, potentially involve penalties, fines or punitive damages, or could result in a change in business practice. The Company classifies certain specified litigation charges and gains related to significant legal matters as certain litigation charges, net in the consolidated statements of income. The Company recognized no certain litigation charges and $81 million of certain litigation charges during the three and six months ended October 25, 2024, respectively, whereas the Company recognized $65 million and $105 million of certain litigation charges during the three and six months ended October 27, 2023, respectively. At October 25, 2024 and April 26, 2024, accrued litigation was approximately $0.2 billion. The ultimate cost to the Company with respect to accrued litigation could be materially different than the amount of the current estimates and accruals and could have a material adverse impact on the Company’s consolidated earnings, financial position, and/or cash flows. The Company includes accrued litigation in other accrued expenses and other liabilities on the consolidated balance sheets. While it is not possible to predict the outcome for most of the legal matters discussed below, the Company believes it is possible that the costs associated with these matters could have a material adverse impact on the Company’s consolidated earnings, financial position, and/or cash flows.
Intellectual Property Matters
At any given time, the Company is involved in litigation relating to patents, trademarks, copyrights, trade secrets, and other intellectual property (IP) rights, and licenses, acquisitions or other agreements relating to such rights. This litigation includes, but is not limited to, alleged infringement or misappropriation of IP rights, or breach of obligations related to IP rights, or other claims asserted by competitors, individuals, or, consistent with a growing trend across technology-intensive industries, other entities created specifically to fund IP litigation. While the outcome of these litigation matters is inherently uncertain, it is possible that the results of such litigation could require the Company to pay significant monetary damages and/or royalty payments, and negatively impact the Company's ability to sell current or future products, which could have a material adverse impact on the Company's business, results of operations, financial condition, and cash flows.
Colibri
The Company is a defendant in patent litigation brought by Colibri Heart Valve LLC (Colibri) in the U.S. District Court for the Central District of California. Colibri alleges infringement of one patent by the Company’s Evolut family of transcatheter aortic valve replacement devices. The patent asserted by Colibri has expired. On February 8, 2023, a jury returned a verdict against the Company for approximately $106 million. In July 2023, the Company filed its appeal with the U.S. Court of Appeals for the Federal Circuit. The Company has not recognized an expense in connection with this matter because it does not currently believe a loss is probable.
Product Liability Matters
Hernia Mesh Litigation
Starting in fiscal year 2020, plaintiffs began filing lawsuits against certain subsidiaries of the Company in U.S. state and federal courts that allege personal injury from hernia mesh products sold by those subsidiaries. As of October 30, 2024, the Company and certain of its subsidiaries have been named as defendants in lawsuits filed on behalf of approximately 8,800 individual plaintiffs, and certain plaintiffs’ law firms have advised the Company that they may file additional cases in the future. Approximately 6,850 plaintiffs have pending lawsuits in a coordinated proceeding in Massachusetts state court, where they have been consolidated before a single judge. Approximately 500 plaintiffs have pending lawsuits in a coordinated action in Minnesota state court, and there are approximately 1,450 actions coordinated in a federal Multidistrict Litigation in the U.S. District Court for the District of Massachusetts plus fewer than ten one-off cases filed in other courts. The pending lawsuits relate almost entirely to hernia mesh products that have not been subject to recalls, withdrawals, or other adverse regulatory action. The Company has not recorded an expense related to damages in connection with these matters because any potential loss is not currently probable and reasonably estimable. Additionally, the Company is unable to reasonably estimate the range of loss, if any, that may result from these matters.
Medtronic plc
Notes to Consolidated Financial Statements
(Unaudited)
Diabetes Pump Retainer Ring Litigation
Starting in fiscal year 2021, plaintiffs began filing lawsuits against the Diabetes operating unit in U.S. state and federal courts alleging personal injury from Series 600 insulin pumps with allegedly defective clear retainer rings that were subject to field corrective actions in 2019 and 2021. As of November 4, 2024, after a number of recent dismissals of individual plaintiffs, there are 23 lawsuits filed on behalf of 76 individuals. Plaintiffs’ firms previously notified the Company that they may file additional lawsuits in the future on behalf of thousands of additional claimants. Most of the filed suits are coordinated in California state court. The Company has not recorded an expense related to damages in connection with these matters because any potential loss is not currently probable and reasonably estimable. Additionally, the Company is unable to reasonably estimate the range of loss, if any, that may result from these matters.
Environmental Proceedings
The Company is a successor to several investigation and cleanup actions at various stages related to environmental remediation matters at a number of sites, including in Orrington, Maine. These projects relate to a variety of activities, including removal of solvents, metals and other hazardous substances from soil and groundwater. The ultimate cost of site cleanup and timing of future cash flows is difficult to predict given uncertainties regarding the extent of the required cleanup, the interpretation of applicable laws and regulations, and alternative cleanup methods.
The Company is also a successor to a party named in a lawsuit filed in the U.S. District Court for the District of Maine in the early 2000's by the Natural Resources Defense Council and the Maine People's Alliance relating to mercury contamination of the Penobscot River and Bay and options for remediating such contamination. In October 2022, the court issued a final order approving the settlement and the parties are working with consultants on implementation of remedial activities. The final court order did not result in a change to the Company's previous accrual for this matter.
The Company's accrued expenses for these various environmental proceedings are included within accrued litigation as discussed above.
Anti-Corruption Matters
The Company has regular and ongoing interactions with governmental agencies, and its practice is to cooperate with such inquiries. In addition, from time to time, the Company self-discloses potential concerns to governmental regulators. Like many in the medical device industry or with international operations, the Company engages in periodic discussions with the U.S. Securities and Exchange Commission, U.S. Department of Justice, and various authorities in China regarding certain activities, including in China. The Company is committed to regularly evaluating and, as appropriate, strengthening its anti-corruption compliance programs and practices. Any possible future determination that certain of our operations and activities, and/or those of our third-party distributors, are not in compliance with existing laws could result in the imposition of fines, penalties, and equitable remedies in the United States or in other jurisdictions. The Company has not recorded an expense in connection with these matters because any potential loss is not currently probable and reasonably estimable. Additionally, the Company is unable to reasonably estimate the range of loss, if any, that may result from these matters.
Other Matters
Italian Payback
In 2015, “payback” legislation was enacted in Italy requiring companies selling medical devices to make payments to the Italian state if Italy’s medical device expenditures exceed annual regional maximum ceilings. The payment amounts are calculated based upon the amount by which the regional ceilings were exceeded for any given year. There has been significant scrutiny on the legality and enforceability of the payback law since its inception, and litigation challenging the law has been proceeding through the Italian Courts. Since the law was enacted, the Company has recognized an estimate for the amount of variable consideration but has not made any payments under the payback law. In July 2024, two rulings by the Constitutional Court of Italy found that the medical device payback law is constitutional. Therefore, the Company increased its liability pertaining primarily to certain prior years since 2015 by $90 million during the three months ended July 26, 2024, as a reduction to net sales in the consolidated statements of income. As litigation before Italian Courts is still pending, final resolution is unknown at this time, and it is possible that the amount of the Company’s liability could differ from the amount currently accrued.
Income Taxes
In March 2009, the IRS issued its audit report on Medtronic, Inc. for fiscal years 2005 and 2006. Medtronic, Inc. reached agreement with the IRS on some, but not all matters related to these fiscal years. The remaining unresolved issue for fiscal years 2005 and 2006 relates to the allocation of income between Medtronic, Inc. and its wholly-owned subsidiary operating in Puerto Rico, which is one of the Company's key manufacturing sites. The U.S. Tax Court (Tax Court) reviewed this dispute, and in June 2016, issued an opinion with respect to the allocation of income between the parties for fiscal years 2005 and 2006 whereby it generally rejected the IRS’s position, but also made certain modifications to the Medtronic, Inc. tax returns as filed. In April 2017, the IRS filed a Notice of Appeal to the U.S. Court of Appeals
Medtronic plc
Notes to Consolidated Financial Statements
(Unaudited)
for the Eighth Circuit regarding the Tax Court opinion. The U.S. Court of Appeals issued its opinion in August 2018 and remanded the case back to the Tax Court for additional factual findings. The Tax Court issued its second opinion in August 2022, the IRS filed a Notice of Appeal to the U.S. Court of Appeals for the Eighth Circuit in September 2023, and Medtronic subsequently filed a cross-appeal in October 2023.
The IRS has issued its audit reports on Medtronic, Inc. for fiscal years 2007 through 2016. Medtronic, Inc. and the IRS have reached agreement on all significant issues except for the allocation of income between Medtronic, Inc. and its wholly-owned subsidiary operating in Puerto Rico for the businesses that are the subject of the U.S. Tax Court matter for fiscal years 2005 and 2006.
Medtronic, Inc.’s fiscal years 2017, 2018, and 2019 U.S. federal income tax returns are currently being audited by the IRS.
Covidien LP (a wholly owned subsidiary of Medtronic plc) has either reached agreement with the IRS or the statute of limitations has lapsed on its U.S. federal income tax returns through fiscal year 2020.
Although it is not possible to predict the outcome for most of the income tax matters discussed above, the Company believes it is possible that charges associated with these matters could have a material adverse impact on the Company’s consolidated earnings, financial position, and/or cash flows.
Refer to Note 11 for additional discussion of income taxes.
Guarantees
In the normal course of business, the Company and/or its affiliates periodically enter into agreements that require one or more of the Company and/or its affiliates to indemnify customers or suppliers for specific risks, such as claims for injury or property damage arising as a result of the Company or its affiliates’ products, the negligence of the Company's personnel, or claims alleging that the Company's products infringe on third-party patents or other intellectual property. The Company also offers warranties on various products. The Company’s maximum exposure under these guarantees is unable to be estimated. Historically, the Company has not experienced significant losses on these types of guarantees.
The Company believes the ultimate resolution of the above guarantees is not expected to have a material effect on the Company’s consolidated earnings, financial position, and/or cash flows.
17. Segment and Geographic Information
Segment disclosures are on a performance basis consistent with internal management reporting. Net sales of the Company's reportable segments include end-customer revenues from the sale of products the segment develops, manufactures, and distributes. The Company’s management evaluates performance of the segments and allocates resources based on net sales and segment operating profit. Segment operating profit represents income before income taxes, excluding interest income or expense, amortization of intangible assets, centralized distribution costs, currency impact of remeasurement and hedging, non-operating income or expense items, certain corporate charges, stock-based compensation, and other items not allocated to the segments.
The accounting policies of the reportable segments are the same as those described in the summary of significant accounting policies in Note 1 to the consolidated financial statements included in the Company's Annual Report on Form 10-K for the fiscal year ended April 26, 2024. Certain depreciable assets may be recorded by one segment, while the depreciation expense is allocated to another segment. The allocation of depreciation expense is based on the proportion of the assets used by each segment.
There have been no changes to reportable segments during the quarter ended October 25, 2024. We continue to have four reportable segments: Cardiovascular Portfolio, Neuroscience Portfolio, Medical Surgical Portfolio, and Diabetes Operating Unit. Prior period amounts have been recast to conform to the new operating segment structure in the fourth quarter of fiscal year 2024. For further information on the operating segment structure changes, refer to Note 19 to the consolidated financial statements included in the Company's Annual Report on Form 10-K for the fiscal year ended April 26, 2024.
Medtronic plc
Notes to Consolidated Financial Statements
(Unaudited)
The following tables present reconciliations of financial information from the segments to the applicable line items in the Company's consolidated financial statements:
Segment Operating Profit
| | | | | | | | | | | | | | | | | | | | | | | |
| | Three months ended | | Six months ended |
| (in millions) | October 25, 2024 | | October 27, 2023 | | October 25, 2024 | | October 27, 2023 |
| Cardiovascular | $ | 1,164 | | | $ | 1,117 | | | $ | 2,289 | | | $ | 2,208 | |
| Neuroscience | 1,024 | | | 929 | | | 1,985 | | | 1,858 | |
| Medical Surgical | 774 | | | 795 | | | 1,464 | | | 1,521 | |
| Diabetes | 116 | | | 101 | | | 218 | | | 186 | |
| Reportable segment operating profit | 3,079 | | | 2,942 | | | 5,956 | | | 5,773 | |
Other operating segment(1) | 12 | | | 4 | | | 26 | | | 1 | |
| Corporate | (448) | | | (431) | | | (903) | | | (879) | |
| Interest expense, net | (209) | | | (180) | | | (376) | | | (329) | |
| Other non-operating income, net | 173 | | | 154 | | | 330 | | | 230 | |
| Amortization of intangible assets | (413) | | | (425) | | | (827) | | | (855) | |
| Stock-based compensation | (159) | | | (146) | | | (242) | | | (219) | |
| Centralized distribution costs | (405) | | | (391) | | | (800) | | | (786) | |
Currency(2) | (39) | | | 32 | | | (44) | | | 29 | |
| Restructuring and associated costs | (46) | | | (91) | | | (108) | | | (182) | |
| Acquisition and divestiture-related items | 25 | | | (58) | | | 13 | | | (107) | |
| Certain litigation charges, net | — | | | (65) | | | (81) | | | (105) | |
| Medical device regulations | (12) | | | (30) | | | (27) | | | (62) | |
Other adjustments(3) | — | | | — | | | (90) | | | — | |
| Income before income taxes | $ | 1,559 | | | $ | 1,313 | | | $ | 2,827 | | | $ | 2,510 | |
(1)Includes the historical operations and ongoing transition agreements from businesses the Company has exited or divested.
(2)Includes the net impact of remeasurement and the Company's hedging programs recorded in other operating income, net.
(3)Incremental Italian payback accruals resulting from the two July 22, 2024 rulings by the Constitutional Court of Italy relating to certain prior years since 2015.
Geographic Information
Net sales are attributed to the country based on the location of the customer taking possession of the products or in which the services are rendered. The following table presents net sales for the three and six months ended October 25, 2024 and October 27, 2023 for the Company's country of domicile, countries with significant concentrations, and all other countries:
| | | | | | | | | | | | | | | | | | | | | | | |
| | Three months ended | | Six months ended |
| (in millions) | October 25, 2024 | | October 27, 2023 | | October 25, 2024 | | October 27, 2023 |
| Ireland | $ | 29 | | | $ | 29 | | | $ | 59 | | | $ | 58 | |
| | | | | | | |
| United States | 4,304 | | | 4,175 | | | 8,387 | | | 8,099 | |
| Rest of world | 4,070 | | | 3,780 | | | 7,872 | | | 7,528 | |
| Total other countries, excluding Ireland | 8,374 | | | 7,955 | | | 16,259 | | | 15,627 | |
| Total | $ | 8,403 | | | $ | 7,984 | | | $ | 16,318 | | | $ | 15,686 | |
Item 2. Management’s Discussion and Analysis of Financial Condition and Results of Operations
UNDERSTANDING OUR FINANCIAL INFORMATION
The following discussion and analysis provides information management believes to be relevant to understanding the financial condition and results of operations of Medtronic plc and its subsidiaries (Medtronic plc, Medtronic, or the Company, or we, us, or our). For a full understanding of financial condition and results of operations, you should read this discussion along with Management’s Discussion and Analysis of Financial Condition and Results of Operations in our Annual Report on Form 10-K for the fiscal year ended April 26, 2024. In addition, you should read this discussion along with our consolidated financial statements and related notes thereto at and for the three and six months ended October 25, 2024. Amounts reported in millions within this quarterly report are computed based on the amounts in thousands, and therefore, the sum of the components may not equal the total amount reported in millions due to rounding. Additionally, certain columns and rows within tables may not sum due to rounding.
Financial Trends
Throughout this Management’s Discussion and Analysis, we present certain financial measures that facilitate management's review of the operational performance of the Company and as a basis for strategic planning; however, such financial measures are not presented in our financial statements prepared in accordance with accounting principles generally accepted in the United States (U.S.) (U.S. GAAP). These financial measures are considered "non-GAAP financial measures" and are intended to supplement, and should not be considered as superior to, financial measures presented in accordance with U.S. GAAP. We believe that non-GAAP financial measures provide information useful to investors in understanding the Company's underlying operational performance and trends and may facilitate comparisons with the performance of other companies in the medical technologies industry.
As presented in the GAAP to Non-GAAP Reconciliations section on the following pages, our non-GAAP financial measures exclude the impact of amortization of intangible assets and certain charges or benefits that contribute to or reduce earnings and that may affect financial trends and include certain charges or benefits that result from transactions or events that we believe may or may not recur with similar materiality or impact to our operations in future periods (Non-GAAP Adjustments).
In the event there is a Non-GAAP Adjustment recognized in our operating results, the tax cost or benefit attributable to that item is separately calculated and reported. Because the effective rate can be significantly impacted by the Non-GAAP Adjustments that take place during the period, we often refer to our tax rate using both the effective rate and the non-GAAP nominal tax rate (Non-GAAP Nominal Tax Rate). The Non-GAAP Nominal Tax Rate is calculated as the income tax provision, adjusted for the impact of Non-GAAP Adjustments, as a percentage of income before income taxes, excluding Non-GAAP Adjustments.
Free cash flow is a non-GAAP financial measure calculated by subtracting property, plant, and equipment additions from operating cash flows.
Refer to the “GAAP to Non-GAAP Reconciliations," "Income Taxes," and "Free Cash Flow" sections for reconciliations of the non-GAAP financial measures to their most directly comparable financial measures prepared in accordance with U.S. GAAP.
EXECUTIVE LEVEL OVERVIEW
Medtronic is the leading global healthcare technology company — alleviating pain, restoring health, and extending life for millions of people around the world. Our primary products include those for cardiac rhythm disorders, cardiovascular disease, advanced and general surgical care, respiratory and monitoring solutions, neurological disorders, spinal conditions and musculoskeletal trauma, urological and digestive disorders, and ear, nose, and throat, and diabetes conditions.
The following is a summary of revenue and diluted earnings per share for the three months ended October 25, 2024 and October 27, 2023, and operating cash flow for the six months ended October 25, 2024 and October 27, 2023:
GAAP to Non-GAAP Reconciliations
The tables below present our GAAP to Non-GAAP reconciliations for the three months ended October 25, 2024 and October 27, 2023:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Three months ended October 25, 2024 |
| (in millions, except per share data) | Income Before Income Taxes | | Income
Tax Provision
(Benefit) | | Net Income Attributable to Medtronic | | Diluted EPS | | Effective
Tax Rate |
| GAAP | $ | 1,559 | | | $ | 281 | | | $ | 1,270 | | | $ | 0.99 | | | 18.0 | % |
| Non-GAAP Adjustments: | | | | | | | | | |
| Amortization of intangible assets | 413 | | | 75 | | | 338 | | | 0.26 | | | 18.2 | |
Restructuring and associated costs(1) | 46 | | | 9 | | | 37 | | | 0.03 | | | 19.6 | |
Acquisition and divestiture-related items(2) | (25) | | | 5 | | | (30) | | | (0.02) | | | (20.0) | |
| | | | | | | | | |
(Gain)/loss on minority investments(3) | (10) | | | 10 | | | (21) | | | (0.02) | | | (100.0) | |
Medical device regulations(4) | 12 | | | 2 | | | 10 | | | 0.01 | | | 16.7 | |
| | | | | | | | | |
| Certain tax adjustments, net | — | | | (16) | | | 16 | | | 0.01 | | | — | |
| Non-GAAP | $ | 1,995 | | | $ | 366 | | | $ | 1,620 | | | $ | 1.26 | | | 18.3 | % |
| | | | | | | | | |
| | Three months ended October 27, 2023 |
| (in millions, except per share data) | Income Before Income Taxes | | Income
Tax Provision (Benefit) | | Net Income Attributable to Medtronic | | Diluted EPS | | Effective
Tax Rate |
| GAAP | $ | 1,313 | | | $ | 402 | | | $ | 909 | | | $ | 0.68 | | | 30.6 | % |
| Non-GAAP Adjustments: | | | | | | | | | |
| Amortization of intangible assets | 425 | | | 65 | | | 360 | | | 0.27 | | | 15.3 | |
Restructuring and associated costs(1) | 91 | | | 16 | | | 76 | | | 0.06 | | | 17.6 | |
Acquisition and divestiture-related items(2) | 58 | | | 7 | | | 51 | | | 0.04 | | | 12.1 | |
| Certain litigation charges, net | 65 | | | 15 | | | 50 | | | 0.04 | | | 23.1 | |
(Gain)/loss on minority investments(3) | 25 | | | 5 | | | 21 | | | 0.02 | | | 20.0 | |
Medical device regulations(4) | 30 | | | 6 | | | 24 | | | 0.02 | | | 20.0 | |
Certain tax adjustments, net (5) | — | | | (176) | | | 176 | | | 0.13 | | | — | |
| Non-GAAP | $ | 2,008 | | | $ | 339 | | | $ | 1,667 | | | $ | 1.25 | | | 16.9 | % |
(1)Associated costs primarily include salaries and wages for employees supporting the restructuring activities, consulting expenses, and asset write-offs.
(2)The charges primarily include business combination costs, changes in fair value of contingent consideration, and exit of business-related charges. The three months ended October 25, 2024 also includes gains related to certain business or asset sales.
(3)We exclude unrealized and realized gains and losses on our minority investments as we do not believe that these components of income or expense have a direct correlation to our ongoing or future business operations.
(4)The charges represent incremental costs of complying with the new European Union (E.U.) medical device regulations for previously registered products and primarily include charges for contractors supporting the project and other direct third-party expenses. We consider these costs to be duplicative of previously incurred costs and/or one-time costs, which are limited to a specific time period.
(5)The charge primarily relates to the establishment of a valuation allowance against certain net operating losses.
The tables below present our GAAP to Non-GAAP reconciliations for the six months ended October 25, 2024 and October 27, 2023:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Six months ended October 25, 2024 | | | | | | | | | | |
| (in millions, except per share data) | Income Before Income Taxes | | Income
Tax Provision (Benefit) | | Net Income attributable to Medtronic | | Diluted EPS | | Effective Tax Rate | | | | | | | | | | |
| GAAP | $ | 2,827 | | | $ | 500 | | | $ | 2,312 | | | $ | 1.79 | | | 17.7 | % | | | | | | | | | | |
| Non-GAAP Adjustments: | | | | | | | | | | | | | | | | | | | |
| Amortization of intangible assets | 827 | | | 149 | | | 678 | | | 0.52 | | | 18.0 | | | | | | | | | | | |
Restructuring and associated costs(1) | 108 | | | 21 | | | 87 | | | 0.07 | | | 19.4 | | | | | | | | | | | |
Acquisition and divestiture-related items(2) | (13) | | | 6 | | | (19) | | | (0.01) | | | (46.2) | | | | | | | | | | | |
| Certain litigation charges, net | 81 | | | 13 | | | 68 | | | 0.05 | | | 16.0 | | | | | | | | | | | |
(Gain)/loss on minority investments(3) | (27) | | | 10 | | | (38) | | | (0.03) | | | (37.0) | | | | | | | | | | | |
Medical device regulations(4) | 27 | | | 5 | | | 22 | | | 0.02 | | | 18.5 | | | | | | | | | | | |
Other(5) | 90 | | | 20 | | | 70 | | | 0.05 | | | 22.2 | | | | | | | | | | | |
| Certain tax adjustments, net | — | | | (33) | | | 33 | | | 0.03 | | | — | | | | | | | | | | | |
| Non-GAAP | $ | 3,921 | | | $ | 693 | | | $ | 3,213 | | | $ | 2.49 | | | 17.7 | % | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | |
| | Six months ended October 27, 2023 | | | | | | | | | | |
| (in millions, except per share data) | Income Before Income Taxes | | Income
Tax Provision (Benefit) | | Net Income attributable to Medtronic | | Diluted EPS | | Effective Tax Rate | | | | | | | | | | |
| GAAP | $ | 2,510 | | | $ | 802 | | | $ | 1,700 | | | $ | 1.28 | | | 32.0 | % | | | | | | | | | | |
| Non-GAAP Adjustments: | | | | | | | | | | | | | | | | | | | |
| Amortization of intangible assets | 855 | | | 130 | | | 724 | | | 0.54 | | | 15.2 | | | | | | | | | | | |
Restructuring and associated costs(1) | 182 | | | 30 | | | 152 | | | 0.11 | | | 16.5 | | | | | | | | | | | |
Acquisition and divestiture-related items(2) | 107 | | | 10 | | | 97 | | | 0.07 | | | 9.3 | | | | | | | | | | | |
| Certain litigation charges, net | 105 | | | 24 | | | 81 | | | 0.06 | | | 22.9 | | | | | | | | | | | |
(Gain)/loss on minority investments(3) | 89 | | | 5 | | | 85 | | | 0.06 | | | 5.6 | | | | | | | | | | | |
Medical device regulations(4) | 62 | | | 13 | | | 49 | | | 0.04 | | | 21.0 | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | |
Certain tax adjustments, net(6) | — | | | (375) | | | 375 | | | 0.28 | | | — | | | | | | | | | | | |
| Non-GAAP | $ | 3,910 | | | $ | 640 | | | $ | 3,262 | | | $ | 2.45 | | | 16.4 | % | | | | | | | | | | |
(1)Associated costs primarily include salaries and wages for employees supporting the restructuring activities, consulting expenses, and asset write-offs.
(2)The charges primarily include business combination costs, changes in fair value of contingent consideration, and exit of business-related charges. The six months ended October 25, 2024 also includes gains related to certain business or asset sales.
(3)We exclude unrealized and realized gains and losses on our minority investments as we do not believe that these components of income or expense have a direct correlation to our ongoing or future business operations.
(4)The charges represent incremental costs of complying with the new European Union (E.U.) medical device regulations for previously registered products and primarily include charges for contractors supporting the project and other direct third-party expenses. We consider these costs to be duplicative of previously incurred costs and/or one-time costs, which are limited to a specific time period.
(5)Reflects the recognition of incremental Italian payback accruals resulting from the two July 22, 2024 rulings by the Constitutional Court of Italy relating to certain prior years since 2015.
(6)The charge relates to an income tax reserve adjustment associated with the June 2023, Israeli Central-Lod District Court decision, the establishment of a valuation allowance against certain net operating losses and amortization of previously established deferred tax assets from intercompany intellectual property transactions.
Free Cash Flow
Free cash flow, a non-GAAP financial measure, is calculated by subtracting additions to property, plant, and equipment from net cash provided by operating activities. Management uses this non-GAAP financial measure, in addition to U.S. GAAP financial measures, to evaluate our operating results. Free cash flow should be considered supplemental to, and not a substitute for, our reported financial results prepared in accordance with U.S. GAAP. Reconciliations between net cash provided by operating activities (the most comparable U.S. GAAP measure) and free cash flow are as follows:
| | | | | | | | | | | |
| Six months ended |
| (in millions) | October 25, 2024 | | October 27, 2023 |
| Net cash provided by operating activities | $ | 1,944 | | $ | 1,536 |
| Additions to property, plant, and equipment | (924) | | (815) |
| Free cash flow | $ | 1,020 | | $ | 721 |
Refer to the Summary of Cash Flows section for drivers of the change in cash provided by operating activities.
NET SALES
Segment and Division
Certain prior period net sales has been recast to reflect the new reporting structure in the fourth quarter of fiscal year 2024. Refer to Note 17 to the consolidated financial statements for additional information regarding the Company's reporting structure. In addition, starting in the first quarter of fiscal year 2025, the Company combined the non-U.S. developed markets and the emerging markets into an international market geography. Prior period net sales has been recast to conform to the new presentation. The charts below illustrate the percent of net sales by segment for the three months ended October 25, 2024 and October 27, 2023:
The table below illustrates net sales by segment and division for the three and six months ended October 25, 2024 and October 27, 2023:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Three months ended | | | | Six months ended | | |
| (in millions) | October 25, 2024 | | October 27, 2023 | | % Change | | October 25, 2024 | | October 27, 2023 | | % Change |
| Cardiac Rhythm & Heart Failure | $ | 1,578 | | | $ | 1,492 | | | 6 | % | | $ | 3,114 | | | $ | 2,938 | | | 6 | % |
| Structural Heart & Aortic | 881 | | | 819 | | | 8 | | | 1,736 | | | 1,633 | | | 6 | |
| Coronary & Peripheral Vascular | 643 | | | 613 | | | 5 | | | 1,259 | | | 1,202 | | | 5 | |
| Cardiovascular | 3,102 | | | 2,923 | | | 6 | | | 6,108 | | | 5,773 | | | 6 | |
| Cranial & Spinal Technologies | 1,234 | | | 1,157 | | | 7 | | | 2,382 | | | 2,260 | | | 5 | |
| Specialty Therapies | 737 | | | 705 | | | 5 | | | 1,450 | | | 1,400 | | | 4 | |
| Neuromodulation | 480 | | | 426 | | | 13 | | | 937 | | | 846 | | | 11 | |
| Neuroscience | 2,451 | | | 2,288 | | | 7 | | | 4,768 | | | 4,506 | | | 6 | |
| Surgical & Endoscopy | 1,649 | | | 1,641 | | | 1 | | | 3,193 | | | 3,187 | | | — | |
| Acute Care & Monitoring | 478 | | | 462 | | | 4 | | | 930 | | | 921 | | | 1 | |
| Medical Surgical | 2,128 | | | 2,103 | | | 1 | | | 4,123 | | | 4,107 | | | — | |
| Diabetes | 686 | | | 610 | | | 12 | | | 1,333 | | | 1,189 | | | 12 | |
| Reportable segment net sales | 8,366 | | | 7,923 | | | 6 | | | 16,333 | | | 15,575 | | | 5 | |
Other operating segment(1) | 37 | | | 61 | | | (39) | | | 75 | | | 111 | | | (32) | |
Other adjustments(2) | — | | | — | | | — | | | (90) | | | — | | | 100 | |
| Total net sales | $ | 8,403 | | | $ | 7,984 | | | 5 | % | | $ | 16,318 | | | $ | 15,686 | | | 4 | % |
(1)Includes the historical operations and ongoing transition agreements from businesses the Company has exited or divested.
(2)Incremental Italian payback accruals resulting from the two July 22, 2024 rulings by the Constitutional Court of Italy relating to certain prior years since 2015.
Segment and Market Geography
The charts below illustrate the percent of net sales by market geography for the three months ended October 25, 2024 and October 27, 2023:
The table below includes net sales by market geography for each of our segments for the three and six months ended October 25, 2024 and October 27, 2023:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | U.S.(1) | | International(2) | | |
| Three months ended | | Three months ended | | |
| (in millions) | October 25, 2024 | | October 27, 2023 | | % Change | | October 25, 2024 | | October 27, 2023 | | % Change | | | | | | |
| Cardiovascular | $ | 1,434 | | | $ | 1,427 | | | 1 | % | | $ | 1,668 | | | $ | 1,496 | | | 12 | % | | | | | | |
| Neuroscience | 1,677 | | | 1,560 | | | 8 | | | 774 | | | 728 | | | 6 | | | | | | | |
| Medical Surgical | 944 | | | 948 | | | — | | | 1,183 | | | 1,155 | | | 3 | | | | | | | |
| Diabetes | 232 | | | 217 | | | 7 | | | 455 | | | 394 | | | 16 | | | | | | | |
| Reportable segment net sales | 4,286 | | | 4,151 | | | 3 | | | 4,080 | | | 3,772 | | | 8 | | | | | | | |
Other operating segment(3) | 18 | | | 23 | | | (22) | | | 19 | | | 37 | | | (49) | | | | | | | |
| | | | | | | | | | | | | | | | | |
| Total net sales | $ | 4,304 | | | $ | 4,175 | | | 3 | % | | $ | 4,099 | | | $ | 3,809 | | | 8 | % | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| | U.S.(1) | | International(2) | | |
| Six months ended | | Six months ended | | |
| (in millions) | October 25, 2024 | | October 27, 2023 | | % Change | | October 25, 2024 | | October 27, 2023 | | % Change | | | | | | |
| Cardiovascular | $ | 2,836 | | | $ | 2,776 | | | 2 | % | | $ | 3,272 | | | $ | 2,996 | | | 9 | % | | | | | | |
| Neuroscience | 3,242 | | | 3,057 | | | 6 | | | 1,526 | | | 1,449 | | | 5 | | | | | | | |
| Medical Surgical | 1,825 | | | 1,815 | | | 1 | | | 2,298 | | | 2,292 | | | — | | | | | | | |
| Diabetes | 447 | | | 405 | | | 10 | | | 886 | | | 784 | | | 13 | | | | | | | |
| Reportable segment net sales | 8,350 | | | 8,054 | | | 4 | | | 7,983 | | | 7,521 | | | 6 | | | | | | | |
Other operating segment(3) | 37 | | | 45 | | | (19) | | | 38 | | | 66 | | | (42) | | | | | | | |
Other adjustments(4) | — | | | — | | | — | | | (90) | | | — | | | 100 | | | | | | | |
| Total net sales | $ | 8,387 | | | $ | 8,099 | | | 4 | % | | $ | 7,931 | | | $ | 7,587 | | | 5 | % | | | | | | |
(1)U.S. includes the United States and U.S. territories.
(2)Includes all other non-U.S. countries and U.S. territories.
(3)Includes historical operations and ongoing transition agreements from businesses the Company has exited or divested.
(4)Incremental Italian payback accruals resulting from the two July 22, 2024 rulings by the Constitutional Court of Italy relating to certain prior years since 2015.
The increase in net sales for the three and six months ended October 25, 2024, as compared to the corresponding periods in the prior fiscal year, was driven primarily by growth in most businesses, including strong growth in Diabetes, Cranial & Spinal Technologies, Neuromodulation, TAVR, Cardiac Rhythm Management, and Cardiac Surgery. The increase in net sales for the six months ended October 25, 2024, was partially offset by the $90 million incremental Italian payback accruals resulting from the two July 22, 2024 rulings by the Constitutional Court of Italy relating to certain prior years since 2015.
Looking ahead, a number of macro-economic and geopolitical factors could negatively impact our business, including without limitation:
•Competitive product launches and pricing pressure, geographic macro-economic developments including changes in global trade policies and fluctuations in currency exchange rates, general price inflation, changes in interest rates, reimbursement challenges, impacts from changes in the mix of our product offerings, delays in product registration approvals, replacement cycle challenges, and supply chain challenges from time to time;
•National and provincial tender pricing for certain products, particularly in China;
•The sanctions and other measures being imposed in response to the Russia-Ukraine conflict are having, and could continue to have impacts on revenue and supply chain. The financial impact of the conflict in the second quarter of fiscal year 2025, including on accounts receivable and inventory reserves, was not material, and for the three and six months ended October 25, 2024, the business of the Company in these countries represented less than 1% of the Company's consolidated revenues and assets. Although the implications of this conflict are difficult to predict at this time, the ongoing conflict may increase pressure on the global economy and supply chains, resulting in increased future volatility risk for our business operations and performance.
•Although the long-term implications of Israel's recent conflicts are difficult to predict at this time, the financial impact of the conflicts in the second quarter of fiscal year 2025, including on accounts receivable and inventory reserves, was not material. As of October 25, 2024, the Company had 6 facilities and approximately 1,500 employees in Israel. For the three and six months ended October 25, 2024, the business of the Company in Israel represented less than 1% of the Company's consolidated revenues and assets.
Cardiovascular
Cardiovascular products include pacemakers, insertable cardiac monitors, cardiac resynchronization therapy devices, implantable cardioverter defibrillators, leads and delivery systems, products for the treatment of atrial fibrillation, information systems for the management of patients with Cardiac Rhythm & Heart Failure devices, products designed to reduce surgical site infections, coronary and peripheral stents and related delivery systems, balloons and related delivery systems, endovascular stent graft systems, heart valve replacement technologies, cardiac tissue ablation systems, and open heart and coronary bypass grafting surgical products. Cardiovascular also includes Care Management Services and Cath Lab Managed Services (CLMS) within the Cardiac Rhythm & Heart Failure division. Cardiovascular's net sales for the three and six months ended October 25, 2024 were $3.1 billion and $6.1 billion, an increase of 6 percent for both periods as compared to the corresponding periods in the prior fiscal year. The net sales increase was primarily due to strong performance of TAVR, Cardiac Rhythm Management, and Cardiac Surgery.
The graphs below illustrate the percent of Cardiovascular net sales by division for the three months ended October 25, 2024 and October 27, 2023:
Cardiac Rhythm & Heart Failure (CRHF) net sales for the three and six months ended October 25, 2024 increased 6 percent for both periods as compared to the corresponding periods in the prior fiscal year. The net sales increase was driven by Cardiac Pacing Therapies and Defibrillation Solutions with growth in Micra transcatheter pacing systems, Aurora extravascular implantable cardioverter defibrillator (EV-ICD) system, and TRYX. Cardiac Ablation Solutions experienced strong growth in PulseSelect Pulsed Field Ablation with offsetting declines in cryoablation.
Structural Heart & Aortic (SHA) net sales for the three and six months ended October 25, 2024 increased 8 percent and 6 percent, respectively, as compared to the corresponding periods in the prior fiscal year. The net sales increase was driven by U.S. and international growth in Structural Heart from adoption of Evolut FX+ TAVR system and in Cardiac Surgery driven by growth in Perfusion and Surgical Valves.
Coronary & Peripheral Vascular (CPV) net sales for the three and six months ended October 25, 2024 increased 5 percent for both periods as compared to the corresponding periods in the prior fiscal year. The increase in net sales was driven by growth in Coronary led by guide catheters and balloons, in addition to growth in Peripheral Vascular and Vascular Embolization.
In addition to the macro-economic and geopolitical factors described in the Executive Level Overview, looking ahead, we expect Cardiovascular could be affected by the following:
•Continued global penetration of our Micra transcatheter pacing portfolio.
•Continued acceptance and growth from the Azure XT and Azure S SureScan pacing systems and the 3830 lead.
•Global adoption of Aurora Extravascular ICD.
•Growth of the Cobalt and Crome portfolio of ICDs and CRT-Ds.
•Growth of the CRT-P quadripolar pacing system.
•Continued growth, adoption, and utilization of the TYRX Envelope for implantable devices.
•Continued use and acceptance of Reveal LINQ and expansion of the LINQ II cardiac monitor.
•Continued acceptance, adoption, and growth of our innovative portfolio of products in the electrophysiology (EP) segment, including the Arctic Front cryoablation system, PulseSelect PFA, and Affera mapping and ablation system. The Affera mapping and ablation system and Sphere-9 catheter received U.S. FDA approval in late October 2024.
•Continued acceptance and growth of the self-expanding CoreValve Evolut transcatheter aortic valve replacement platform. This includes Evolut PRO which provides enhanced hemodynamics, reliable delivery, enhanced durability, advanced sealing, and Evolut FX, a system designed to improve the overall procedural experience through enhancements in deliverability, implant visibility, and deployment stability. The Evolut FX+ TAVR system maintains the valve performance benefits of the legacy Evolut TAVR platform and is designed to facilitate coronary access. The system was approved by the U.S. FDA in March 2024 and received CE Mark in late October 2024.
•Market acceptance and reimbursement for the Symplicity Spyral renal denervation system, also known as the Symplicity blood pressure procedure, for the treatment of hypertension.
•Continued acceptance and growth of the Onyx Frontier DES platform. Onyx Frontier is a DES that introduces an enhanced delivery system and is used for complex percutaneous coronary intervention (PCI).
•Acceptance and growth of IN.PACT 018 drug-coated balloons (DCB). IN.PACT 018 adds to the existing IN.PACT Admiral DCB portfolio and is used to treat femoropopliteal disease.
•Our ability to meet growing demand for our existing products and to successfully develop, obtain regulatory approval of and commercialize the products within our pipeline.
Neuroscience
Neuroscience's products include various spinal implants, bone graft substitutes, biologic products, image-guided surgery and intra-operative imaging systems, robotic guidance systems used in the robot-assisted spine procedures, and systems that incorporate advanced energy surgical instruments. Neuroscience's products also focus on therapies to treat the diseases of the vasculature in and around the brain, including coils, neurovascular stents, and flow diversion products, as well as products to treat ear, nose, and throat (ENT), and the treatment of overactive bladder and urinary retention. Neuroscience also manufactures products related to implantable neurostimulation therapies and drug delivery systems for the treatment of chronic pain, movement disorders, and epilepsy. Neuroscience’s net sales for the three and six months ended October 25, 2024 were $2.5 billion and $4.8 billion, respectively, an increase of 7 percent and 6 percent, respectively, as compared to the corresponding periods in the prior fiscal year. The net sales increase for both periods was primarily due to growth in Neuromodulation, Spine and Biologics, Neurosurgery, and Hemorrhagic Stroke.
The graphs below illustrate the percent of Neuroscience net sales by division for the three months ended October 25, 2024 and October 27, 2023:
Cranial & Spinal Technologies (CST) net sales for the three and six months ended October 25, 2024 increased 7 percent and 5 percent, respectively, as compared to the corresponding periods in the prior fiscal year. The net sales increase was driven by the continued adoption of the AiBLE ecosystem of spine implants and enabling technology with growth in Core Spine, Biologics, and Neurosurgery.
Specialty Therapies (Specialty) net sales for the three and six months ended October 25, 2024 increased 5 percent and 4 percent, respectively, as compared to the corresponding periods in the prior fiscal year. The net sales increase was driven by hemorrhagic stroke products, growth on continued adoption of the Interstim X system, and ENT.
Neuromodulation (NM) net sales for the three and six months ended October 25, 2024 increased 13 percent and 11 percent, respectively, as compared to the corresponding periods in the prior fiscal year. The net sales increase was driven by the continued launch of both the Inceptiv spinal cord stimulator in the U.S. and the Percept RC deep brain neurostimulator.
In addition to the macro-economic and geopolitical factors described in the Executive Level Overview, looking ahead we expect Neuroscience could be affected by the following:
•Continued adoption and growth of our integrated solutions through the AiBLE offering, which integrates spinal implants with enabling technologies (StealthStation, O-arm Imaging Systems, and Midas), Mazor robotics, and UNiD Adaptive Spine Intelligence AI-driven technology for surgical planning and personalized spinal implants.
•Market acceptance and continued global adoption of innovative new spine products and procedural solutions within our CST operating unit, such as Catalyft PL, ModuLeX, CD Horizon Voyager System, and our Infinity OCT System, as well as continued growth from Titan spine titanium interbody implants with Nanolock technology.
•Continued growth of Pipeline Embolization Devices, endovascular treatments for large or giant wide-necked brain aneurysms.
•Continued acceptance and growth of the Solitaire X revascularization device for treatment of acute ischemic stroke and our React Catheter and Riptide aspiration system.
•Continued acceptance and growth of our Pelvic Health therapies, including our InterStim therapy with InterStim X and InterStim II recharge-free neurostimulators and InterStim Micro rechargeable neurostimulator for patients suffering from overactive bladder, (non-obtrusive) urinary retention, and chronic fecal incontinence.
•Continued acceptance and growth of our ENT therapies, including capital equipment sales of the StealthStation ENT surgical navigation system and intraoperative NIM nerve monitoring system, and the Propel sinus implants used in the treatment of chronic rhinosinusitis.
•Continued acceptance and growth from spinal cord stimulation (SCS) therapy for treating chronic pain and Diabetic Peripheral Neuropathy (DPN) on the Inceptiv closed-loop rechargeable neurostimulator, Intellis rechargeable neurostimulator and Vanta recharge-free neurostimulator. The Inceptiv closed-loop rechargeable SCS received U.S. FDA approval in April 2024.
•Continued acceptance and growth of our Percept family of deep brain stimulation (DBS) devices with proprietary BrainSense technology for objectifying and personalizing the treatment of Parkinson's Disease, epilepsy, and other movement disorders. In August 2024, the U.S. FDA approved Asleep DBS surgery for people with Parkinson's and people with essential tremor.
•Our ability to meet growing demand for our existing products and to successfully develop, obtain regulatory approval of and commercialize the products within our pipeline, which include hemorrhagic stroke intravascular device and our next-generation spine enabling technologies.
Medical Surgical
Medical Surgical’s products span the entire continuum of patient care from diagnosis to recovery, with a focus on diseases of the gastrointestinal tract, lungs, pelvic region, obesity, and preventable complications. The products include those for advanced and general surgical products, surgical stapling devices, vessel sealing instruments, wound closure, electrosurgery products, hernia mechanical devices, mesh implants, advanced ablation, interventional lung, airway products, and sensors and monitors for pulse oximetry, capnography, level of consciousness and cerebral oximetry. Medical Surgical's net sales for the three and six months ended October 25, 2024 were $2.1 billion and $4.1 billion, an increase of 1 percent and flat, respectively, as compared to the corresponding periods in the prior fiscal year. The net sales were primarily impacted by growth in Advanced Energy and Blood Oxygen Management with partially offsetting declines in Stapling.
The graphs below illustrate the percent of Medical Surgical net sales by division for the three months ended October 25, 2024 and October 27, 2023:
Surgical & Endoscopy (SE) net sales for the three and six months ended October 25, 2024 increased 1 percent and flat, respectively, as compared to the corresponding periods in the prior fiscal year. The net sales for both periods were impacted by growth in Advanced Energy, driven by continued adoption of the LigaSure Maryland XP vessel sealer, and strength in Hernia and Wound Management products. The net sales results were partially offset by declines in Advanced Stapling and Endoscopy.
Acute Care & Monitoring (ACM) net sales for the three and six months ended October 25, 2024 increased 4 percent and 1 percent, respectively, as compared to the corresponding periods in the prior fiscal year. The net sales increase for both periods was largely due to growth in Nellcor pulse oximetry driven by strong sensor sales and continued adoption of the RespArray patient monitor in addition to strength in Bispectral Index monitoring system (BIS).
In addition to the macro-economic and geopolitical factors described in the Executive Level Overview, looking ahead we expect Medical Surgical could be affected by the following:
•Acceptance and continued growth of Open-to-MIS (minimally invasive surgery) techniques and tools through our efforts to transition open surgery to MIS. Open-to-MIS initiative focuses on capturing the market opportunity that exists in transitioning open procedures to MIS, whether through traditional MIS, advanced instrumentation, or robotics. Through our approach, in parallel, we also expand our presence and optimize open surgery in current open surgery markets.
•Continued global acceptance and future growth of powered stapling and energy platform.
•Our ability to execute ongoing strategies addressing the near-term pressures to bariatric surgery procedure volumes in the U.S. from pharmaceuticals, and growth of surgical soft tissue robotics procedures in the U.S.
•Our ability to create markets and drive products and procedures into emerging markets with our high quality and cost-effective surgical products designed for customers in emerging markets.
•Continued acceptance and growth in patient monitoring and airway management. Key products in this area include Microstream Capnography, Nellcor pulse oximetry system with OxiMax technology, Shiley tracheostomy and endotracheal tubes, and McGRATH MAC video laryngoscopes.
•Acceptance of less invasive standards of care in chronic and colorectal, as well as hepatology products, including products that span the care continuum from diagnostics to therapeutics. Recently launched products include GI Genius.
•Expanding the use of less invasive treatments and furthering our commitment to improving options for women with abnormal uterine bleeding. Our expanded and strengthened surgical offerings complement our global gynecology business.
•Global adoption of robotic-assisted surgery and installations of Hugo robotic assisted surgery (RAS) system for urologic, bariatric, gynecologic, hernia, and general surgery procedures. This includes continued integration and adoption of Touch Surgery Enterprise with the first artificial intelligence powered surgical videos and analytics platform to make it easier to train and discover new techniques within the robotics platform. The Hugo RAS system, which received CE Mark in October 2021, as well as secured additional regulatory approvals outside the U.S., is designed to help reduce unwanted variability, improve patient outcomes, and, by extension, lower per procedure cost.
•Our ability to meet growing demand for our existing products and to successfully develop, obtain regulatory approval of and commercialize the products within our pipeline, which include our Hugo RAS system in the U.S., the adoption of AI in Endoscopy, Signia powered stapling devices, and our next-gen Ligasure and Sonicision vessel sealing devices.
Diabetes
Diabetes' products include insulin pumps, continuous glucose monitoring (CGM) systems, and consumables. Diabetes' net sales for the three and six months ended October 25, 2024 were $686 million and $1.3 billion, respectively, an increase of 12 percent for both periods as compared to the corresponding periods in the prior fiscal year. The increase in net sales for both periods was primarily driven by strong U.S growth as a result of the continued adoption of the MiniMed 780G automated insulated delivery (AID) system, and strong international growth in CGM systems from increased attachment rates and adoption of Simplera Sync.
In addition to the macro-economic and geopolitical factors described in the Executive Level Overview, looking ahead we expect Diabetes could be affected by the following:
•Continued acceptance and growth for the MiniMed 780G insulin pump system, which is powered by SmartGuard technology and features the added benefits of meal detection technology that automatically adjusts and corrects sugar levels every five minutes. The global adoption of our AID systems has resulted in strong sensor attachment rates. The MiniMed 780G insulin pump system with the Guardian 4 Sensor is available in the U.S., and the MiniMed 780G insulin pump system with Simplera Sync received CE Mark in early January 2024.
•Continued acceptance and growth of the Guardian Connect CGM system, which displays glucose information directly to a smartphone to provide patients access to their glucose levels seamlessly and discretely. The Guardian Connect CGM system is available on both Apple iOS and Android devices.
•Market acceptance and growth of our sensor Simplera, which received U.S FDA approval in August 2024 and CE Mark in September 2023.
•Market acceptance and growth of our InPen smart pen system, which allows users to have their Medtronic CGM readings in real-time alongside insulin dose information, all in one view.
•Continued pump, CGM, and consumable competition in an expanding global market.
•Changes in medical reimbursement policies and programs, along with additional payor coverage on insulin pumps.
•Our ability to meet growing demand for our existing products and to successfully develop, obtain regulatory approval of and commercialize the products within our pipeline, including our Minimed 780G insulin pump system with Simplera Sync in the U.S., and the products resulting from our partnership with Abbott to expand CGM options for people living with diabetes.
COSTS AND EXPENSES
The following is a summary of cost of products sold, research and development, and selling, general, and administrative expenses as a percent of net sales for the three and six months ended October 25, 2024 and October 27, 2023:
Cost of Products Sold Cost of products sold for the three and six months ended October 25, 2024 was $2.9 billion and $5.7 billion, respectively, as compared to $2.8 billion and $5.4 billion, respectively, for the corresponding periods in the prior fiscal year. The increase in cost products sold as a percentage of net sales for the three and six months ended October 25, 2024 was primarily due to unfavorable currency impact.
Research and Development Expense We remain committed to deliver the best possible experiences for patients, physicians, and caregivers we serve; to create technologies that expand what’s possible across the human body to transform lives; to turn data and insights into real action to serve patient needs, improving care; and to expand healthcare access and deliver positive outcomes. Research and development expense for the three and six months ended October 25, 2024 was $697 million and $1.4 billion, respectively, as compared to $698 million and $1.4 billion, respectively, for the corresponding periods in the prior fiscal year.
Selling, General, and Administrative Expense Our goal is to continue to leverage selling, general, and administrative expense management initiatives. Selling, general, and administrative expense primarily consists of salaries and wages, other administrative costs, such as professional fees and marketing expenses, certain acquisition and divestiture-related costs, and restructuring associated expenses. Selling, general, and administrative expense for the three and six months ended October 25, 2024 was $2.8 billion and $5.4 billion, respectively, as compared to $2.7 billion and $5.3 billion, respectively, for the corresponding periods in the prior fiscal year. The increase in selling, general, and administrative expense for both periods is primarily due to new product launches and commercialization activities.
The following is a summary of other costs and expenses (income): | | | | | | | | | | | | | | | | | | | | | | | |
| Three months ended | | Six months ended |
| (in millions) | October 25, 2024 | | October 27, 2023 | | October 25, 2024 | | October 27, 2023 |
| Amortization of intangible assets | $ | 413 | | | $ | 425 | | | $ | 827 | | | $ | 855 | |
| Restructuring charges, net | 30 | | | 40 | | | 77 | | | 94 | |
| Certain litigation charges, net | — | | | 65 | | | 81 | | | 105 | |
| Other operating income, net | (34) | | | (31) | | | (33) | | | (30) | |
| Other non-operating income, net | (173) | | | (154) | | | (330) | | | (230) | |
| Interest expense, net | 209 | | | 180 | | | 376 | | | 329 | |
Amortization of Intangible Assets Amortization of intangible assets includes the amortization expense of our definite-lived intangible assets, consisting of purchased patents, trademarks, tradenames, customer relationships, purchased technology, and other intangible assets.
Restructuring Charges, Net For the three and six months ended October 25, 2024 and October 27, 2023, restructuring costs primarily related to employee termination benefits and facility consolidations to support cost reduction initiatives.
For additional information about our restructuring activities, refer to Note 5 to the current period's consolidated financial statements.
Certain Litigation Charges, Net We classify specified certain litigation charges and gains related to significant legal matters as certain litigation charges, net in the consolidated statements of income. For additional information, refer to Note 16 in the current period's consolidated financial statements.
Other Operating Income, Net Other operating income, net primarily includes expenses associated with royalties paid for the in-license of intellectual property from third parties, currency remeasurement and derivative gains and losses, changes in the fair value of contingent consideration, certain acquisition and divestiture-related items, and income from funded research and development arrangements.
For the three and six months ended October 25, 2024, the increase in other operating income, net was largely driven by insignificant gains from certain business or asset sales in the Cardiovascular and Neuroscience Portfolio. The increase in other operating, income net was partially offset by the net impact of currency remeasurement and our hedging programs, which resulted in net expense of $39 million and $44 million for the three and six months ended October 25, 2024, respectively, as compared to a net gain of $32 million and $29 million, respectively, in the corresponding periods in the prior fiscal year.
Other Non-Operating Income, Net Other non-operating income, net includes the non-service component of net periodic pension and postretirement benefit cost, investment gains and losses, and interest income.
For the three months and six months ended October 25, 2024, the increase in other non-operating income, net is primarily attributable to an increase in net gains on our minority investment portfolio. Net gains on minority investments were $10 million and $27 million for the three and six months ended October 25, 2024, respectively, compared to net losses of $25 million and $89 million for the three and six months ended October 27, 2023, respectively.
Interest Expense, Net Interest expense, net includes interest incurred on our outstanding borrowings, amortization of debt issuance costs and debt premiums or discounts, and amortization of amounts excluded from the effectiveness assessment of certain net investment and fair value hedges.
For the three and six months ended October 25, 2024, the increase in interest expense, net was primarily driven by the €3.0 billion debt issuance on June 3, 2024.
INCOME TAXES
| | | | | | | | | | | | | | | | | | | | | | | |
| Three months ended | | Six months ended |
| (in millions) | October 25, 2024 | | October 27, 2023 | | October 25, 2024 | | October 27, 2023 |
| Income tax provision | $ | 281 | | | $ | 402 | | | $ | 500 | | | $ | 802 | |
| Income before income taxes | 1,559 | | | 1,313 | | | 2,827 | | | 2,510 | |
| Effective tax rate | 18.0 | % | | 30.6 | % | | 17.7 | % | | 32.0 | % |
| | | | | | | |
| Non-GAAP income tax provision | $ | 366 | | | $ | 339 | | | $ | 693 | | | $ | 640 | |
| Non-GAAP income before income taxes | 1,995 | | | 2,008 | | | 3,921 | | | 3,910 | |
| Non-GAAP Nominal Tax Rate | 18.3 | % | | 16.9 | % | | 17.7 | % | | 16.4 | % |
| | | | | | | |
| Difference between the effective tax rate and Non-GAAP Nominal Tax Rate | 0.3 | % | | (13.7) | % | | — | % | | (15.6) | % |
The Organization for Economic Co-operation and Development (OECD) published Pillar Two Model Rules defining the global minimum tax, which calls for the taxation of large multinational corporations at a minimum rate of 15% in each jurisdiction in which the group operates. The OECD has since issued administrative guidance providing transition and safe harbor rules around the implementation of the Pillar Two global minimum tax. A number of countries, including Ireland, have enacted legislation to implement the core elements of Pillar Two, which are effective for Medtronic in fiscal year 2025. We will continue to monitor the impacts of further legislation, regulatory guidance, and regulations issued in the countries in which we do business.
The Israeli Central-Lod District Court issued its decision in Medtronic Ventor Technologies Ltd (Ventor) v. Kfar Saba Assessing Office on June 1, 2023. The court determined that there was a deemed taxable transfer of intellectual property. As a result, the Company recorded a $187 million income tax charge during the first quarter of fiscal year 2024 and has filed an appeal with the Supreme Court of Israel.
Our effective tax rate for the three and six months ended October 25, 2024 was 18.0% and 17.7%, respectively, as compared to 30.6% and 32.0% for the three and six months ended October 27, 2023, respectively. The decrease in our effective tax rate for the three months ended October 25, 2024 primarily relates to the establishment of a valuation allowance on certain net operating losses recorded during the three months ended October 27, 2023, which was partially offset by the implementation of the Pillar Two global minimum tax. In addition to the items discussed in the current quarter, the decrease in the effective tax rate for the six months ended October 25, 2024 was also attributable to an income tax reserve adjustment made during the six months ended October 27, 2023 associated with the Ventor court decision noted above.
Our Non-GAAP Nominal Tax Rate for the three and six months ended October 25, 2024 was 18.3% and 17.7%, respectively, as compared to 16.9% and 16.4% for the three and six months ended October 27, 2023, respectively. The change in our Non-GAAP Nominal Tax Rate was primarily due to the implementation of the Pillar Two global minimum tax and year-over-year changes in operational results by jurisdiction. An increase in our Non-GAAP Nominal Tax Rate of 1 percent would result in an additional income tax provision for the three and six months ended October 25, 2024 of approximately $20 million and $39 million, respectively.
LIQUIDITY AND CAPITAL RESOURCES
We are currently in a strong financial position, and we believe our balance sheet and liquidity as of October 25, 2024 provide us with flexibility, and our cash, cash equivalents, and current investments, along with our credit facility and related commercial paper programs will satisfy our foreseeable operating needs.
Our liquidity and capital structure are evaluated regularly within the context of our annual operating and strategic planning processes. We consider the liquidity necessary to fund our operations, which includes working capital needs, investments in research and development, property, plant, and equipment, and other operating costs. We also consider capital allocation alternatives that balance returning value to shareholders through dividends and share repurchases, satisfying maturing debt, and acquiring businesses and technology.
Summary of Cash Flows
The following is a summary of cash provided by (used in) operating, investing, and financing activities, the effect of exchange rate changes on cash and cash equivalents, and the net change in cash and cash equivalents:
| | | | | | | | | | | |
| | Six months ended |
| (in millions) | October 25, 2024 | | October 27, 2023 |
| Cash provided by (used in): | | | |
| Operating activities | $ | 1,944 | | | $ | 1,536 | |
| Investing activities | (604) | | | (963) | |
| Financing activities | (1,265) | | | (591) | |
| Effect of exchange rate changes on cash and cash equivalents | 35 | | | (214) | |
| Net change in cash and cash equivalents | $ | 110 | | | $ | (232) | |
Operating Activities The $408 million increase in net cash provided was primarily driven by an increase in cash collected from customers due to an increase in sales and a decrease in cash paid to vendors, which was partially offset by an increase in cash paid for taxes and increased annual incentive payouts.
Investing Activities The $359 million decrease in cash used was primarily attributable to an increase in net sales and maturities of investments of $386 million partially offset by an increase in net additions to property, plant, and equipment of $109 million.
Financing Activities There was a $674 million increase in net cash used during the six months ended October 25, 2024, as compared to the corresponding period in the prior fiscal year. In the current period, there was a decrease in total short-term borrowings of $67 million, as compared to an increase of $1.3 billion in the prior year. Additionally, on June 3, 2024, Medtronic Inc. issued four tranches of EUR-denominated Senior Notes with an aggregate principal of €3.0 billion, or $3.2 billion, which was partially offset by a $2.3 billion increase in net share repurchases during the six months ended October 25, 2024, as compared to the corresponding period in the prior fiscal year. For more information on commercial paper and Senior Notes issued, refer to the Debt and Capital section below.
Debt and Capital
Our capital structure consists of equity and interest-bearing debt. We primarily utilize unsecured senior debt obligations to meet our financing needs and, to a lesser extent, bank borrowings. From time to time, we may repurchase our outstanding debt obligations in the open market or through privately negotiated transactions.
Total debt at October 25, 2024 was $28.3 billion as compared to $25.0 billion at April 26, 2024. The increase in total debt was primarily driven by issuance of Euro-denominated Senior Notes and fluctuations in exchange rates.
On June 3, 2024, Medtronic Inc. issued four tranches of EUR-denominated Senior Notes with an aggregate principal of €3.0 billion, with maturities ranging from fiscal year 2030 to 2054, resulting in cash proceeds of approximately $3.2 billion, net of discounts and issuance costs. In anticipation of the Euro-denominated debt issuance, the Company entered into forward currency exchange rate contracts to manage the exposure to exchange rate movements. These contracts were settled in conjunction with the issuance of the June 2024 Notes.
We repurchase our ordinary shares on occasion as part of our focus on returning value to our shareholders. In March 2024, the Company's Board of Directors authorized the repurchase of $5.0 billion of the Company's ordinary shares. There is no specific time period associated with these repurchase authorizations. During the six months ended October 25, 2024, the Company repurchased a total of 33 million shares under this program at an average price of $82.61. At October 25, 2024, we had approximately $2.6 billion remaining under the share repurchase program authorized by our Board of Directors.
For more information on credit arrangements, refer to Note 7 to the current period's consolidated financial statements and Note 6 to the consolidated financial statements included in our Annual Report on Form 10-K for the fiscal year ended April 26, 2024.
Liquidity
Our liquidity sources at October 25, 2024 included $1.4 billion of cash and cash equivalents and $6.6 billion of current investments. Additionally, we maintain commercial paper programs and a Credit Facility.
Our investments primarily include available-for-sale debt securities, including U.S. and non-U.S. government and agency securities, corporate debt securities, mortgage-backed securities, and other asset-backed securities. Refer to Note 6 to the current period's consolidated financial statements for additional information regarding fair value measurements.
We maintain multicurrency commercial paper programs for short-term financing, which allow us to issue unsecured commercial paper notes on a private placement basis up to a maximum aggregate amount outstanding at any time of $3.5 billion. At October 25, 2024 and April 26, 2024, we had $899 million and $1.1 billion commercial paper outstanding, respectively. The issuance of commercial paper reduces the amount of credit available under our existing line of credit, as explained below.
We also have a $3.5 billion five-year syndicated credit facility (Credit Facility), which expires in December 2028. At each anniversary date of the Credit Facility we can request a one-year extension of the maturity date. The Credit Facility provides backup funding for the commercial paper programs and may also be used for general corporate purposes. The Credit Facility provides us with the ability to increase our borrowing capacity by an additional $1.0 billion at any time during the term of the agreement. At October 25, 2024 and April 26, 2024, no amounts were outstanding under the Credit Facility.
Interest rates on advances of our Credit Facility are determined by a pricing matrix based on our long-term debt ratings assigned by Standard & Poor's Ratings Services (S&P) and Moody's Investors Service (Moody’s). Facility fees are payable on the Credit Facility and are determined in the same manner as the interest rates. We are in compliance with all covenants related to the Credit Facility.
The following table is a summary of our S&P and Moody's long-term debt ratings and short-term debt ratings:
| | | | | | | | | | | | | | |
| | Agency Rating(1) |
| | October 25, 2024 | | April 26, 2024 |
| Standard & Poor's Ratings Services | | | | |
| Long-term debt | | A | | A |
| Short-term debt | | A-1 | | A-1 |
| | | | |
| Moody's Investors Service | | | | |
| Long-term debt | | A3 | | A3 |
| Short-term debt | | P-2 | | P-2 |
(1) Agency ratings are subject to change, and there may be no assurance that an agency will continue to provide ratings and/or maintain its current ratings. A security rating is not a recommendation to buy, sell or hold securities, and may be subject to revision or withdrawal at any time by the rating agency, and each rating should be evaluated independently of any other rating.
S&P and Moody's long-term debt ratings and short-term debt ratings at October 25, 2024 were unchanged as compared to the ratings at April 26, 2024. We do not expect the S&P and Moody's ratings to have a significant impact on our liquidity or future flexibility to access additional liquidity given our balance sheet, Credit Facility, and related commercial paper programs.
We have future contractual obligations and other minimum commercial commitments that are entered into in the normal course of business. We believe our off-balance sheet arrangements do not have a material current or anticipated future effect on our consolidated earnings, financial position, and/or cash flows. Refer to the Debt and Capital section above for changes in debt obligations during the first quarter of fiscal year 2025; there have been no other material changes to our long-term contractual obligations as reported in our most recent Annual Report filed on Form 10-K for the fiscal year ended April 26, 2024.
ACQUISITIONS AND DISPOSITIONS
Information regarding acquisitions and dispositions activity is included in Note 4 to the current period's consolidated financial statements.
GOODWILL
We assess goodwill and indefinite-lived intangible assets for impairment annually in the third quarter of the fiscal year and whenever an event occurs or circumstances change that would indicate the carrying amount may be impaired.
The Company calculates the excess of each reporting unit's fair value over its carrying amount, including goodwill, utilizing a discounted cash flow analysis and revenue and earnings multiples using comparable public company information. The test for impairment of goodwill requires the Company to make several estimates related to projected future cash flows and appropriate multiples to determine the fair value of the goodwill reporting units. Significant assumptions used in the reporting unit fair value measurements include forecasted cash flows, including revenue and expense growth rates, discount rates, and revenue and earnings multiples. An impairment loss is recognized when the carrying amount of the reporting unit’s net assets exceeds the estimated fair value of the reporting unit.
Definite-lived intangible assets are tested for impairment whenever events or changes in circumstances indicate that the carrying amount of an intangible asset (asset group) may not be recoverable. There were no impairments of intangible assets in the current period. Further adverse changes to macroeconomic conditions or significant changes to our current and future expected financial performance could lead to goodwill or intangible asset impairment charges in future periods, and such charges could be material to our results of operations.
CRITICAL ACCOUNTING ESTIMATES
We have used various accounting policies to prepare the consolidated financial statements in accordance with U.S. GAAP. Our significant accounting policies are disclosed in Note 1 to the consolidated financial statements included in our Annual Report on Form 10-K for the fiscal year ended April 26, 2024.
The preparation of the consolidated financial statements, in conformity with U.S. GAAP, requires us to use judgment in making estimates and assumptions that affect the reported amounts of assets, liabilities, revenues, and expenses. These estimates reflect our best judgment about economic and market conditions and the potential effects on the valuation and/or carrying value of assets and liabilities based upon relevant information available. We base our estimates on historical experience and on various assumptions that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources.
As of October 25, 2024, there were no material changes to our critical accounting estimates.
NEW ACCOUNTING PRONOUNCEMENTS
Information regarding new accounting pronouncements is included in Note 2 to the current period's consolidated financial statements.
SUPPLEMENTAL GUARANTOR FINANCIAL INFORMATION
Medtronic plc and Medtronic Global Holdings S.C.A. (Medtronic Luxco), a wholly-owned subsidiary guarantor, each have provided full and unconditional guarantees of the obligations of Medtronic, Inc., a wholly-owned subsidiary issuer, under the Senior Notes (Medtronic Senior Notes) and full and unconditional guarantees of the obligations of Covidien International Finance S.A. (CIFSA), a wholly-owned subsidiary issuer, under the Senior Notes (CIFSA Senior Notes). The guarantees of the CIFSA Senior Notes are in addition to the guarantees of the CIFSA Senior Notes by Covidien Ltd. and Covidien Group Holdings Ltd., both of which are wholly-owned subsidiary guarantors of the CIFSA Senior Notes. Medtronic plc and Medtronic, Inc. each have provided a full and unconditional guarantee of the obligations of Medtronic Luxco under the Senior Notes (Medtronic Luxco Senior Notes). The following is a summary of these guarantees:
Guarantees of Medtronic Senior Notes
•Parent Company Guarantor – Medtronic plc
•Subsidiary Issuer – Medtronic, Inc.
•Subsidiary Guarantor – Medtronic Luxco
Guarantees of Medtronic Luxco Senior Notes
•Parent Company Guarantor – Medtronic plc
•Subsidiary Issuer – Medtronic Luxco
•Subsidiary Guarantor – Medtronic, Inc.
Guarantees of CIFSA Senior Notes
•Parent Company Guarantor – Medtronic plc
•Subsidiary Issuer – CIFSA
•Subsidiary Guarantors – Medtronic Luxco, Covidien Ltd., and Covidien Group Holdings Ltd. (CIFSA Subsidiary Guarantors)
The following tables present summarized financial information for the six months ended October 25, 2024 and summarized balance sheet information at October 25, 2024 and April 26, 2024 for the obligor groups of Medtronic and Medtronic Luxco Senior Notes, and CIFSA Senior Notes. The obligor group consists of the parent company guarantor, subsidiary issuer, and subsidiary guarantors for the applicable senior notes. The summarized financial information is presented after elimination of (i) intercompany transactions and balances among the guarantors and issuers and (ii) equity in earnings from and investments in any subsidiary that is a non-guarantor or issuer.
The summarized results of operations information for the six months ended October 25, 2024 was as follows:
| | | | | | | | | | | |
| (in millions) | Medtronic & Medtronic Luxco Senior Notes (1) | | CIFSA Senior Notes (2) |
| Net sales | $ | 1,373 | | | $ | — | |
| Operating profit | 173 | | | 98 | |
| (Loss) income before income taxes | (181) | | | 113 | |
| Net (loss) income attributable to Medtronic | (151) | | | 118 | |
The summarized balance sheet information at October 25, 2024 was as follows:
| | | | | | | | | | | |
| (in millions) | Medtronic & Medtronic Luxco Senior Notes (1) | | CIFSA Senior Notes (2) |
Total current assets(3) | $ | 15,037 | | | $ | 4,146 | |
Total noncurrent assets(4) | 11,462 | | | 5,338 | |
Total current liabilities(5) | 22,215 | | | 5,858 | |
Total noncurrent liabilities(6) | 37,793 | | | 24,463 | |
| Noncontrolling interests | 222 | | | 222 | |
(1)The Medtronic Senior Notes and Medtronic Luxco Senior Notes obligor group consists of the following entities: Medtronic plc, Medtronic Luxco, and Medtronic, Inc. Refer to the guarantee summary above for further details.
(2)The CIFSA Senior Notes obligor group consists of the following entities: Medtronic plc, Medtronic Luxco, CIFSA, and CIFSA Subsidiary Guarantors. Refer to the guarantee summary above for further details.
(3)Includes receivables due from non-guarantor subsidiaries of $12.0 billion and $1.7 billion for Medtronic & Medtronic Luxco Senior Notes, and CIFSA Senior Notes, respectively.
(4)Includes loans receivable due from non-guarantor subsidiaries of $5.2 billion and $5.2 billion for Medtronic & Medtronic Luxco Senior Notes, and CIFSA Senior Notes, respectively.
(5)Includes payables due to non-guarantor subsidiaries of $16.7 billion and $2.1 billion for Medtronic & Medtronic Luxco Senior Notes, and CIFSA Senior Notes, respectively.
(6)Includes loans payable due to non-guarantor subsidiaries of $11.3 billion and $7.7 billion for Medtronic & Medtronic Luxco Senior Notes, and CIFSA Senior Notes, respectively.
The summarized balance sheet information at April 26, 2024 was as follows:
| | | | | | | | | | | |
| (in millions) | Medtronic & Medtronic Luxco Senior Notes (1) | | CIFSA Senior Notes (2) |
Total current assets(3) | $ | 17,389 | | | $ | 4,179 | |
Total noncurrent assets(4) | 11,548 | | | 19,246 | |
Total current liabilities(5) | 25,228 | | | 43,416 | |
Total noncurrent liabilities(6) | 33,508 | | | 26,995 | |
| Noncontrolling interests | 206 | | | 206 | |
(1)The Medtronic Senior Notes and Medtronic Luxco Senior Notes obligor group consists of the following entities: Medtronic plc, Medtronic Luxco, and Medtronic, Inc. Refer to the guarantee summary above for further details.
(2)The CIFSA Senior Notes obligor group consists of the following entities: Medtronic plc, Medtronic Luxco, CIFSA, and CIFSA Subsidiary Guarantors. Refer to the guarantee summary above for further details.
(3)Includes receivables due from non-guarantor subsidiaries of $14.3 billion and $1.7 billion for Medtronic & Medtronic Luxco Senior Notes, and CIFSA Senior Notes, respectively.
(4)Includes loans receivable due from non-guarantor subsidiaries of $5.2 billion and $19.1 billion for Medtronic & Medtronic Luxco Senior Notes, and CIFSA Senior Notes, respectively.
(5)Includes payables due to non-guarantor subsidiaries of $21.8 billion and $42.1 billion for Medtronic & Medtronic Luxco Senior Notes, and CIFSA Senior Notes, respectively.
(6)Includes loans payable due to non-guarantor subsidiaries of $7.7 billion and $7.7 billion for Medtronic & Medtronic Luxco Senior Notes, and CIFSA Senior Notes, respectively.
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This Quarterly Report on Form 10-Q, and other written reports of Medtronic plc, organized under the laws of Ireland (together with its consolidated subsidiaries, Medtronic, the Company, or we, us, or our), and oral statements made by or with the approval of one of the Company’s executive officers from time to time, may include “forward-looking” statements. All statements other than statements of historical fact contained in this Quarterly Report on Form 10-Q, including statements regarding our future results of operations and financial position, business strategy and plans, objectives of management for future operations and current expectations or forecasts of future results, are forward-looking statements. These statements involve known and unknown risks, uncertainties, and other important factors that may cause our actual results, performance, or achievements to be materially different from any future results, performance, or achievements expressed or implied by the forward-looking statements. Our forward-looking statements may include statements related to our growth and growth strategies, developments in the markets for our products, therapies and services, financial results, product development launches and effectiveness, research and development strategy, regulatory approvals, competitive strengths, the potential or anticipated direct or indirect impact of public health crises and geopolitical conflicts on our business, results of operations and/or financial condition, restructuring and cost-saving initiatives, intellectual property rights, litigation and tax matters, governmental proceedings and investigations, mergers and acquisitions, divestitures, market acceptance of our products, therapies and services, accounting estimates, financing activities, ongoing contractual obligations, working capital adequacy, value of our investments, our effective tax rate, our expected returns to shareholders, and sales efforts. In some cases, such statements may be identified by the use of terminology such as “anticipate,” “believe,” “could,” “estimate,” “expect,” “forecast,” “intend,” “looking ahead,” “may,” “plan,” “possible,” “potential,” “project,” “should,” “will,” and similar words or expressions. Forward-looking statements in this Quarterly Report include, but are not limited to, statements regarding: our ability to drive long-term shareholder value; development and future launches of products and continued or future acceptance of products, therapies and services in our segments; expected timing for completion of research studies relating to our products; integration of new technologies, including artificial intelligence (AI) and data analytics, into our products, therapies and services; market positioning and performance of our products, including stabilization of certain product markets; divestitures and the potential benefits thereof; the costs and benefits of integrating previous acquisitions; anticipated timing for United States (U.S.) Food and Drug Administration (U.S. FDA) and non-U.S. regulatory approval of new products; increased presence in new markets, including markets outside the U.S.; changes in the market and our market share; our ability to meet growing demand for our existing products; acquisitions and investment initiatives, including the timing of regulatory approvals as well as integration of acquired companies into our operations; the resolution of tax matters; the effectiveness of our development activities in reducing patient care costs and hospital stay lengths; our approach towards cost containment; our expectations regarding healthcare costs, including potential changes to reimbursement policies and pricing pressures; our expectations regarding changes to patient standards of care; our ability to identify and maintain successful business partnerships; the elimination of certain positions or costs related to restructuring initiatives; outcomes in our litigation matters and governmental proceedings and investigations; general economic conditions; the adequacy of available working capital and our working capital needs; our payment of dividends and redemption of shares; the continued strength of our balance sheet and liquidity; our accounts receivable exposure; our human capital management with respect to our global workforce; and the potential impact of our compliance with governmental regulations and accounting guidance.
We have based these forward-looking statements largely on our current expectations and projections about future events and financial trends that we believe may affect our business, results of operations, financial condition, and/or cash flows. These forward-looking statements speak only as of the date of this Quarterly Report on Form 10-Q and are subject to a number of risks, uncertainties and assumptions described in the “Risk Factors” section and elsewhere in our Annual Report on Form 10-K. Because forward-looking statements are inherently subject to risks and uncertainties, some of which cannot be predicted or quantified, you should not rely on these forward-looking statements as predictions of future events. One must carefully consider forward-looking statements and understand that such forward-looking statements are inherently subject to risks and uncertainties, some of which cannot be predicted or quantified, and involve a variety of risks and uncertainties, known and unknown, including, among others, those discussed in the sections entitled “Government Regulation” within “Item 1. Business” and “Item 1A. Risk Factors” in our Annual Report on Form 10-K, as well as those related to:
•competition in the medical device industry,
•delays in regulatory approvals,
•public health crises,
•reduction or interruption in our supply,
•failure to complete or achieve the intended benefits of acquisitions or divestitures,
•adverse regulatory action,
•laws and governmental regulations,
•litigation results,
•quality problems,
•healthcare policy changes,
•cybersecurity and privacy incidents,
•international operations, including the impact of armed conflicts,
•self-insurance,
•commercial insurance,
•changes in applicable tax rates,
•positions taken by taxing authorities,
•decreasing selling prices and pricing pressure,
•liquidity shortfalls,
•fluctuations in currency exchange rates,
•inflation, or
•disruption of our current plans and operations.
Consequently, no forward-looking statement may be guaranteed, and actual results may vary materially from those projected in the forward-looking statements. We intend to take advantage of the Safe Harbor provisions of the Private Securities Litigation Reform Act of 1995 regarding our forward-looking statements and are including this sentence for the express purpose of enabling us to use the protections of the safe harbor with respect to all forward-looking statements. While we may elect to update these forward-looking statements at some point in the future, whether as a result of any new information, future events, or otherwise, we have no current intention of doing so except to the extent required by applicable law.
Item 3. Quantitative and Qualitative Disclosures About Market Risk
CURRENCY EXCHANGE RATE RISK
Due to the global nature of our operations, we are exposed to currency exchange rate changes, which may cause fluctuations in earnings and cash flows. Fluctuations in the currency exchange rates of currency exposures that are unhedged, such as in certain emerging markets, may result in future earnings and cash flow volatility. The gross notional amount of all currency exchange rate derivative instruments outstanding at October 25, 2024 and April 26, 2024 was $24.6 billion and $23.7 billion, respectively. At October 25, 2024, these contracts were in a net unrealized gain position of $464 million. Additional information regarding our currency exchange rate derivative instruments is included in Note 8 to the current period's consolidated financial statements.
A sensitivity analysis of changes in the fair value of all currency exchange rate derivative contracts at October 25, 2024 and April 26, 2024 indicates that, if the U.S. dollar uniformly strengthened/weakened by 10 percent against all currencies, the fair value of these contracts would increase/decrease by approximately $1.7 billion. Any gains and losses on the fair value of derivative contracts would generally be offset by gains and losses on the underlying transactions. These offsetting gains and losses are not reflected in the above analysis.
INTEREST RATE RISK
We are subject to interest rate risk on our short-term investments and our borrowings. We manage interest rate risk in the aggregate, while focusing on our immediate and intermediate liquidity needs. Our debt portfolio at October 25, 2024 was comprised of debt predominantly denominated in U.S. dollars and Euros, which is primarily fixed rate debt. We are also exposed to interest rate changes affecting our investments in interest rate sensitive instruments, which include our marketable debt securities.
A sensitivity analysis of the impact on our interest rate-sensitive financial instruments of a hypothetical 50 basis point change in interest rates, as compared to interest rates at October 25, 2024 and April 26, 2024, indicates that the fair value of these instruments would correspondingly change by $65 million and $64 million, respectively.
For a discussion of current market conditions and the impact on our financial condition and results of operations, please see the “Liquidity” section of the current period's Management's Discussion and Analysis. For additional discussion of market risk, refer to Notes 6 and 8 to the current period's consolidated financial statements.
Item 4. Controls and Procedures
EVALUATION OF DISCLOSURE CONTROLS AND PROCEDURES
Our management, with the participation of our Chief Executive Officer and Chief Financial Officer, has evaluated the effectiveness of the design and operation of our disclosure controls and procedures (as defined in Rule 13a-15(e) under the Securities Exchange Act of 1934, as amended (the Exchange Act)) and changes in the Company’s internal control over financial reporting (as defined in Rule 13a-15(f) under the Exchange Act) as of the end of the period covered by this report. Based upon that evaluation, the Chief Executive Officer and Chief Financial Officer have concluded that, as of the end of the period covered by this quarterly report, our disclosure controls and procedures (as defined in Rule 13a-15(e) of the Exchange Act) are effective.
CHANGES IN INTERNAL CONTROL OVER FINANCIAL REPORTING
There have been no changes in our internal control over financial reporting (as defined in Rules 13a-15(f) under the Exchange Act) during the period covered by this Quarterly Report on Form 10-Q that have materially affected, or are reasonably likely to materially affect, the Company's internal control over financial reporting.
PART II — OTHER INFORMATION
Item 1. Legal Proceedings
In accordance with Item 103 of Regulation S-K, we have adopted a $1 million disclosure threshold for proceedings under environmental laws to which a governmental authority is a party, as we believe matters under this threshold are not material to the Company. A discussion of the Company’s legal proceedings and other loss contingencies are described in Note 16 to the current period's consolidated financial statements.
Item 2. Unregistered Sales of Equity Securities and Use of Proceeds
Issuer Purchases of Equity Securities
The following table provides information about the shares repurchased by the Company during the second quarter of fiscal year 2025: | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Fiscal Period | | Total Number of
Shares Purchased | | Average Price
Paid per Share | | Total Number of Shares
Purchased as a Part of
Publicly Announced
Program | | Maximum Approximate Dollar Value of Shares that may yet be Purchased Under the Program |
| 7/27/2024-8/23/2024 | | 1,380,200 | | | $ | 82.71 | | | 1,380,200 | | | $ | 2,690,475,982 | |
| 8/24/2024-9/27/2024 | | 807,700 | | | 89.42 | | | 807,700 | | | 2,618,252,054 | |
| 9/28/2024-10/25/2024 | | 691,854 | | | 89.66 | | | 691,854 | | | 2,556,222,068 | |
| Total | | 2,879,754 | | | $ | 86.26 | | | 2,879,754 | | | $ | 2,556,222,068 | |
In March 2024, the Company's Board of Directors authorized the repurchase of $5.0 billion of the Company's ordinary shares. There is no specific time period associated with these repurchase authorizations.
Item 5. Other Information
Rule 10b5-1 Director and Officer Trading Arrangements
During the three months ended October 25, 2024, certain of our officers and directors adopted “Rule 10b5-1 trading arrangements,” intended to satisfy the affirmative defense of Rule 10b5-1(c) under the Exchange Act as follows.
On October 10, 2024, Brett Wall, Executive Vice President and President Neuroscience Portfolio, adopted a Rule 10b5-1 trading plan. Mr. Wall's trading plan provides for the sale of up to 22,287 shares of the Company's common stock prior to the plan's August 1, 2025 termination date.
Item 6. Exhibits | | | | | | | | | | | | | | |
| (a) | | Exhibits | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | 101.SCH | | Inline XBRL Schema Document. |
| | | 101.CAL | | Inline XBRL Calculation Linkbase Document. |
| | | 101.DEF | | Inline XBRL Definition Linkbase Document. |
| | | 101.LAB | | Inline XBRL Label Linkbase Document. |
| | | 101.PRE | | Inline XBRL Presentation Linkbase Document. |
| | 104 | | Cover Page Interactive Data File (formatted as Inline XBRL and contained in Exhibit 101) |
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned authorized officer. | | | | | | | | | | | |
| | | Medtronic plc | |
| | | (Registrant) | |
| | | | |
| Date: | November 26, 2024 | /s/ Jennifer M. Kirk | |
| | Jennifer M. Kirk | |
| | Senior Vice President, Global Controller and Chief Accounting Officer (Principal Accounting Officer) | |
| | | |