As filed with the U.S. Securities and Exchange Commission on December 20, 2024.
Registration No. 333-
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
_______________________
FORM F-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
_______________________
WORK Medical Technology Group LTD
(Exact name of registrant as specified in its charter)
_______________________
Cayman Islands | | 5047 | | Not Applicable |
(State or other jurisdiction of
incorporation or organization) | | (Primary Standard Industrial
Classification Code Number) | | (I.R.S. Employer
Identification Number) |
Floor 23, No. 2 Tonghuinan Road
Xiaoshan District, Hangzhou City, Zhejiang Province
The People’s Republic of China
+86-571-82613568
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices)
_______________________
Cogency Global Inc.
122 East 42nd Street, 18th Floor
New York, NY 10168
Telephone: (800) 221-0102
(Name, address, including zip code, and telephone number, including area code, of agent for service)
_______________________
With a Copy to:
Ying Li, Esq.
Lisa Forcht, Esq.
Hunter Taubman Fischer & Li LLC
950 Third Avenue, 19th Floor
New York, NY 10022
212-530-2206 | | David E. Danovitch, Esq.
Michael DeDonato, Esq.
Hermione Krumm, Esq.
Sullivan & Worcester LLP
1251 Avenue of the Americas
New York, NY 10022
212-660-3027 |
_______________________
Approximate date of commencement of proposed sale to the public: As soon as practicable after the effective date of this registration statement.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933 check the following box. ☒
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering ☐
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933
Emerging growth company ☒
If an emerging growth company that prepares its financial statements in accordance with U.S. GAAP, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act ☐
The Registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act, or until the registration statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to such Section 8(a), may determine.
Table of Contents
The information in this prospectus is not complete and may be changed. We may not sell the securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and it is not soliciting any offer to buy these securities in any jurisdiction where such offer or sale is not permitted.
SUBJECT TO COMPLETION | | PRELIMINARY PROSPECTUS DATED December 20, 2024 |
2,500,000 Ordinary Units (each Ordinary Unit consisting of one Ordinary Share, one Series A Warrant to purchase one Ordinary Share and one Series B Warrant to purchase one Ordinary Share)
Up to 2,500,000 Pre-Funded Ordinary Units (each Pre-Funded Ordinary Unit consisting of one
Pre-Funded Warrant, one Series A Warrant to purchase one Ordinary Share and
one Series B Warrant to purchase one Ordinary Share)
Up to 7,500,000 Ordinary Shares underlying the Series A Warrants, Series B Warrants and
Pre-Funded Warrants

WORK Medical Technology Group LTD
We are offering in a firm commitment offering 2,500,000 ordinary units (each, an “Ordinary Unit,” and, collectively, the “Ordinary Units”), with each Ordinary Unit consisting of (i) one ordinary share, par value $0.0005 per share (each, an “Ordinary Share,” and, collectively, the “Ordinary Shares”), (ii) one Series A warrant to purchase one Ordinary Share (each, a “Series A Warrant,” and, collectively, the “Series A Warrants”), and (iii) one Series B warrant to purchase one Ordinary Share (each, a “Series B Warrant,” and, collectively, the “Series B Warrants”), at the assumed public offering price of $6.00 per Ordinary Unit. To the extent that the purchase of Ordinary Units would cause the beneficial ownership of a purchaser in this offering, together with its affiliates, to exceed 4.99% (or, at the election of the purchaser, 9.99%) of the Ordinary Shares immediately following the consummation of this offering, the Company agrees to issue, at the election of the purchasers, a number of pre-funded ordinary units (each, a “Pre-Funded Ordinary Unit,” and, collectively, the “Pre-Funded Ordinary Units;” together with the Ordinary Units, the “Units”) in lieu of the Ordinary Units. Each Pre-Funded Ordinary Unit consists of (i) one pre-funded warrant to purchase one Ordinary Share (each, a “Pre-Funded Warrant,” and, collectively, the “Pre-Funded Warrants; and together with the Series B Warrants and the Series A Warrants, the “Warrants”), (ii) one Series A Warrant, and (iii) one Series B Warrant. The purchase price of each Pre-Funded Ordinary Unit will equal the price per Ordinary Unit, minus $0.0005, and the exercise price of each Pre-Funded Warrant included in the Pre-Funded Ordinary Unit will be $0.0005 per share. The Pre-Funded Warrants offered hereby will be immediately exercisable and may be exercised at any time until exercised in full. For each Pre-Funded Warrant that we sell, the number of Ordinary Units that we are offering will be decreased on a one-for-one basis.
The Series A Warrants will have five-year terms, will be immediately exercisable after issuance and have an initial exercise price equal to the actual public offering price of the Ordinary Units. Such exercise price will be reset immediately following the thirtieth (30th) trading day following the date of closing of this offering (the “Reset Date”) to a price equal to 105% of the average of the sum of the three lowest per share volume-weighted average prices (the “VWAP”) of the Ordinary Shares on the trading market for the twenty (20) trading days immediately prior to the Reset Date; provided that the exercise price will not be lower than 20% of the closing price of the Ordinary Shares on the Nasdaq Capital Market immediately prior to the pricing of this offering (such price, the “Series A Floor Price”). Notwithstanding the foregoing, the Company may reduce the Series A Floor Price to any amounts set forth in a written notice to the holder of the Series A Warrants; provided that such reduction shall be in compliance with applicable Nasdaq rules, irrevocable and not be subject to increase thereafter.
The Series B Warrants will have five-year terms and will be immediately exercisable after issuance at an exercise price equal to the actual public offering price of the Ordinary Units. The Series B Warrants may also be exercised in whole by means of a one-time only “alternative cashless exercise” in which the holder will be entitled to receive a number of Ordinary Shares equal to the product of (a) the aggregate number of Ordinary Shares that would be issuable upon exercise of the Series B Warrants if such exercise were by means of a cash exercise rather than a cashless exercise; multiplied by (b) the quotient obtained by dividing (i) the exercise price minus the lowest VWAP of the Ordinary Shares over the 10 trading days immediately prior to the exercise date by (ii) 50% of the lowest VWAP of the Ordinary Shares over the 10 trading days immediately prior to the exercise date. Such alternative cashless exercise is subject to the Beneficial Ownership Limitation (as defined below), and will only be available at a time when the applicable VWAP is lower than
Table of Contents
the then applicable exercise price. The maximum number of Ordinary Shares issuable pursuant to such alternative cashless exercise will be equal to the quotient obtained by dividing (a) the aggregate exercise price of the Series B Warrant as of its issuance date, by (b) 20% of the actual offering price of the Ordinary Units (as adjusted for reverse and forward splits and similar transactions, the “Series B Floor Price”). Notwithstanding the foregoing, the Company may reduce the Series B Floor Price to any amounts set forth in a written notice to the holder of the Series B Warrants; provided that such reduction shall be in compliance with appliable Nasdaq rules, irrevocable and not be subject to increase thereafter.
We are also registering all of the Ordinary Shares issuable from time to time upon full exercise of each of the Warrants included in the Units offered hereby. See “Description of Share Capital — Units Being Offered” in this prospectus for more information.
The Units do not have stand-alone rights and will not be certificated or issued as stand-alone securities. The Ordinary Shares (or, if applicable, the Pre-Funded Warrants), the Series A Warrants and the Series B Warrants included in the Units are immediately separable, and will be issued separately in this offering.
Our Ordinary Shares are listed on the Nasdaq Capital Market under the symbol “WOK.”
The number of Units offered in this prospectus and all other applicable information has been determined based on the assumed public offering price of $6.00 per Ordinary Unit.
There is no established trading market for the Units or the Warrants, and we do not expect an active trading market to develop. We do not intend to list the Units or the Warrants on any securities exchange or other trading market. Without an active trading market, the liquidity of such securities will be limited.
Unless otherwise stated, as used in this prospectus, the terms “we,” “us,” “our,” “Work Cayman,” “our Company,” and the “Company” refer to WORK Medical Technology Group LTD, an exempted company limited by shares incorporated under the laws of the Cayman Islands and not a Chinese operating company. Work Cayman conducts its operations through Work (Hangzhou) Medical Treatment Equipment Co., Ltd. and its subsidiaries in China (collectively referred to herein as “the PRC subsidiaries”).
Our Ordinary Shares began trading on the Nasdaq Capital Market under the symbol “WOK” on August 23, 2024. On August 26, 2024, the Company closed its initial public offering (the “IPO”) of 2,000,000 ordinary shares at a price of $4.00 per share. On August 28, 2024, the underwriter for the IPO exercised its over-allotment option, in part, to purchase an additional 91,942 Ordinary Shares at a price of $4.00 per Ordinary Share. The total gross proceeds received from the IPO, including proceeds from the exercise of the over-allotment option, was $8,367,768.
Investing in our securities involves a high degree of risk, including the risk of losing your entire investment. See “Risk Factors” beginning on page 17 to read about factors you should consider before buying our securities.
We are both an “emerging growth company” and a “foreign private issuer” as defined under the U.S. federal securities laws and, as such, may elect to comply with certain reduced public company reporting requirements for this and future filings. See “Prospectus Summary — Implications of Being an Emerging Growth Company” on page 12, and “Prospectus Summary — Implications of Being a Foreign Private Issuer” on page 13.
Our Ordinary Shares offered in this prospectus are shares of our Cayman Islands holding company, which has no operations of its own and conducts all of its operations through the PRC subsidiaries, namely, Work (Hangzhou) Medical Treatment Technology Co., Ltd. (“Work Hangzhou”), our wholly owned subsidiary, and its subsidiaries, Hangzhou Shanyou Medical Equipment Co., Ltd. (“Hangzhou Shanyou”), Shanghai Chuqiang Medical Equipment Co., Ltd. (“Shanghai Chuqiang”), Hangzhou Hanshi Medical Equipment Co., Ltd. (“Hangzhou Hanshi”), Hangzhou Woli Medical Treatment Technology Co., Ltd. (“Hangzhou Woli”), Shanghai Saitumofei Medical Treatment Technology Co., Ltd. (“Shanghai Saitumofei”), Huangshan Saitumofei Medical Technology Co., Ltd. (“Huangshan Saitumofei”), and Hunan Saitumofei Medical Treatment Technology Co., Ltd. (“Hunan Saitumofei”). The operations of the PRC subsidiaries could affect other parts of our business. Investors in our Ordinary Shares should be aware that they will not directly hold equity interests in the PRC subsidiaries, but rather are purchasing equity solely in WORK Medical Technology Group LTD, a Cayman Islands holding company, which indirectly owns 100% equity interests in such PRC subsidiaries. For a description of our corporate structure, see “Corporate History and Structure — Corporate Structure” beginning on page 68.
In addition, as we conduct all of our operations through the PRC subsidiaries in China, we and the PRC subsidiaries are subject to legal and operational risks associated with being based in China, including risks related to the legal, political and economic policies of the Chinese government, the relations between China and the United States, or Chinese or United States regulations, which risks could result in a material change in the PRC subsidiaries’ operations and/or cause the value of our Ordinary Shares to significantly decline or become worthless and affect our ability to offer or continue to offer securities to investors. Recently, the PRC government initiated a series of regulatory actions
Table of Contents
and made a number of public statements on the regulation of business operations in China with little advance notice, including cracking down on illegal activities in the securities market, enhancing supervision over China-based companies listed overseas, adopting new measures to extend the scope of cybersecurity reviews, and expanding efforts in anti-monopoly enforcement. We do not believe that we or the PRC subsidiaries are directly subject to these regulatory actions or statements, as neither we nor the PRC subsidiaries have implemented any monopolistic behavior, and the PRC subsidiaries’ business does not implicate cybersecurity, because the PRC subsidiaries currently engage in the manufacture and sale of medical devices and neither we nor the PRC subsidiaries possess the personal information of over one million users, nor are we or the PRC subsidiaries involved in any type of restricted industries. On September 8, 2006, the Regulations on Mergers and Acquisitions of Domestic Enterprises by Foreign Investors (the “M&A Rules”) which was jointly adopted by six PRC regulatory agencies came into effect. The M&A Rules include, among other things, provisions that purport to require that offshore special purpose vehicles (each, an “SPV”) that are controlled by PRC entities or individuals and that have been formed for overseas listing purposes through acquisitions of PRC domestic interests held by such PRC entities or individuals, obtain the approval of the China Securities Regulatory Commission (the “CSRC”) prior to the listing and trading of any such SPV’s securities on an overseas stock exchange. On September 21, 2006, the CSRC published on its official website procedures regarding its approval of overseas listings by SPVs. Since the Company is neither controlled by PRC entities or individuals, nor formed for overseas listing purposes through acquisitions of PRC domestic interests held by such PRC entities or individuals, we believe, as advised by our PRC counsel, AllBright Law Offices (Fuzhou), that the Company is not an SPV, and, therefore, the M&A Rules do not apply to us, and we do not need to obtain the approval from the CSRC under the M&A Rules. However, substantial uncertainty remains regarding the scope and applicability of the M&A Rules to offshore SPVs. Furthermore, on March 31, 2023, the Trial Administrative Measures of Overseas Securities Offering and Listing by Domestic Companies (the “Trial Administrative Measures”) and relevant supporting guidelines (collectively, the “New Administrative Rules Regarding Overseas Listings”) issued by the CSRC came into force. According to the New Administrative Rules Regarding Overseas Listings, among other things, a domestic company in the PRC that seeks to offer and list securities on overseas markets shall fulfill the filing procedures with the CSRC as per requirement of the Trial Administrative Measures. This includes subsequent securities offerings of the company in the same overseas market where it has previously offered and listed securities, which requires a company, such as ours, to file with the CSRC within three working days after the subsequent securities offering is completed. On the same day, the Provisions on Strengthening Confidentiality and Archives Administration of Overseas Securities Offering and Listing by Domestic Companies (the “Confidentiality and Archives Administration Provisions”) promulgated by the CSRC became effective. According to the Confidentiality and Archives Administration Provisions, domestic companies that seek overseas offering and listing (either in direct or indirect means) and the securities companies and securities service (either incorporated domestically or overseas) providers that undertake relevant businesses shall not leak any state secret or working secret of government agencies, or harm national security and public interests. Furthermore, a domestic company that provides accounting archives or copies of accounting archives to any entities, including securities companies, securities service providers and overseas regulators and individuals, shall fulfill due procedures in compliance with applicable regulations. We believe that this offering does not involve the leaking of any state secret or working secret of government agencies, or the harming of national security and public interests. However, we may be required to perform additional procedures in connection with the provision of accounting archives. The specific requirements of the relevant procedures are currently unclear and we cannot be certain whether we will be able to perform the relevant procedures. We believe, based on the advice of our PRC counsel, AllBright Law Offices (Fuzhou), that (i) as this offering is regarded as a subsequent securities offering in the same overseas market under the Trial Administrative Measures, we are required to complete the filing procedures with the CSRC in accordance with the Trial Administrative Measures with respect to this offering, and we will submit our filing application to the CSRC within three working days after the completion of this offering; (ii) neither we nor the PRC subsidiaries are subject to cybersecurity review with the Cyberspace Administration of China (the “CAC”), pursuant to the Measures for Cybersecurity Review (2021 version), which became effective on February 15, 2022, since the PRC subsidiaries currently engage in the manufacture and sale of medical devices and neither we nor the PRC subsidiaries possess personal information of over one million users; and (iii) there are substantial uncertainties regarding the interpretation and application of PRC laws and regulations and future PRC laws and regulations, and there can be no assurance that the relevant government agencies will take a view that is contrary to, or otherwise different from, the conclusions stated above. If the relevant government agencies take a view that is contrary to, or otherwise different from, the foregoing conclusions, it could have a material adverse effect on the PRC subsidiaries’ business, operating results and reputation, as well as the trading price of our Ordinary Shares. See “Risk Factors — Risks Related to Doing Business in China — Any requirement to obtain prior approval under the M&A Rules and/or any other regulations promulgated by relevant PRC regulatory agencies in the future could delay this offering and failure to obtain any such approvals, if required, could have a material adverse effect on the PRC subsidiaries’ and our business, operating results and
Table of Contents
reputation, as well as the trading price of our Ordinary Shares, and could also create uncertainties for this offering and affect our ability to offer or continue to offer securities to investors outside China” on page 22; “Risk Factors — Risks Related to Doing Business in China — There are uncertainties regarding the interpretation and enforcement of PRC laws, rules and regulations” on page 17; and “Risk Factors — Risks Related to Doing Business in China — Additional compliance procedures may be required in connection with this offering, due to the promulgation of the new filing-based administrative rules for overseas offering and listing by domestic companies in China, which could significantly limit or completely hinder our ability to offer or continue to offer our Ordinary Shares to investors and could cause the value of our Ordinary Shares to significantly decline or become worthless” on page 20.
However, since these statements and regulatory actions by the PRC government are newly published and official guidance and related implementation rules have not been issued, it is highly uncertain what the potential impact such modified or new laws and regulations will have on the PRC subsidiaries’ daily business operations, the ability to accept foreign investments and list on an U.S. exchange. Moreover, the Standing Committee of the National People’s Congress (the “SCNPC”) or other PRC regulatory authorities may in the future promulgate laws or regulations or implementing rules that require our Company, or any of our subsidiaries to obtain regulatory approval from Chinese authorities before listing in the U.S. Although the Company is currently not required to obtain permission or approval from any of the PRC central or local governmental authorities, except for completing the filing procedures with the CSRC, and it has not received any denial to list on a U.S. exchange, the PRC subsidiaries’ operations could be adversely affected, directly or indirectly; our ability to offer, or continue to offer, securities to investors would be potentially hindered; and the value of our securities might significantly decline or be worthless, by existing or future laws and regulations relating to the business of the PRC subsidiaries or our industry or by intervention or interruption by PRC governmental authorities, if we or the PRC subsidiaries (i) do not receive or maintain such permissions or approvals, (ii) inadvertently conclude that such permissions or approvals are not required, (iii) applicable laws, regulations, or interpretations change and we or the PRC subsidiaries are required to obtain such permissions or approvals in the future, or (iv) due to any intervention or interruption by PRC governmental with little advance notice. See “Risk Factors” beginning on page 17 for a discussion of these legal and operational risks and other information that should be considered before making a decision to purchase our securities.
Moreover, the Chinese government may exert substantial influence over the manner in which the PRC subsidiaries conduct their business activities. The PRC government may also intervene or influence the PRC subsidiaries’ operations and this offering at any time, which could result in a material change in the PRC subsidiaries’ operations and our Ordinary Shares could decline in value significantly or become worthless. See “— Summary of Risks Factors — Risk Related to Doing Business in China — The PRC government exerts substantial influence over the manner in which the PRC subsidiaries conduct their business activities. The PRC government may also intervene or influence the PRC subsidiaries’ operations and this offering at any time, which could result in a material change in the PRC subsidiaries’ operations and our Ordinary Shares could significantly decline in value or become worthless” on page 19; and “Risk Factors — Risks Related to Doing Business in China — The PRC government exerts substantial influence over the manner in which the PRC subsidiaries conduct their business activities. The PRC government may also intervene or influence the PRC subsidiaries’ operations and this offering at any time, which could result in a material change in the PRC subsidiaries’ operations and our Ordinary Shares could significantly decline in value or become worthless” on page 19.
In addition, although Work Medical Technology Group (China) Limited (“Work Medical Technology”), our Hong Kong subsidiary, is an investment holding company, the legal and operational risks associated with operating in mainland China may also apply to the future activities (if any) in Hong Kong of Work Medical Technology, to the extent that they are made applicable to such entity and its anticipated operations. Work Medical Technology, as of the date of this prospectus, has yet to commence operations, and is expected to be limited to operating as an investment holding company in the future without any substantive or data-related operations in Hong Kong. However, such operations may be affected if Hong Kong adopts rules, regulations or policy guidance with respect to currency exchange control. Furthermore, as of the date of this prospectus, we do not expect that any regulatory actions related to data security or anti-monopoly concerns in Hong Kong will impact the Company’s ability to conduct its business, accept foreign investments, or list on a U.S. or foreign exchange, because we have never had and do not plan to have any material operations in Hong Kong. See “Risk Factors — Risks Related to Doing Business in China” on page 17.
Hong Kong was established as a special administrative region of the PRC in accordance with Article 31 of the Constitution of the PRC. The Basic Law of the Hong Kong Special Administrative Region of the PRC (the “Basic Law”) was adopted and promulgated on April 4, 1990 and became effective on July 1, 1997, when the PRC resumed the exercise of sovereignty over Hong Kong. Pursuant to the Basic Law, Hong Kong is authorized by the National
Table of Contents
People’s Congress of the PRC to exercise a high degree of autonomy and enjoy executive, legislative, and independent judicial power, under the principle of “one country, two systems,” and the PRC laws and regulations shall not be applied in Hong Kong except for those listed in Annex III of the Basic Law (which is confined to laws relating to national defense, foreign affairs, and other matters that are not within the scope of autonomy of Hong Kong). While the National People’s Congress of the PRC has the power to amend the Basic Law, the Basic Law also expressly provides that no amendment to the Basic Law shall contravene the established basic policies of the PRC regarding Hong Kong. As a result, national laws of the PRC not listed in Annex III of the Basic Law do not apply to our Hong Kong subsidiary, Work Medical Technology. However, there is no assurance that certain PRC laws and regulations, including existing laws and regulations and those enacted or promulgated in the future, will not be applicable to Work Medical Technology due to changes in the current political arrangements between mainland China and Hong Kong or other unforeseeable reasons. The application of such laws and regulations may have a material adverse impact on Work Medical Technology, as relevant authorities may impose fines and penalties upon Work Medical Technology, delay or restrict the repatriation of the proceeds from this offering into mainland China and Hong Kong, and any failure by us to fully comply with any such new regulatory requirements may significantly limit or completely hinder our ability to offer or continue to offer our Ordinary Shares, cause significant disruption to our business operations, and severely damage our reputation, which would materially and adversely affect our financial condition and results of operations and cause our Ordinary Shares to significantly decline in value or in extreme cases, become worthless.
Furthermore, as more stringent criteria have been imposed by the U.S. Securities and Exchange Commission (the “SEC”) and the Public Company Accounting Oversight Board (the “PCAOB”) recently, our securities may be prohibited from trading on a national exchange or over-the-counter under the Holding Foreign Companies Accountable Act (“the HFCA Act”), if the PCAOB is unable to inspect our auditors for two consecutive years. As a result, an exchange may determine to delist our securities. Pursuant to the HFCA Act, if the PCAOB is unable to inspect an issuer’s auditors for two consecutive years, the issuer’s securities are prohibited to trade on a U.S. stock exchange. The PCAOB issued a Determination Report on December 16, 2021 which found that the PCAOB is unable to inspect or investigate completely registered public accounting firms headquartered in: (1) mainland China of the People’s Republic of China because of a position taken by one or more authorities in mainland China; and (2) Hong Kong, a Special Administrative Region and dependency of the PRC, because of a position taken by one or more authorities in Hong Kong. Furthermore, the PCAOB’s report identified the specific registered public accounting firms which are subject to these determinations. On June 22, 2021, United States Senate passed the Accelerating Holding Foreign Companies Accountable Act. On December 29, 2022, legislation entitled “Consolidated Appropriations Act, 2023” (the “Consolidated Appropriations Act”), was signed into law by President Biden. The Consolidated Appropriations Act contained, among other things, an identical provision to the Accelerating Holding Foreign Companies Accountable Act, which reduces the number of consecutive non-inspection years required for triggering the prohibitions under the HFCA Act from three years, as was formerly required under the HFCA Act before such amendment, to two consecutive years. According to the Consolidated Appropriations Act, any foreign jurisdiction could be the reason why the PCAOB does not have complete access to inspect or investigate a company’s auditor. As it was originally enacted, the HFCA Act applied only if the PCAOB’s inability to inspect or investigate was due to a position taken by an authority in the foreign jurisdiction where the relevant public accounting firm is located. As a result of the Consolidated Appropriations Act, the HFCA Act now also applies if the PCAOB’s inability to inspect or investigate the relevant accounting firm is due to a position taken by an authority in any foreign jurisdiction. The denying jurisdiction does not need to be where the accounting firm is located. As of the date of this prospectus, our auditor WWC, P.C., is not on the list published by the PCAOB subject to the determinations as to inability to inspect or investigate completely, as announced by the PCAOB on December 16, 2021, and it is based in the U.S. and is registered with the PCAOB and subject to PCAOB inspection, having its latest inspection completed in December 2021. However, recently developments with respect to audits of China-based companies, create uncertainty about the ability of our auditor, to fully cooperate with the PCAOB’s request for audit workpapers without the approval of the Chinese authorities. In the event it is later determined that the PCAOB is unable to inspect or investigate completely the Company’s auditor because of a position taken by an authority in a foreign jurisdiction, then such lack of inspection could cause trading in the Company’s securities to be prohibited under the HFCA Act, and ultimately result in a determination by a securities exchange to delist the Company’s securities. The delisting of our Ordinary Shares, or the threat of their being delisted, may materially and adversely affect the value of your investment, even making it worthless. On August 26, 2022, the CSRC, the Ministry of Finance of the PRC (the “MOF”), and the PCAOB signed a Statement of Protocol (the “Protocol”), which sets out specific arrangements on conducting inspections and investigations over relevant audit firms within the jurisdiction of the PRC and the U.S, including the audit firms based in mainland China and Hong Kong, taking the first step toward opening access for the PCAOB to inspect and investigate registered public accounting firms headquartered in mainland China and Hong Kong. On December 15, 2022, the PCAOB Board determined that the PCAOB was able to
Table of Contents
secure complete access to inspect and investigate registered public accounting firms headquartered in mainland China and Hong Kong and voted to vacate its previous determinations to the contrary. However, should PRC authorities obstruct or otherwise fail to facilitate the PCAOB’s access in the future, the PCAOB Board will consider the need to issue a new determination. Notwithstanding the foregoing, in the event it is later determined that the PCAOB is unable to inspect or investigate completely our auditor, then such lack of inspection could cause our securities to be delisted from the stock exchange. See “Risk Factors — Risks Related to Doing Business in China — Our Ordinary Shares may be delisted under the Holding Foreign Companies Accountable Act if the PCAOB is unable to inspect our auditors. The delisting of our Ordinary Shares, or the threat of their being delisted, may materially and adversely affect the value of your investment. Furthermore, on June 22, 2021, the U.S. Senate passed the Accelerating Holding Foreign Companies Accountable Act, and on December 29, 2022, the Consolidated Appropriations Act was signed into law by President Biden. The Consolidated Appropriations Act contained, among other things, an identical provision to the Accelerating Holding Foreign Companies Accountable Act, which reduces the number of consecutive non- inspection years required for triggering the prohibitions under the HFCA Act from three years to two.” on page 30.
As a holding company, we may rely on dividends and other distributions on equity paid by the PRC subsidiaries for our cash and financing requirements. If any of the PRC subsidiaries incurs debt on its own behalf in the future, the instruments governing such debt may restrict their ability to pay dividends to us. However, none of the PRC subsidiaries have made any dividends or other distributions to the Company or any U.S. investors as of the date of this prospectus. In the future, cash proceeds raised from overseas financing activities, including this offering, may be transferred by us to the PRC subsidiaries via capital contribution or shareholder loans, as the case may be.
The transfers and distributions among the Company and its subsidiaries include: (i) proceeds from the IPO, which were transferred from the Company to Work BVI and subsequently to Work Medical Technology; and (ii) funds transferred among five PRC subsidiaries, Hangzhou Shanyou, Hangzhou Hanshi, Hangzhou Woli, Shanghai Saitumofei, and Work Hangzhou for operational purposes. The aggregate principal amounts of funds transferred among the PRC subsidiaries were $10,301,861, $8,262,606, and $84,234 for the six months ended March 31, 2024, and the fiscal years ended September 30, 2023 and 2022, respectively. As of the date of this prospectus, there have been no other transfers or distributions made or dividends paid among the Company and its subsidiaries, except as described above.
This prospectus does not constitute, and there will not be, an offering of securities to the public in the Cayman Islands.
| | Per Unit | | Per Pre-Funded
Ordinary Unit | | Total |
Public offering price | | $ | | | $ | | | $ | |
Underwriters’ discounts and commissions(1) | | $ | | | $ | | | $ | |
Proceeds to our Company before expenses(2) | | $ | | | $ | | | $ | |
The underwriters are expected to deliver the securities included in the Units against payment in U.S. dollars in New York, New York on or about , 2024.
Neither the U.S. Securities and Exchange Commission nor any state securities commission nor any other regulatory body has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
Univest Securities, LLC

Prospectus dated , 2024
Table of Contents
i
Table of Contents
About this Prospectus
We and the underwriters have not authorized anyone to provide any information or to make any representations other than those contained in this prospectus or in any free writing prospectuses prepared by us or on our behalf or to which we have referred you. We take no responsibility for, and can provide no assurance as to the reliability of, any other information that others may give you. This prospectus is an offer to sell only the securities offered hereby, but only under circumstances and in jurisdictions where it is lawful to do so. We are not making an offer to sell these securities in any jurisdiction where the offer or sale is not permitted or where the person making the offer or sale is not qualified to do so or to any person to whom it is not permitted to make such offer or sale. For the avoidance of doubt, no offer or invitation to subscribe for the securities is made to the public in the Cayman Islands. The information contained in this prospectus is current only as of the date on the front cover of the prospectus. Our business, financial condition, results of operations, and prospects may have changed since that date.
Neither we nor the underwriters have taken any action to permit a public offering of the securities outside the United States or to permit the possession or distribution of this prospectus or any filed free-writing prospectus outside the United States. Persons outside the United States who come into possession of this prospectus or any filed free writing prospectus must inform themselves about, and observe any restrictions relating to, the offering of the securities and the distribution of this prospectus or any filed free-writing prospectus outside the United States.
Conventions that Apply to this Prospectus
Unless otherwise indicated, in this prospectus, references to:
• “China” or the “PRC” are to the People’s Republic of China, including the special administrative regions of Hong Kong, Macau and Taiwan. The term “Chinese” has a correlative meaning for the purpose of this prospectus. When used in the case of laws, regulations and rules, of “China” or the “PRC”, it refers to only such laws, regulations and rules of mainland China. When used in the case of government, governmental authorities, regulatory agencies, courts, jurisdictions, tax, entities, enterprises, individuals and residents of “China” or the “PRC” or “Chinese”, it refers to only such government, governmental authorities, regulatory agencies, courts, jurisdictions, tax, entities, enterprises, individuals and residents of mainland China;
• “Class I medical device” are to a medical device with a low level of risks and whose safety and effectiveness can be ensured through routine administration, pursuant to the Regulations on the Supervision and Administration of Medical Devices (as amended in 2021) (the “2021 Medical Device Regulation”);
• “Class II medical device” are to a medical device with moderate risks that must be strictly controlled and regulated to ensure its safety and effectiveness, pursuant to the 2021 Medical Device Regulation;
• “Class III medical device” are to a medical device with relatively high risks that must be strictly controlled and regulated through special measures to ensure its safety and effectiveness, pursuant to the 2021 Medical Device Regulation;
• “Code” are to the United States Internal Revenue Code of 1986, as amended;
• “Exchange Act” are to the Securities Exchange Act of 1934, as amended;
• “FDA” are to the U.S. Food and Drug Administration;
• “Group” are to the WORK Medical Technology Group LTD, Work Medical Technology Group Limited, and the PRC subsidiaries, collectively;
• “Hangzhou Hanshi” are to Hangzhou Hanshi Medical Equipment Co., Ltd.;
• “Hangzhou Shanyou” are to Hangzhou Shanyou Medical Equipment Co., Ltd.;
• “Hangzhou Woli” are to Hangzhou Woli Medical Treatment Technology Co., Ltd.;
• “Hangzhou Youshunhe” are to Hangzhou Youshunhe Technology Co., Ltd.;
• “Huangshan Saitumofei” are to Huangshan Saitumofei Medical Technology Co., Ltd.;
ii
Table of Contents
• “Hunan Saitumofei” are to Hunan Saitumofei Medical Treatment Technology Co., Ltd.;
• “mainland China” or “Chinese mainland” are to the People’s Republic of China, excluding, solely for the purpose of this prospectus, the special administrative regions of Hong Kong, Macau and Taiwan. The term “mainland Chinese” has a correlative meaning for the purpose of this prospectus;
• “Nasdaq” are to the Nasdaq Capital Market;
• “Ordinary Shares” are to ordinary shares of the Company, par value $0.0005 per share;
• “PFIC” are to a passive foreign investment company;
• “PRC subsidiaries” are to Work Hangzhou, Hangzhou Shanyou, Shanghai Chuqiang, Hangzhou Hanshi, Hangzhou Woli, Shanghai Saitumofei, and Hunan Saitumofei, collectively;
• “RMB” or the “Renminbi” are to the legal currency of China;
• “SEC” are to the United States Securities and Exchange Commission;
• “Securities Act” are to the Securities Act of 1933, as amended;
• “Shanghai Chuqiang” are to Shanghai Chuqiang Medical Equipment Co., Ltd.;
• “Shanghai Saitumofei” are to Shanghai Saitumofei Medical Treatment Technology Co., Ltd.;
• “US$,” “USD,” “U.S. dollars,” “$,” and “dollars” are to the legal currency of the United States;
• “Work BVI” are to Work Medical Technology Group Limited;
• “WFOE” or “Work Age” are to Work Age (Hangzhou) Medical Treatment Technology Co., Ltd, which is a limited liability company formed in China; and
• “Work Hangzhou” are to Work (Hangzhou) Medical Treatment Technology Co., Ltd.
Our reporting and functional currency is the Renminbi, or RMB. Solely for the convenience of the reader, this prospectus contains translations of some RMB amounts into U.S. dollars, at specified rates. For the figures as of March 31, 2024, translations from RMB to U.S. dollars are made at RMB7.2203 to US$1.00, the rate published by the Federal Reserve Board on March 29, 2024. Except as otherwise stated in this prospectus, all other translations from RMB to U.S. dollars are made at RMB7.2960 to US$1.00, the rate published by the Federal Reserve Board on September 30, 2023. No representation is made that the RMB amounts referred to in this prospectus could have been or could be converted into U.S. dollars at such rate.
Our fiscal year end is September 30. References to a particular “fiscal year” are to our fiscal year ended September 30 of that calendar year. Our audited consolidated financial statements have been prepared in accordance with the generally accepted accounting principles in the United States (the “U.S. GAAP”).
The PRC subsidiaries have proprietary rights to trademarks used in this prospectus that are important to their business, many of which are registered under applicable intellectual property laws. Solely for convenience, the trademarks, service marks and trade names referred to in this prospectus are without the®, ™ and other similar symbols, but such references are not intended to indicate, in any way, that we will not assert, to the fullest extent under applicable law, the PRC subsidiaries’ rights or the rights of the applicable licensors to these trademarks, service marks and trade names.
This prospectus contains additional trademarks, service marks and trade names of others. All trademarks, service marks and trade names appearing in this prospectus are, to our knowledge, the property of their respective owners. We do not intend our use or display of other companies’ trademarks, service marks or trade names to imply a relationship with, or endorsement or sponsorship of us by, any other person.
iii
Table of Contents
PROSPECTUS SUMMARY
Investors are cautioned that you are buying securities of a Cayman Islands holding company with no operations of its own that holds 100% of the shares of a China-based operating company.
This summary highlights certain information contained elsewhere in this prospectus. You should read the entire prospectus carefully, including our financial statements and related notes and the risks described under “Risk Factors.” Our actual results and future events may differ significantly based upon a number of factors. The reader should not put undue reliance on the forward-looking statements in this document, which speak only as of the date on the cover of this prospectus.
Overview
We are a holding company incorporated in the Cayman Islands. As a holding company with no material operations of our own, we conduct all of our operations through our operating entities established in the PRC, primarily Work Hangzhou, our wholly owned subsidiary, and its subsidiaries (collectively referred to herein as the “PRC subsidiaries”). The operations of our PRC subsidiaries could affect other parts of our business.
We are a supplier of medical devices in China. We develop and manufacture Class I and II medical devices and sell Class I and II disposable medical devices through operating subsidiaries in China. The PRC subsidiaries’ products include, to name a few, medical face masks, artery compression tourniquets for bleeding control, disposable breathing circuits for delivering oxygen and anesthetic gases, laryngeal mask airways for keeping patients’ airways open during anesthesia and endotracheal tubes for keeping the trachea open for air to get to the lungs.
The PRC subsidiaries have Class I, II and III disposable medical device qualifications, including filing certificates for Class I products and registration certificates for Class II products, and medical device production and operation licenses in China. For more information about the qualifications, please refer to “Business — Certification of Production and Products.”
The PRC subsidiaries have been providing medical devices to hospitals, pharmacies, and medical institutions since 2002. The PRC subsidiaries currently have a total of 21 medical devices in their product portfolio. All of them are sold domestically, and 15 of them are sold internationally.
In the Chinese market, the PRC subsidiaries’ products are sold in 34 provincial-level administrative regions. Internationally, the products are exported to more than 30 countries in Asia, Africa, Europe, North America, South America, and Oceania. In the meantime, the PRC subsidiaries have established a strict quality management system. The PRC subsidiaries have 18 products that have passed the inspections administered by local authorities in Zhejiang province and obtained the registration certificates. The PRC subsidiaries have also received international “CE” certification and ISO 13485 system certification. Furthermore, the PRC subsidiaries have registered with the FDA for 17 products.
Our Revenue Model
We primarily generate our revenue through the PRC subsidiaries’ sales of medical devices.
The PRC subsidiaries sell medical devices both domestically and internationally. For the six months ended March 31, 2024, and the fiscal years ended September 30, 2023 and 2022, the revenue from domestic sales was $4,630,186, $12,598,261, and $18,291,527, accounting for 87%, 93% and 93%, respectively, of our revenue, and the revenue from international sales was $678,909, $967,690, and $1,419,763, accounting for 13%, 7%, and 7%, respectively, of our revenue.
For the six months ended March 31, 2024 and the fiscal years ended September 30, 2023 and 2022, we recognized approximately $5,309,095, $13,565,951, and $19,711,290 in revenue, respectively.
1
Table of Contents
Customers
The PRC subsidiaries have three types of customers, i) direct end-user customers, which include hospitals, pharmacies, and medical institutions, ii) domestic distributor customers that distribute the PRC subsidiaries’ products to end-user customers in China, and iii) export distributor customers that distribute the PRC subsidiaries’ products to end-user customers in Asia, Africa, Europe, North America, South America, and Oceania. The top ten countries and regions outside of mainland China where these products are sold are Saudi Arabia, Germany, Switzerland, Hong Kong, France, Poland, Netherlands, Mexico, Romania and Russia.
As of March 31, 2024, the PRC subsidiaries had a total of 913 customers, of which, 42 are direct end-user customers, 849 are domestic distributor customers, and 22 are export distributor customers. For the six months ended March 31, 2024, the top three customers accounted for 5%, 5%, and 4%, respectively, of the revenue. For the fiscal year ended September 30, 2023, the top three customers accounted for 9%, 6%, and 8%, respectively, of the revenue. For the fiscal year ended September 30, 2022, the top three customers accounted for 6%, 4%, and 4%, respectively, of the revenue.
Marketing and Sales
The PRC subsidiaries market and sell their products through their sales team and distribution network, including their domestic and export distributors.
Sales Team
As of the date of this prospectus, the PRC subsidiaries have a sales team of 38 employees. There are four team leaders leading their respective teams to market the PRC subsidiaries’ products, both domestically and internationally.
Distribution Network
For the six months ended March 31, 2024 and the fiscal years ended September 30, 2023 and 2022, the PRC subsidiaries had approximately 849, 892, and 867 domestic distributors and 22, 22, and 37 exporting distributors, respectively. According to the Company, it is a common practice in the industry of medical devices for companies to rely on a considerable number of distributors to sell their products. Distributors usually purchase products from the PRC subsidiaries at a discounted price and then resell the products to end customers, both domestically and internationally.
The PRC subsidiaries’ domestic distributors cover 34 provincial-level administrative regions of PRC for the resales of the products in the Chinese market. They market and distribute the products in the regions where they are located and secured approximately 865, 1,032, and 1,021 domestic customers for the PRC subsidiaries for the six months ended March 31, 2024 and the fiscal years ended September 30, 2023 and 2022, respectively, including hospitals and medical institutions.
The PRC subsidiaries’ exporting distributors can be classified into two categories: those located outside of China that only distribute the products internationally, and those located in China that distribute the products, both domestically and internationally. The total number of direct and indirect customer relationships established overseas through the PRC subsidiaries’ exporting distributors was approximately 48, 50, and 100 as of March 31, 2024, September 30, 2023 and 2022, respectively. The decline was attributed to a decreased demand for masks and other medical devices overseas following the easing of COVID-19 restrictions and the diminishing impact of the pandemic since the first quarter of 2023. Additionally, the PRC subsidiaries have placed more emphasis on screening client qualifications, preferring to collaborate with major clients, which has resulted in reduced number of clients. We do not anticipate the continuation of this trend in future financial periods for two main reasons: (i) before the COVID-19 pandemic, the PRC subsidiaries primarily engaged with exporting distributors that specialized in selling medical devices other than masks. As the pandemic escalated and the demand for masks surged, the PRC subsidiaries terminated most of their relationships with distributors of medical devices (not including masks), pivoting towards distributors of masks. With the ongoing easing of COVID-19 restrictions and the diminishing impact of the pandemic, the PRC subsidiaries have since the first calendar quarter of 2023, been gradually reinstating their ties with exporting distributors that focus on
2
Table of Contents
medical devices other than masks, thereby enhancing the customer relationships established through these distributors; and (ii) the PRC subsidiaries are actively expanding their overseas sales networks and are in the process of securing new exporting distributors.
See “Business — Marketing and Sales — Distribution Network” and “Risk Factors — Risks Related to the PRC Subsidiaries’ Business and Industry — Our PRC subsidiaries rely in part on third-party distributors to place their products into the market and they may not be able to control their distributors.”
Competitive Strengths
We believe that the following competitive strengths have contributed to our success and differentiated us from our competitors:
• a deep understanding of the industry;
• cost-effective masks;
• customized and multifunctional masks; and
• wide distribution network.
Growth Strategies
We intend to develop our business and strengthen brand loyalty by implementing the following strategies:
• continue to invest in research and department team;
• expand sales and distribution network; and
• strengthen quality control system and uphold the commitment to product quality.
Our Corporate History and Structure
WORK Medical Technology Group LTD, or Work Cayman, is a Cayman Islands exempted company incorporated on March 1, 2022. Work Medical Technology Group Limited, or Work BVI, is our wholly-owned subsidiary formed in the British Virgin Islands on March 15, 2022. Work Medical Technology Group (China) Limited (“Work Medical Technology”) is Work BVI’s wholly-owned subsidiary formed in Hong Kong on April 19, 2022. WFOE is Work Medical Technology’s wholly-owned subsidiary formed in Hangzhou on April 28, 2022. Work Hangzhou is WFOE’s wholly-owned subsidiary formed in Hangzhou on November 10, 2021. Work Hangzhou and its subsidiaries contributed 100% of our consolidated revenue. We operate our business through the operating subsidiaries in China, namely 1) Hangzhou Shanyou, a PRC company formed on April 29, 2002, located in Hangzhou, Zhejiang Province, the PRC; 2) Hangzhou Hanshi, a PRC company formed on July 22, 2019, located in Hangzhou, Zhejiang Province, the PRC; 3) Shanghai Chuqiang Medical Equipment Co., Ltd. (“Shanghai Chuqiang”), a PRC company formed on March 12, 2018, located in Shanghai, the PRC; 4) Shanghai Saitumofei Medical Treatment Technology Co., Ltd. (“Shanghai Saitumofei”), a PRC company formed on May 15, 2019, located in Shanghai, PRC; 5) Hunan Saitumofei, a PRC company formed on April 2, 2021, located in Changsha, Hunan Province, the PRC; and 6) Hangzhou Woli, a PRC company formed on July 29, 2022, located in Hangzhou, Zhejiang Province, the PRC. In addition to the above subsidiaries, there are also two subsidiaries, Hangzhou Youshunhe Technology Co., Ltd. (“Hangzhou Youshunhe”), which is owned by Hangzhou Shanyou, and Huangshan Saitumofei Medical Technology Co., Ltd. (“Huangshan Saitumofei”), a wholly owned subsidiary of Shanghai Saitumofei, which have had no operations as of the date of this prospectus. Work Cayman, Work BVI and the PRC subsidiaries are collectively referred to herein as the “Group”.
3
Table of Contents
The chart below summarizes our corporate structure as of the date of this prospectus.

For more details regarding our corporate structure and related changes, see “Corporate History and Structure.”
Work Hangzhou and its subsidiaries contributed 100% of our consolidated revenue for the six months ended March 31, 2024 and fiscal years ended September 30, 2023 and 2022; and accounted for 97.90%, 99.48%, and 100% of our consolidated total assets for the six months ended March 31, 2024 and the fiscal years ended September 30, 2023 and 2022, respectively, and accounted for 96.28% and 100% liabilities for the six months ended March 31, 2024 and each of the fiscal years ended September 30, 2023 and 2022. The following financial information of Work Hangzhou and its subsidiaries was included in the consolidated financial statements:
| | As of
September 30, |
| | | 2023 | | 2022 |
Total Assets | | $ | 29,958,901 | | $ | 25,541,469 |
Total Liabilities | | $ | 18,972,986 | | $ | 14,350,869 |
4
Table of Contents
| | Fiscal Years Ended
September 30, |
| | | 2023 | | 2022 |
Revenue | | $ | 13,565,951 | | $ | 19,711,290 |
Net profit | | $ | 63,383 | | $ | 944,126 |
| | Fiscal Years Ended
September 30, |
| | | 2023 | | 2022 |
Net cash provided by (used in) operating activities | | $ | 2,209,736 | | | $ | (2,258,948 | ) |
Net cash used in investing activities | | | (583,304 | ) | | | (1,347,165 | ) |
Net cash (used in) provided by financing activities | | | (728,719 | ) | | | 3,566,223 | |
Effect of foreign currency translations | | | (33,852 | ) | | | (59,819 | ) |
Net increase (decrease) in cash and cash equivalents | | $ | 863,861 | | | $ | (99,709 | ) |
| | As of
March 31,
2024 | | As of
September 30,
2023 |
Total Assets | | $ | 32,494,480 | | $ | 29,958,901 |
Total Liabilities | | $ | 21,158,984 | | $ | 18,972,986 |
| | Fiscal Six Months Ended
March 31, |
| | | 2024 | | 2023 |
Revenue | | $ | 5,309,095 | | $ | 9,011,404 |
Net profit | | $ | 234,854 | | $ | 1,433,365 |
| | Fiscal Six Months Ended
March 31, |
| | | 2024 | | 2023 |
Net cash (used in) provided by operating activities | | $ | (1,264,684 | ) | | $ | 719,455 | |
Net cash used in investing activities | | | (5,856,936 | ) | | | (522,487 | ) |
Net cash provided (used in) by financing activities | | | 6,556,704 | | | | (87,108 | ) |
Effect of foreign currency translations | | | 8,272 | | | | (44,829 | ) |
Net (decrease) increase in cash and cash equivalents | | $ | (556,644 | ) | | $ | 65,031 | |
Neither we nor the PRC subsidiaries operate in an industry that prohibits or limits foreign investment. As a result, neither we nor the PRC subsidiaries are required to obtain any permission from Chinese authorities to operate, other than those permissions a domestic company in mainland China will need to engage in businesses similar to our PRC subsidiaries’. As of the date of the prospectus, such licenses and permissions include a Business License, Medical Device Registration Certificate, Medical Device Production Certificate, Medical Device Selling Certificate, Record Form of Medical Device Products, Certificate of Class I medical device production recordation, Certificate of Class II medical device selling recordation, Record Registration Form for Foreign Trade Business Operators and Certificate of the Customs of the People’s Republic of China on Registration of a Customs Declaration Entity. The PRC subsidiaries have obtained the above licenses and permissions to conduct their business in mainland China. See “Business — Licenses and Permissions” on page 86.
Furthermore, we believe, based on the advice of our PRC counsel, AllBright Law Offices (Fuzhou), that (i) since the Company is neither controlled by PRC entities or individuals, nor formed for overseas listing purposes through acquisitions of PRC domestic interests held by such PRC entities or individuals, the Company is not an SPV, and therefore, the M&A Rules do not apply to us, and we do not need to obtain the approval from the CSRC under the M&A Rules; (ii) as this offering is regarded as a subsequent securities offering in the same overseas market under the Trial Administrative Measures, we are required to complete the filing procedures with the CSRC in accordance with the Trial Administrative Measures within three working days after the completion of this offering; (iii) we are not subject to cybersecurity review with the CAC, under the Measures for Cybersecurity Review (2021 version), which became effective on February 15, 2022, because the PRC subsidiaries currently engage in the manufacture and sale of medical devices and we or the PRC subsidiaries do not possess personal information of over one million users; and (iv) there are substantial uncertainties regarding the interpretation and application of PRC laws and regulations and prospects for future PRC laws and regulations, and there can be no assurance that the relevant government agencies will take a view that is not contrary to or otherwise different from the conclusions stated above. See “Risk Factors — Risks
5
Table of Contents
Related to Doing Business in China — Any requirement to obtain prior approval under the M&A Rules and/or any other regulations promulgated by relevant PRC regulatory agencies in the future could delay this offering and failure to obtain any such approvals, if required, could have a material adverse effect on the PRC subsidiaries’ and our business, operating results and reputation, as well as the trading price of our Ordinary Shares, and could also create uncertainties for this offering and affect our ability to offer or continue to offer securities to investors outside China” on page 22; and “Risk Factors — Risks Related to Doing Business in China — There are uncertainties regarding the interpretation and enforcement of PRC laws, rules and regulations” on page 17.
The PRC government may take actions to exert more oversight and control over offerings by China based issuers conducted overseas and/or foreign investment in such companies. In particular, additional compliance procedures may be required in connection with this offering, due to the promulgation of the new filing-based administrative rules for overseas offering and listing by domestic companies in China. The New Administrative Rules Regarding Overseas Listings could significantly limit or completely hinder our ability to offer or continue to offer securities to investors outside China and cause the value of our securities to significantly decline or become worthless. See “Risk Factors — Risks Related to Doing Business in China — Any requirement to obtain prior approval under the M&A Rules and/or any other regulations promulgated by relevant PRC regulatory agencies in the future could delay this offering and failure to obtain any such approvals, if required, could have a material adverse effect on the PRC subsidiaries’ and our business, operating results and reputation, as well as the trading price of our Ordinary Shares, and could also create uncertainties for this offering and affect our ability to offer or continue to offer securities to investors outside China” on page 22, and “Risk Factors — Risks Related to Doing Business in China — Additional compliance procedures may be required in connection with this offering, due to the promulgation of the new filing-based administrative rules for overseas offering and listing by domestic companies in China, which could significantly limit or completely hinder our ability to offer or continue to offer our Ordinary Shares to investors and could cause the value of our Ordinary Shares to significantly decline or become worthless” on page 20.
On February 17, 2023, the CSRC issued the New Administrative Rules Regarding Overseas Listings, which came into force on March 31, 2023. According to the New Administrative Rules Regarding Overseas Listings, among other things, a domestic company in the PRC that seeks to offer and list securities on overseas markets shall fulfill the filing procedures with the CSRC as per requirement of the Trial Administrative Measures. In accordance with the New Trial Administrative Rules Regarding Overseas Listings, we will submit our filing application to the CSRC within three working days after the completion of this offering. On February 24, 2023, the CSRC promulgated the Confidentiality and Archives Administration Provisions, which also became effective on March 31, 2023. According to the Confidentiality and Archives Administration Provisions, domestic companies that seek overseas offering and listing (either in direct or indirect means) and the securities companies and securities service (either incorporated domestically or overseas) providers that undertake relevant businesses shall institute a sound confidentiality and archives administration system, and take necessary measures to fulfill confidentiality and archives administration obligations. They shall not leak any state secret or working secret of government agencies, or harm national security and public interests. Furthermore, a domestic company that provides accounting archives or copies of accounting archives to any entities, including securities companies, securities service providers and overseas regulators and individuals, shall fulfill due procedures in compliance with applicable regulations. Working papers produced in mainland China by securities companies and securities service providers in the process of undertaking businesses related to overseas offering and listing by domestic companies shall be retained in mainland China. Where such documents need to be transferred or transmitted to areas outside of mainland China, relevant approval procedures stipulated by regulations shall be followed. We believe that this offering does not involve the leaking of any state secret or working secret of government agencies, or the harming of national security and public interests. However, we may be required to perform additional procedures in connection with the provision of accounting archives. The specific requirements of the relevant procedures are currently unclear and we cannot be certain whether we will be able to perform the relevant procedures. Except for the filing procedures with the CSRC, as of the date of this prospectus, we are not required to obtain any permission from any PRC governmental authorities to offer securities to foreign investors. We have been closely monitoring regulatory developments in China regarding any necessary approvals from the CSRC or other PRC governmental authorities required for overseas listings, including this offering. As of the date of this prospectus, neither we nor the PRC subsidiaries have received any inquiry, notice, warning, sanctions or regulatory objection to this offering from the CSRC or other PRC governmental authorities. However, there remains significant uncertainty as to the enactment, interpretation and implementation of regulatory requirements related to overseas securities offerings and other capital markets activities. Moreover, on December 28, 2021, the Measures for Cybersecurity Review (2021 version) were promulgated and took effect on February 15, 2022, which provide that any “online platform operators” controlling personal information of more than one million users which seeks to list in a foreign stock exchange should also be subject to cybersecurity review. We do not expect to be subject to cybersecurity
6
Table of Contents
review, because the PRC subsidiaries currently engage in the manufacture and sale of medical devices and neither we nor the PRC subsidiaries possess personal information of over one million users. As of the date of this prospectus, we have not been involved in any investigations on cybersecurity review initiated by the Cyberspace Administration of China, and we have not received any warning, sanction or penalty in such respect. If it is determined in the future that the approval of the Cyberspace Administration of China or any other regulatory authority is required for this offering, we may face sanctions by the Cyberspace Administration of China or other PRC regulatory agencies. These regulatory agencies may impose fines and penalties on the PRC subsidiaries’ operations in China, limit the PRC subsidiaries’ ability to pay dividends outside of China, limit the PRC subsidiaries’ operations, delay or restrict the repatriation of the proceeds from this offering into China or take other actions that could have a material adverse effect on our or the PRC subsidiaries’ business, financial condition, results of operations and prospects, as well as the trading price of our securities. The CSRC, the Cyberspace Administration of China or other PRC regulatory agencies also may take actions requiring us, or making it advisable for us, to halt this offering before settlement and delivery of our Ordinary Shares. Consequently, if you engage in market trading or other activities in anticipation of and prior to settlement and delivery, you do so at the risk that settlement and delivery may not occur. In addition, if the Cyberspace Administration of China or other PRC regulatory agencies later promulgate new rules requiring that we obtain their approvals for this offering, we may be unable to obtain such approvals or a waiver of such approval requirements, if and when procedures are established to obtain such a waiver. Any uncertainties and/or negative publicity regarding such approval requirement could have a material adverse effect on the trading price of our securities. See “— Summary of Risks Factors — Risk Related to Doing Business in China — The PRC government exerts substantial influence over the manner in which the PRC subsidiaries conduct their business activities. The PRC government may also intervene or influence the PRC subsidiaries’ operations and this offering at any time, which could result in a material change in the PRC subsidiaries’ operations and our Ordinary Shares could significantly decline in value or become worthless” on page 19; and “Risk Factors — Risks Related to Doing Business in China — The PRC government exerts substantial influence over the manner in which the PRC subsidiaries conduct their business activities. The PRC government may also intervene or influence the PRC subsidiaries’ operations and this offering at any time, which could result in a material change in the PRC subsidiaries’ operations and our Ordinary Shares could significantly decline in value or become worthless” on page 19.
Additionally, our PRC subsidiaries are subject to various laws and regulations related to their operating activities. Failure to comply with these laws and regulations could cause material changes in the PRC subsidiaries’ operations. Our PRC subsidiaries are subject to fire protection laws, for example, Hangzhou Shanyou, the only PRC subsidiary that manages production lines, has not prepared the required regulatory reports in connection with fire protection laws and regulations, and, as a consequence, may be ordered to stop use of such production lines by PRC regulatory authorities. Since all of its products are manufactured by operation of such production lines, any such development could materially and adversely affect our business, financial condition and results of operations. See “Risk Factors — The PRC subsidiaries are subject to a variety of fire protection laws that could be costly for them to comply with, and they could incur liability if they fail to comply with such laws, which could adversely affect the Group as a whole” on page 42.
Change in Authorized Share Capital and Share Subdivision
On April 6, 2023, the shareholders of the Company unanimously passed resolutions effecting the subdivision of the Company’s authorized and issued share capital and the adoption of the amended and restated memorandum of association, pursuant to which, (1) the Company effectuated a 1:2000 share subdivision, whereupon the Company’s authorized share capital was amended from US$50,000 divided into 50,000 shares of par value US$1.00 each to US$50,000 divided into 100,000,000 Ordinary Shares of a par value of $0.0005 each; and (2) immediately after the share subdivision, the shareholders voluntarily surrendered, on a pro rata basis, a total of 87,500,000 Ordinary Shares of a par value of $0.0005 each, after which, the Company had an aggregate of 12,500,000 Ordinary Shares issued and outstanding.
Dividends and Other Distributions
We are a holding company with no material operations of our own and do not generate any revenue. We currently conduct all of our operations through Work Hangzhou, our wholly owned subsidiary and its subsidiaries. We are permitted under PRC laws and regulations to provide funding to PRC subsidiaries only through loans or capital contributions, and only if we satisfy the applicable government registration and approval requirements. See “Risk Factors — Risks Related to Doing Business in China — PRC regulation of loans to and direct investment in PRC entities by offshore holding companies and governmental control of currency conversion may delay us from using the proceeds of this offering to make loans or additional capital contributions to the PRC subsidiaries, which could materially and adversely affect our liquidity and our ability to fund and expand the PRC subsidiaries’ business” on page 26.
7
Table of Contents
We have not installed any cash management policies that dictate how funds are transferred among Work Cayman and its subsidiaries, and thus, we do not have any procedures governing fund transfers. Under our current corporate structure, we rely on dividend payments from the PRC subsidiaries to fund any cash and financing requirements we may have, including the funds necessary to pay dividends and other cash distributions to our shareholders or to service any debt we may incur. Our subsidiaries in the PRC generate and retain cash generated from operating activities and re-invest it in their business. If any of the PRC subsidiaries incurs debt on its own behalf in the future, the instruments governing such debt may restrict their ability to pay dividends to us.
The transfers and distributions among the Company and its subsidiaries include: (i) proceeds from the Company’s IPO, which were transferred from the Company to Work BVI and subsequently to Work Medical Technology; and (ii) funds transferred among five PRC subsidiaries, Hangzhou Shanyou, Hangzhou Hanshi, Hangzhou Woli, Shanghai Saitumofei, and Work Hangzhou for operational purposes. The aggregate principal amounts of funds transferred among the PRC subsidiaries were $10,301,861, $8,262,606, and $84,234 for the six months ended March 31, 2024, and the fiscal years ended September 30, 2023 and 2022, respectively. As of the date of this prospectus, there have been no other transfers or distributions made or dividends paid among the Company and its subsidiaries, except as described above.
The PRC subsidiaries are permitted to pay dividends only out of their retained earnings. However, each of the PRC subsidiaries is required to set aside at least 10% of its after-tax profits each year, after making up for previous year’s accumulated losses, if any, to fund certain statutory reserves, until the aggregate amount of such funds reaches 50% of its registered capital. This portion of the PRC subsidiaries’ respective net assets are prohibited from being distributed to their shareholders as dividends. See “Regulations — Regulations on Dividend Distributions.” However, none of our subsidiaries has made any dividends or other distributions to the Company or any U.S. investors as of the date of this prospectus. See also “Risk Factors — Risks Related to Doing Business in China — We rely to a significant extent on dividends and other distributions on equity paid by our subsidiaries to fund offshore cash and financing requirements and any limitation on the ability of the PRC subsidiaries to make remittance to pay dividends to us could limit our ability to access cash generated by the operations of those entities” on page 27.
The PRC subsidiaries have made no further plans to pay dividends since January 31, 2021, and do not expect to do so unless and until they have generated sufficient accumulated profits and have met the requirements for statutory reserve funds. We intend to retain all of our available funds and any future earnings after this offering and cash proceeds from overseas financing activities, including this offering, to fund the development and growth of the PRC subsidiaries’ business. As a result, we do not expect to pay any cash dividends in the foreseeable future.
In addition, the PRC government imposes controls on the convertibility of the Renminbi into foreign currencies and, in certain cases, the remittance of currency out of mainland China. If the foreign exchange control system prevents us from obtaining sufficient foreign currencies to satisfy our foreign currency demands, we may not be able to pay dividends in foreign currencies to our shareholders. See “Risk Factors — Risks Related to Doing Business in China — Restrictions on currency exchange may limit our ability to utilize our revenue effectively” on page 29.
A 10% PRC withholding tax is applicable to dividends payable to investors that are non-resident enterprises. Any gain realized on the transfer of Ordinary Shares by such investors is also subject to PRC tax at a current rate of 10% which in the case of dividends will be withheld at source if such gain is regarded as income derived from sources within the PRC. See also “Risk Factors — Risks Related to Doing Business in China — Dividends payable to our foreign investors and gains on the sale of our Ordinary Shares by our foreign investors may be subject to PRC tax” on page 28.
Recent Developments
The Ordinary Shares began trading on August 23, 2024 on Nasdaq under the ticker symbol “WOK.” On August 26, 2024, the Company completed its IPO of 2,000,000 Ordinary Shares at a price of $4.00 per share. On August 28, 2024, the underwriter for the Company’s IPO exercised its over-allotment option in part to purchase 91,942 Ordinary Shares at a price of $4.00. The total gross proceeds received from the IPO, including proceeds from the exercise of the over-allotment option, was $8,367,768.
8
Table of Contents
Summary of Risk Factors
Investing in our securities involves significant risks. You should carefully consider all of the information in this prospectus before making an investment in our securities. Below please find a summary of the principal risks we face, organized under relevant headings. These risks are discussed more fully in the section titled “Risk Factors.”
Risks Related to Doing Business in China
Risks and uncertainties related to doing business in China include, but are not limited to, the following:
• Changes in the political and economic policies of the PRC government or in relations between China and the United States may materially and adversely affect the PRC subsidiaries’ and our business, financial condition and results of operations and may result in the PRC subsidiaries’ inability to sustain their growth and expansion strategies.
• There are uncertainties regarding the interpretation and enforcement of PRC laws, rules and regulations.
• The PRC government exerts substantial influence over the manner in which the PRC subsidiaries conduct their business activities. The PRC government may also intervene or influence the PRC subsidiaries’ operations and this offering at any time, which could result in a material change in the PRC subsidiaries’ operations and our Ordinary Shares could significantly decline in value or become worthless.
• Additional compliance procedures may be required in connection with this offering, due to the promulgation of the new filing-based administrative rules for overseas offering and listing by domestic companies in China, which could significantly limit or completely hinder our ability to offer or continue to offer our Ordinary Shares to investors and could cause the value of our Ordinary Shares to significantly decline or become worthless.
• You may experience difficulties in effecting service of legal process, enforcing foreign judgments or bringing actions in China against us or our directors and officers named in the prospectus based on foreign laws.
• On December 28, 2021, the Measures for Cybersecurity Review (2021 version) were promulgated and took effect on February 15, 2022, which provide that any “online platform operators” controlling personal information of more than one million users which seeks to list in a foreign stock exchange should also be subject to cybersecurity review. We do not believe that the PRC subsidiaries are subject to cybersecurity review, because they currently engage in the manufacture and sale of medical devices and do not possess personal information of over one million users. As of the date of this prospectus, neither we nor the PRC subsidiaries have been involved in any investigations on cybersecurity review initiated by the Cyberspace Administration of China, and neither we nor the PRC subsidiaries have received any warning, sanction, or penalty in such respect. Any requirement to obtain prior approval under the M&A Rules and/or any other regulations promulgated by relevant PRC regulatory agencies in the future could delay this offering and failure to obtain any such approvals, if required, could have a material adverse effect on the PRC subsidiaries’ and our business, operating results and reputation, as well as the trading price of our Ordinary Shares, and could also create uncertainties for this offering and affect our ability to offer or continue to offer securities to investors outside China.
• PRC regulation of loans to and direct investment in PRC entities by offshore holding companies and governmental control of currency conversion may delay us from using the proceeds of this offering to make loans or additional capital contributions to the PRC subsidiaries, which could materially and adversely affect our liquidity and our ability to fund and expand the PRC subsidiaries’ business.
• Our Ordinary Shares may be delisted under the HFCA Act if the PCAOB is unable to inspect our auditors. The delisting of our Ordinary Shares, or the threat of their being delisted, may materially and adversely affect the value of your investment. Furthermore, on June 22, 2021, the U.S. Senate passed the Accelerating Holding Foreign Companies Accountable Act, and on December 29, 2022, the Consolidated Appropriations Act was signed into law by President Biden. The Consolidated Appropriations Act contained, among other things, an identical provision to the Accelerating Holding Foreign Companies Accountable Act, which reduces the number of consecutive non-inspection years required for triggering the prohibitions under the HFCA Act from three years to two”.
9
Table of Contents
Risks Related to the PRC Subsidiaries’ Business and Industry:
Risks and uncertainties related to the PRC Subsidiaries’ business and industry include, but are not limited to, the following:
• Failure to maintain the quality and safety of the PRC subsidiaries’ products could have a material and adverse effect on the PRC subsidiaries’ and our reputation, financial condition and results of operations.
• The PRC subsidiaries may experience significant liability claims or complaints from customers, doctors and patients, litigation and regulatory investigations and proceedings, such as claiming in relation to medical device safety, or adverse publicity involving their products, which could adversely affect the PRC subsidiaries’ and our financial condition and results of operations.
• The PRC subsidiaries face the risk of fluctuations in the cost, availability and quality of their raw materials, which could adversely affect their results of operations, and thus, adversely affect the Group as a whole.
• The PRC subsidiaries do not have long-term contracts with their suppliers and the suppliers can reduce order quantities or terminate sales to the PRC subsidiaries at any time.
• If the PRC subsidiaries fail to identify, acquire and develop other products, they may be unable to grow their business.
• The PRC subsidiaries’ international sales are subject to a variety of risks that could adversely affect their profitability and operating results, which may adversely affect the profitability and operating results of the Group.
• If the PRC subsidiaries fail to timely renew their medical device licenses or registration certificates, it could adversely affect the PRC subsidiaries’ and our reputation, financial condition and results of operations.
Risks Relating to this Offering and the Trading Market
In addition to the risks described above, we are subject to general risks and uncertainties relating to this offering and the trading market, including, but not limited to, the following:
• There is no public market for the Units or the Warrants.
• The Warrants in this offering are speculative in nature.
• The holders of the Warrants will not have rights of holders of our Ordinary Shares until such Warrants are exercised.
• You will experience immediate and substantial dilution in the net tangible book value of Ordinary Shares included in the Units and may experience additional dilution of your investment in the future.
• Substantial future issuances and sales of our Ordinary Shares, including as a result of certain provisions contained in the Series A Warrants and Series B Warrants, or the anticipation of future sales of our Ordinary Shares in the public market could cause the price of our Ordinary Shares to decline.
• We do not intend to pay dividends for the foreseeable future.
• If we cease to qualify as a foreign private issuer, we would be required to comply fully with the reporting requirements of the Exchange Act applicable to U.S. domestic issuers, and we would incur significant additional legal, accounting and other expenses that we would not incur as a foreign private issuer.
• Because we are a foreign private issuer and are exempt from certain Nasdaq corporate governance standards applicable to U.S. issuers, you will have less protection than you would have if we were a domestic issuer.
• We are an “emerging growth company” within the meaning of the Securities Act, and if we take advantage of certain exemptions from disclosure requirements available to emerging growth companies, this will make it more difficult to compare our performance with other public companies. In addition, we may not be subject to requirements that other public companies are subject to, which could affect investor confidence in us and our Ordinary Shares.
10
Table of Contents
• The laws of the Cayman Islands may not provide our shareholders with benefits comparable to those provided to shareholders of corporations incorporated in the United States.
Recent Regulatory Developments in China
Recently, the PRC government initiated a series of regulatory actions and made a number of public statements on the regulation of business operations in China with little advance notice, including cracking down on illegal activities in the securities market, enhancing supervision over China-based companies listed overseas, adopting new measures to extend the scope of cybersecurity reviews, and expanding efforts in anti-monopoly enforcement.
Among other things, the M&A Rules and the Anti-Monopoly Law of the People’s Republic of China promulgated by the SCNPC, which took effect in 2008 and was amended on June 24, 2022, which amendment became effective August 1, 2022 (the “Anti-Monopoly Law”), established additional procedures and requirements that could make merger and acquisition activities by foreign investors more time-consuming and complex. Under the Anti-Monopoly Law, companies undertaking acquisitions relating to businesses in China must notify the State Council’s anti-monopoly law enforcement authority, in advance of any transaction where the parties’ revenue in the China market exceed certain thresholds and the buyer would obtain control of, or decisive influence over, the target. In addition, the Rules of Ministry of Commerce on Implementation of Security Review System of Mergers and Acquisitions of Domestic Enterprises by Foreign Investors, or the security review rules, issued by the MOFCOM that took effect in September 2011 specify that if a merger and acquisition of domestic enterprise by a foreign investor falls within the M&A safety review scope, the foreign investor shall file an application for M&A safety review to the Ministry of Commerce. The M&A safety review scope is as follows: foreign investors’ M&A of domestic military industry enterprises and military industry support enterprises, enterprises around key and sensitive military facilities, and other units which have impact on national defense security; and foreign investors’ M&A of domestic enterprises, which have impact on the national security, in fields of important agricultural products, important energy and resources, important infrastructure, important transport service, key technology and major equipment manufacturing, etc., and such M&A may result in foreign investors’ acquirement of actual control over the enterprises.
On July 6, 2021, the relevant PRC government authorities made public the Opinions on Strictly Cracking Down Illegal Securities Activities in Accordance with the Law. These opinions emphasized the need to strengthen the administration over illegal securities activities and the supervision on overseas listings by China-based companies and proposed to take effective measures, such as promoting the construction of relevant regulatory systems to deal with the risks and incidents faced by China-based overseas-listed companies. Pursuant to the Opinions, Chinese regulators are required to accelerate rulemaking related to the overseas issuance and listing of securities, and update the existing laws and regulations related to data security, cross-border data flow, and management of confidential information. Numerous regulations, guidelines and other measures are expected to be adopted under the umbrella of or in addition to the Cybersecurity Law and Data Security Law. See “Risk Factors — Risks Related to Doing Business in China — Any requirement to obtain prior approval under the M&A Rules and/or any other regulations promulgated by relevant PRC regulatory agencies in the future could delay this offering and failure to obtain any such approvals, if required, could have a material adverse effect on the PRC subsidiaries’ and our business, operating results and reputation, as well as the trading price of our Ordinary Shares, and could also create uncertainties for this offering and affect our ability to offer or continue to offer securities to investors outside China” on page 22.
In addition, on July 10, 2021, the Cyberspace Administration of China issued the Measures for Cybersecurity Review (Revision Draft for Comments), or the Measures, for public comments, which propose to authorize the relevant government authorities to conduct cybersecurity review on a range of activities that affect or may affect national security, including listings in foreign countries by companies that possess the personal data of more than one million users. On December 28, 2021, the Measures for Cybersecurity Review (2021 version) were promulgated and took effect on February 15, 2022, which provide that any “online platform operators” controlling personal information of more than one million users which seeks to list in a foreign stock exchange should also be subject to cybersecurity review. The Measures for Cybersecurity Review (2021 version), further elaborate on the factors to be considered when assessing the national security risks of the relevant activities, including, among others, (i) the risk of core data, important data or a large amount of personal information being stolen, leaked, destroyed, and illegally used or exited the country; and (ii) the risk of critical information infrastructure, core data, important data or a large amount of personal information being affected, controlled, or maliciously used by foreign governments after listing abroad.
11
Table of Contents
On February 17, 2023, the CSRC issued the New Administrative Rules Regarding Overseas Listings, which became effective on March 31, 2023. These rules propose to establish a new filing-based regime to regulate overseas offerings and listings by Chinese domestic companies. Under the New Administrative Rules Regarding Overseas Listings, a domestic company in the PRC that seeks to offer and list securities on overseas markets shall fulfill the filing procedures with the CSRC as per requirement of the Trial Administrative Measures. This includes subsequent securities offerings of the company in the same overseas market where it has previously offered and listed securities, which requires a company, such as ours, to file with the CSRC within three working days after the subsequent securities offering is completed. In the opinion of our PRC legal counsel, AllBright Law Offices (Fuzhou), as this offering constitutes a subsequent offering by us, we are required to file with the CSRC in accordance with the Trial Administrative Measures within three working days after this offering is completed. We cannot assure you that we can complete the required filing procedures with the CSRC or receive any other approvals or complete other compliance procedures in a timely manner, or at all, or that any completion of filing or approval or other compliance procedures would not be rescinded. Any such failure to do so would subject us to sanctions by the CSRC or other PRC regulatory authorities. These regulatory authorities may impose restrictions and penalties on our operations in China, significantly limit or completely hinder our ability to launch any new offering of our securities, limit our ability to pay dividends outside China, delay or restrict the repatriation of the proceeds from future capital raising activities into China, or take other actions that could materially and adversely affect our business, results of operations, financial condition and prospects, as well as the trading price of our Ordinary Shares. Furthermore, the PRC government authorities may further strengthen oversight and control over listings and offerings that are conducted overseas. Any such action may adversely affect our operations and significantly limit or completely hinder our ability to offer or continue to offer securities to you and cause the value of such securities to significantly decline or be worthless. See “Risk Factors — Risks Related to Doing Business in China — Additional compliance procedures may be required in connection with this offering, due to the promulgation of the new filing-based administrative rules for overseas offering and listing by domestic companies in China, which could significantly limit or completely hinder our ability to offer or continue to offer our Ordinary Shares to investors and could cause the value of our Ordinary Shares to significantly decline or become worthless” on page 20. See “Risk Factors” for a discussion of these legal and operational risks and other information that should be considered before making a decision to purchase our securities.
Implications of Being an Emerging Growth Company
We had less than $1.235 billion in revenue during our last fiscal year. As a result, we qualify as an “emerging growth company” as defined in the Jumpstart Our Business Startups Act of 2012 (the “JOBS Act”), and may take advantage of reduced public reporting requirements. These provisions include, but are not limited to:
• being permitted to present only two years of audited financial statements and only two years of related Management’s Discussion and Analysis of Financial Condition and Results of Operations in our filings with the SEC;
• not being required to comply with the auditor attestation requirements in the assessment of our internal control over financial reporting;
• reduced disclosure obligations regarding executive compensation in periodic reports, proxy statements and registration statements; and
• exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously approved.
We may take advantage of these provisions until the last day of our fiscal year following the fifth anniversary of the date of the first sale of our Ordinary Shares pursuant to this offering. However, if certain events occur before the end of such five-year period, including if we become a “large accelerated filer,” if our annual gross revenue exceed $1.235 billion or if we issue more than $1.0 billion of non-convertible debt in any three-year period, we will cease to be an emerging growth company before the end of such five-year period.
Section 107 of the JOBS Act provides that an emerging growth company can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act of 1933, as amended (the “Securities Act”), for complying with new or revised accounting standards. We have elected to take advantage of this extended transition period and acknowledge such election is irrevocable pursuant to Section 107 of the JOBS Act.
12
Table of Contents
Implications of Being a Foreign Private Issuer
We report under the Exchange Act, as a non-U.S. company with “foreign private issuer” status. Even after we no longer qualify as an emerging growth company, so long as we qualify as a foreign private issuer under the Exchange Act, we will be exempt from certain provisions of the Exchange Act and the rules thereunder that are applicable to U.S. domestic public companies, including:
• the rules under the Exchange Act that require U.S. domestic public companies to issue financial statements prepared under U.S. GAAP;
• the sections of the Exchange Act that regulate the solicitation of proxies, consents or authorizations in respect of any securities registered under the Exchange Act;
• the sections of the Exchange Act that require insiders to file public reports of their share ownership and trading activities and that impose liability on insiders who profit from trades made in a short period of time; and
• the rules under the Exchange Act that require the filing with the SEC of quarterly reports on Form 10-Q, containing unaudited financial and other specified information, and current reports on Form 8-K, upon the occurrence of specified significant events.
We will file with the SEC, within four months after the end of each fiscal year (or such other reports required by the SEC), an annual report on Form 20-F containing financial statements audited by an independent registered public accounting firm.
We may take advantage of these exemptions until such time as we are no longer a foreign private issuer. We would cease to be a foreign private issuer at such time as more than 50% of our outstanding voting securities are held by U.S. residents and any of the following three circumstances applies: (i) the majority of our executive officers or directors are U.S. citizens or residents, (ii) more than 50% of our assets are located in the United States, or (iii) our business is administered principally in the United States.
Both foreign private issuers and emerging growth companies are also exempt from certain of the more extensive SEC executive compensation disclosure rules. Therefore, if we no longer qualify as an emerging growth company but remain a foreign private issuer, we will continue to be exempt from such rules and will continue to be permitted to follow our home country practice as to the disclosure of such matters.
Corporate Information
Our principal executive offices are located at Floor 23, No. 2 Tonghuinan Road, Hangzhou, Zhejiang Province, the PRC, and our telephone number is +86-571-82613568. Our registered office in the Cayman Islands is at the offices of Tricor Services (Cayman Islands) Limited, Second Floor, Century Yard, Cricket Square, P.O. Box 902, Grand Cayman, KY1-1103, Cayman Islands. Our agent for service of process in the United States is Cogency Global Inc., located at 122 East 42nd Street, 18th Floor, New York, NY 10168.
13
Table of Contents
THE OFFERING
Ordinary Units offered by us | | 2,500,000 Ordinary Units, based on an assumed public offering price of $6.00 per Ordinary Unit, with each Ordinary Unit consisting of (i) one Ordinary Share, (ii) one Series A Warrant, and (iii) one Series B Warrant. The Ordinary Units will not be certificated, and each of the Ordinary Shares, the Series A Warrants, and the Series B Warrants are immediately separable and will be issued separately in this offering. |
Pre-Funded Ordinary Units offered by us | |
We are also offering to those purchasers, if any, whose purchase of the Ordinary Shares in this offering would result in the purchaser, together with its affiliates, beneficially owning more than 4.99% (or at the election of the purchaser, 9.99%) of our outstanding Ordinary Shares immediately following the consummation of this offering, the opportunity to purchase, if they so choose, Pre-Funded Ordinary Units in lieu of the Ordinary Units that would otherwise result in ownership in excess of 4.99% (or 9.99%, as applicable) of our outstanding Ordinary Shares immediately following the consummation of this offering. Each Pre-Funded Ordinary Unit consists of one Pre-Funded Warrant, one Series A Warrant and one Series B Warrant. The Pre-Funded Ordinary Units have no stand-alone rights and will not be certificated or issued as stand-alone securities. The Pre-Funded Warrants can each be purchased in this offering only with the accompanying Warrants as part of the Pre-Funded Ordinary Units, but the component parts of the Pre-Funded Ordinary Units will be immediately separable and issued separately in this offering.
For each Pre-Funded Ordinary Unit that we sell, the number of Ordinary Units that we are offering will be decreased on a one-for-one basis. Each Pre-Funded Warrant will be immediately exercisable and may be exercised at any time, subject to ownership limitations. The purchase price of each Pre-Funded Ordinary Unit is equal to the price per Ordinary Unit being sold to the public in this offering, minus $0.0005, and the exercise price of each Pre-Funded Warrant included in the Pre-Funded Ordinary Unit is $0.0005 per share. Because we will issue both a Series A Warrant and Series B Warrant as part of each Ordinary Unit or Pre-Funded Ordinary Unit, the number of Warrants sold in this offering will not change as a result of a change in the mix of the Ordinary Units and Pre-Funded Ordinary Units sold. |
Assumed public offering price
per Ordinary Unit and per Pre-Funded Ordinary Unit | |
$6.00 per Ordinary Unit and $5.9995 per Pre-Funded Ordinary Unit
|
Ordinary Shares included in the Ordinary Units offered by us (assuming no sale of the Pre-Funded Warrants, and no exercise of the Warrants included in the Units) | |
2,500,000 Ordinary Shares, assuming a $6.00 public offering price.
|
Pre-Funded Warrants included in the Pre-Funded Ordinary Units offered by us | |
The aggregate exercise price of the Pre-Funded Warrant, except for a nominal exercise price of $0.0005 per Ordinary Share, was pre-funded to the Company on or prior to its issuance date, consequently, no additional consideration (other than the nominal exercise price of $0.0005 per Ordinary Share) shall be required to be paid by the holder to effect any exercise of the Pre-Funded Warrant.
|
14
Table of Contents
Series A Warrants and Series B Warrants included in the Ordinary Units offered by us | |
The Series A Warrants will have five-year terms, will be immediately exercisable upon issuance, and have an initial exercise price equal to the actual public offering price of the Ordinary Units. Such exercise price will be reset on the Reset Date to a price that is equal to 105% of the arithmetic average of the sum of the three lowest per share VWAPs of the Ordinary Shares on the trading market for the twenty (20) trading days immediately prior to the Reset Date; provided that the exercise price shall not be lower than the Series A Floor Price. Notwithstanding the foregoing, the Company may reduce the Series A Floor Price to any amounts set forth in a written notice to the holder of the Series A Warrants; provided that such reduction shall be in compliance with applicable Nasdaq rules, irrevocable and not be subject to increase thereafter.
The Series B Warrants will have five-years terms and will be immediately exercisable upon issuance at an exercise price equal to the actual public offering price of the Ordinary Units. The Series B Warrants may also be exercised in whole by means of a one-time only “alternative cashless exercise” in which the holder shall be entitled to receive a number of Ordinary Shares equal to the product of (a) the aggregate number of Ordinary Shares that would be issuable upon exercise of the Series B Warrants if such exercise were by means of a cash exercise rather than a cashless exercise; multiplied by (b) the quotient obtained by dividing (i) the exercise price minus the lowest VWAP of the Ordinary Shares over the 10 trading days immediately prior to the exercise date by (ii) 50% of the lowest VWAP of the Ordinary Shares over the 10 trading days immediately prior to the exercise date. Such alternative cashless exercise is subject to the Beneficial Ownership Limitation, and shall only be available at a time when the applicable VWAP is lower than the then applicable exercise price. The maximum number of Ordinary Shares issuable pursuant to such alternative cashless exercise shall be equal to the quotient obtained by dividing (a) the aggregate exercise price of the Series B Warrant as of its issuance date, by (b) the Series B Floor Price. Notwithstanding the foregoing, the Company may reduce the Series B Floor Price to any amounts set forth in a written notice to the holder of the Series B Warrants; provided that such reduction shall be in compliance with applicable Nasdaq rules, irrevocable and not be subject to increase thereafter. |
| | | For more information regarding the Warrants, you should carefully read the section titled “Description of Share Capital — Warrants” in this prospectus, and the form of the Pre-Funded Warrants, Series A Warrants, and Series B Warrants, which are filed as exhibits to the registration statement of which this prospectus is a part. |
Ordinary Shares Outstanding Immediately Before This Offering | |
14,591,942
|
Ordinary Shares Outstanding Immediately After This Offering(1) | |
17,091,942 Ordinary Shares, based on an assumed public offering price of $6.00 per Ordinary Unit, assuming no sale of the Pre-Funded Warrants and no exercise of Warrants.
|
15
Table of Contents
Listing | | Our Ordinary Shares are listed on the Nasdaq Capital Market. There is no established public trading market for the Units or the Warrants, and we do not expect a market to develop. We do not intend to apply for listing of the Units or the Warrants on any securities exchange or other nationally recognized trading system. Without an active trading market, the liquidity of such securities will be limited. |
Ticker symbol | | “WOK” |
Transfer Agent | | VStock Transfer LLC |
Use of proceeds | | We estimate that we will receive net proceeds from this offering of approximately $13,712,279, based on an assumed public offering price of $6.00 per Ordinary Unit, assuming no exercise of the Warrants included in the Units, after deducting an estimated underwriters’ discount and estimated offering expenses payable by us. We intend to use the proceeds from this offering for (i) upgrading production equipment and investing in the PRC subsidiaries’ research and development; (ii) hiring experienced employees to improve our systems of internal control and compliance with U.S. GAAP and the Sarbanes-Oxley Act of 2002; and (iii) working capital and general corporate purposes. See “Use of Proceeds” on page 60 for more information. |
Lock-up Agreements | | Our directors, officers and holders of five percent (5%) or more of our outstanding Ordinary Shares as of the effective date of the registration statement related to this offering will enter into customary “lock-up” agreements in favor of the Representative pursuant to which such persons and entities shall agree, for a period of one hundred eighty (180) days following the closing of the offering of the securities offered hereby, that they shall neither offer, issue, sell, contract to sell, encumber, grant any option for the sale of or otherwise dispose of any of our securities without the Representative’s prior written consent, including the issuance of Ordinary Shares upon the exercise of currently outstanding convertible securities. The Company, on behalf of itself and any successor entity, will not, without the prior written consent of the Representatives, for a period of thirty (30) days from the closing of this offering, (i) offer, pledge, announce the intention to sell, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase, lend, or otherwise transfer or dispose of, directly or indirectly, or file with the SEC a registration statement under the Securities Act relating to, any Ordinary Shares or any securities convertible into or exercisable or exchangeable for Ordinary Shares, (ii) enter into any swap or other agreement that transfers, in whole or in part, any of the economic consequences of ownership of the Ordinary Shares or any such other securities, whether any such transaction described in clause (i), or (ii) above is to be settled by delivery of Ordinary Shares or such other securities, in cash or otherwise, except to the underwriters, or (iii) repurchase any Ordinary Shares. |
Risk Factors | | The securities offered hereby involve a high degree of risk. You should read “Risk Factors” beginning on page 17 for a discussion of factors to consider before deciding to invest in the securities we offered. |
16
Table of Contents
RISK FACTORS
An investment in our securities involves a high degree of risk. Before deciding whether to invest in our securities, you should consider carefully the risks described below, together with all of the other information set forth in this prospectus, including the section titled “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our consolidated financial statements and related notes. If any of these risks actually occurs, our business, financial condition, results of operations, or cash flow could be materially and adversely affected, which could cause the trading price of our Ordinary Shares to decline, resulting in a loss of all or part of your investment. The risks described below and discussed in other parts of this prospectus are not the only ones that we face. Additional risks not presently known to us or that we currently deem immaterial may also affect our business. You should only consider investing in our securities if you can bear the risk of loss of your entire investment.
Risks Related to Doing Business in China
Changes in the political and economic policies of the PRC government or in relations between China and the United States may materially and adversely affect the PRC subsidiaries’ and our business, financial condition and results of operations and may result in the PRC subsidiaries’ inability to sustain their growth and expansion strategies.
All of the operations are conducted in the PRC and a majority of our revenue is sourced from the PRC. Accordingly, our financial condition and results of operations are affected to a significant extent by economic, political and legal developments in the PRC or changes in government relations between China and the United States or other governments. There is significant uncertainty about the future relationship between the United States and China with respect to trade policies, treaties, government regulations and tariffs.
The PRC economy differs from the economies of most developed countries in many respects, including the extent of government involvement, level of development, growth rate, control of foreign exchange and allocation of resources. Although the PRC government has implemented measures emphasizing the utilization of market forces for economic reform, the reduction of state ownership of productive assets, and the establishment of improved corporate governance in business enterprises, a substantial portion of productive assets in China is still owned by the government. In addition, the PRC government continues to play a significant role in regulating industry development by imposing industrial policies. The PRC government also exercises significant control over China’s economic growth by allocating resources, controlling payment of foreign currency-denominated obligations, setting monetary policy, regulating financial services and institutions and providing preferential treatment to particular industries or companies.
While the PRC economy has experienced significant growth in the past four decades, growth has been uneven, both geographically and among various sectors of the economy. The PRC government has implemented various measures to encourage economic growth and guide the allocation of resources. Some of these measures may benefit the overall PRC economy, but may also have a negative effect on the PRC subsidiaries. The PRC subsidiaries’ financial condition and results of operation could be materially and adversely affected by government control over capital investments or changes in tax regulations that are applicable to the PRC subsidiaries. In addition, the PRC government has implemented in the past certain measures, including interest rate increases, to control the pace of economic growth. These measures may cause decreased economic activity.
In July 2021, the SEC has imposed enhanced disclosure requirements on China-based companies seeking to register securities with the SEC. As all of the operations are based in China, any future Chinese, U.S. or other rules and regulations that place restrictions on capital raising or other activities by China based companies could adversely affect the PRC subsidiaries’ business and results of operations. If the business environment in China deteriorates from the perspective of domestic or international investment, or if relations between China and the United States or other governments deteriorate, the Chinese government may intervene with the PRC subsidiaries’ operations and business in China and United States, as well as the market price of our Ordinary Shares, may also be adversely affected.
There are uncertainties regarding the interpretation and enforcement of PRC laws, rules and regulations.
All of the operations are conducted in the PRC, and are governed by PRC laws, rules and regulations. The PRC subsidiaries are subject to laws, rules and regulations applicable to foreign investment in China. The PRC legal system is a civil law system based on written statutes. Unlike the common law system, prior court decisions may be cited for reference but have limited precedential value.
17
Table of Contents
In 1979, the PRC government began to promulgate a comprehensive system of laws, rules and regulations governing economic matters in general. The overall effect of legislation over the past four decades has significantly enhanced the protections afforded to various forms of foreign investment in China. However, China has not developed a fully integrated legal system, and recently enacted laws, rules and regulations may not sufficiently cover all aspects of economic activities in China or may be subject to significant degrees of interpretation by PRC regulatory agencies. In particular, because these laws, rules and regulations are relatively new, and because of the limited number of published decisions and the nonbinding nature of such decisions, and because the laws, rules and regulations often give the relevant regulator significant discretion in how to enforce them, the interpretation and enforcement of these laws, rules and regulations involve uncertainties and can be inconsistent and unpredictable. In addition, the PRC legal system is based in part on government policies and internal rules, some of which are not published on a timely basis or at all, and which may have a retroactive effect. As a result, we or the PRC subsidiaries may not be aware of the violation of these policies and rules until after the occurrence of the violation.
Any administrative and court proceedings in China may be protracted, resulting in substantial costs and diversion of resources and management attention. Since PRC administrative and court authorities have significant discretion in interpreting and implementing statutory and contractual terms, it may be more difficult to evaluate the outcome of administrative and court proceedings and the level of legal protection we or the PRC subsidiaries enjoy than in more developed legal systems. These uncertainties may impede the PRC subsidiaries’ ability to enforce the contracts they have entered into and could materially and adversely affect the PRC subsidiaries’ and our business, financial condition and results of operations.
On July 6, 2021, the General Office of the Central Committee of the Communist Party of China and the General Office of the State Council jointly issued the “Opinions on Severely Cracking Down on Illegal Securities Activities According to Law,” or the Opinions, and made the Opinions available to the public. The Opinions emphasized the need to strengthen the administration over illegal securities activities, and the need to strengthen the supervision over overseas listings by Chinese companies. Effective measures, such as promoting the construction of relevant regulatory systems will be taken to deal with the risks and incidents of China-concept overseas listed companies, and cybersecurity and data privacy protection requirements and similar matters. The Opinions remain unclear on how the law will be interpreted, amended and implemented by the relevant PRC governmental authorities, but the Opinions and any related implementing rules to be enacted may subject us and the PRC subsidiaries to compliance requirements in the future.
On July 10, 2021, the Cyberspace Administration of China issued a revised draft of the Measures for Cybersecurity Review for public comments, which required that, among others, in addition to “operator of critical information infrastructure,” any “data processor” controlling personal information of no less than one million users which seeks to list in a foreign stock exchange should also be subject to cybersecurity review, and further elaborated the factors to be considered when assessing the national security risks of the relevant activities. On December 28, 2021, the Measures for Cybersecurity Review (2021 version) were promulgated and took effect on February 15, 2022, which provide that any “online platform operators” controlling personal information of more than one million users which seeks to list in a foreign stock exchange should be subject to cybersecurity review.
On November 14, 2021, the Cyberspace Administration of China released the Regulations on Network Data Security (draft for public comments) and will accept public comments until December 13, 2021. The draft Regulations on Network Data Security provide that if a data processor that processes personal data of more than one million users intends to list overseas, it shall apply for a cybersecurity review. In addition, data processors that process important data or are listed overseas shall carry out an annual data security assessment on their own or by engaging a data security services institution, and the data security assessment report for the prior year should be submitted to the local cyberspace affairs administration department before January 31 of each year. We do not believe we or any of the PRC subsidiaries may be considered to be an “operator of critical information infrastructure”, or “online platform operators,” as mentioned above; however, Measures for Cybersecurity Review (2021 version) were recently adopted and the Regulations on Network Data Security (draft for public comments) are in the process of being formulated and it remains unclear how the Opinions will be interpreted, amended and implemented by the relevant PRC governmental authorities.
On February 17, 2023, the CSRC issued the New Administrative Rules Regarding Overseas Listings, which came into force on March 31, 2023. According to the New Administrative Rules Regarding Overseas Listings, subsequent securities offerings of the company in the same overseas market where it has previously offered and listed securities
18
Table of Contents
shall file with the CSRC within three working days after the subsequent securities offering is completed. As this offering constitutes a subsequent offering by us, we are required to file with the CSRC in accordance with the Trial Administrative Measures within three working days after this offering is completed. See “— Additional compliance procedures may be required in connection with this offering, due to the promulgation of the new filing-based administrative rules for overseas offering and listing by domestic companies in China, which could significantly limit or completely hinder our ability to offer or continue to offer our Ordinary Shares to investors and could cause the value of our Ordinary Shares to significantly decline or become worthless” on page 20.
Furthermore, if the CSRC or other regulatory agencies later promulgate new rules or explanations requiring that we obtain their approvals for this offering and any follow-on offering, we may be unable to obtain such approvals which could significantly limit or completely hinder our ability to offer or continue to offer securities to our investors.
Furthermore, the PRC government authorities may strengthen oversight and control over offerings that are conducted overseas and/or foreign investment in China-based issuers like us. Such actions taken by the PRC government authorities may intervene or influence the PRC subsidiaries’ operations at any time, which are beyond our control. Therefore, any such action may adversely affect the PRC subsidiaries’ operations and significantly limit or hinder our ability to offer or continue to offer securities to you and reduce the value of such securities.
Uncertainties regarding the enforcement of laws and the fact that rules and regulations in China can change quickly with little advance notice, along with the risk that the Chinese government may intervene or influence the PRC subsidiaries’ operations at any time, or may exert more control over offerings conducted overseas and/or foreign investment in China-based issuers could result in a material change in the PRC subsidiaries’ and our operations, financial performance and/or the value of our Ordinary Shares or impair our ability to raise money.
The PRC government exerts substantial influence over the manner in which the PRC subsidiaries conduct their business activities. The PRC government may also intervene or influence the PRC subsidiaries’ operations and this offering at any time, which could result in a material change in the PRC subsidiaries’ operations and our Ordinary Shares could significantly decline in value or become worthless.
Except for completing the filing procedures with the CSRC, we are currently not required to obtain any other approval from Chinese authorities to list on U.S exchanges. However, if the Company or any of the PRC subsidiaries were required to obtain approval in the future and were denied permission from Chinese authorities to list on U.S. exchanges, we will not be able to continue listing on U.S. exchange, continue to offer securities to investors, or materially affect the interest of the investors and cause significantly depreciation of our price of Ordinary Shares.
The Chinese government has exercised and continues to exercise substantial control over virtually every sector of the Chinese economy through regulation and state ownership. Our ability to operate in China may be harmed by changes in its laws and regulations, including those authority relating to taxation, environmental regulations, land use rights, property and other matters. The central or local governments of these jurisdictions may impose new, stricter regulations or interpretations of existing regulations that would require additional expenditures and efforts on our and the PRC subsidiaries’ part to ensure compliance with such regulations or interpretations. Accordingly, government actions in the future, including any decision not to continue to support recent economic reforms and to return to a more centrally planned economy or regional or local variations in the implementation of economic policies, could have a significant effect on economic conditions in China or particular regions thereof, and could require us to divest ourselves of any interest we then hold in the operations in China.
The Chinese cybersecurity regulator announced on July 2, 2021, that it had begun an investigation of Didi Global Inc. (NYSE: DIDI) and two days later ordered that the company’s app be removed from smartphone app stores. Similarly, the PRC subsidiaries’ business segments may be subject to various government and regulatory interference in the regions in which the PRC subsidiaries operate. The PRC subsidiaries could be subject to regulation by various political and regulatory entities, including various local and municipal agencies and government sub-divisions. The PRC subsidiaries may incur increased costs necessary to comply with existing and newly adopted laws and regulations or penalties for any failure to comply.
Furthermore, it is uncertain when and whether we or the PRC subsidiaries will be required to obtain permission from the PRC government to list on U.S. exchanges in the future, and even when such permission is obtained, whether it will be denied or rescinded. Although neither we nor any of the PRC subsidiaries is currently required to obtain permission from any of the PRC central or local government, except for completing the filing procedures with the CSRC, and
19
Table of Contents
neither we nor any of the PRC subsidiaries has received any denial to list on a U.S. exchange, the PRC subsidiaries’ operations could be adversely affected, directly or indirectly, by existing or future laws and regulations relating to the PRC subsidiaries’ business or industry, which may also adversely affect the Group as a whole. Recent statements by the Chinese government indicating an intent, and the PRC government may take actions to exert more oversight and control over offerings that are conducted overseas and/or foreign investment in China-based issuers, which could significantly limit or completely hinder our ability to offer or continue to offer securities to investors and cause the value of our securities to significantly decline or become worthless.
Additional compliance procedures may be required in connection with this offering, due to the promulgation of the new filing-based administrative rules for overseas offering and listing by domestic companies in China, which could significantly limit or completely hinder our ability to offer or continue to offer our Ordinary Shares to investors and could cause the value of our Ordinary Shares to significantly decline or become worthless.
On February 17, 2023, the CSRC issued the New Administrative Rules Regarding Overseas Listings, which became effective on March 31, 2023. These rules propose to establish a new filing-based regime to regulate overseas offerings and listings by Chinese domestic companies. Under the New Administrative Rules Regarding Overseas Listings, a domestic company in the PRC that seeks to offer and list securities on overseas markets shall fulfill the filing procedures with the CSRC as per requirement of the Trial Administrative Measures. This includes subsequent securities offerings of the company in the same overseas market where it has previously offered and listed securities, which requires a company, such as ours, to file with the CSRC within three working days after the subsequent securities offering is completed. In the opinion of our PRC legal counsel, AllBright Law Offices (Fuzhou), as this offering constitutes a subsequent offering by us, we are required to file with the CSRC in accordance with the Trial Administrative Measures within three working days after this offering is completed. We cannot assure you that we can complete the required filing procedures with the CSRC or receive any other approvals or complete other compliance procedures in a timely manner, or at all, or that any completion of filing or approval or other compliance procedures would not be rescinded. Any such failure to do so would subject us to sanctions by the CSRC or other PRC regulatory authorities. These regulatory authorities may impose restrictions and penalties on our operations in China, significantly limit or completely hinder our ability to launch any new offering of our securities, limit our ability to pay dividends outside China, delay or restrict the repatriation of the proceeds from future capital raising activities into China, or take other actions that could materially and adversely affect our business, results of operations, financial condition and prospects, as well as the trading price of our Ordinary Shares. Furthermore, the PRC government authorities may further strengthen oversight and control over listings and offerings that are conducted overseas. Any such action may adversely affect our operations and significantly limit or completely hinder our ability to offer or continue to offer securities to you and cause the value of such securities to significantly decline or be worthless. Furthermore, on February 24, 2023, the CSRC promulgated the Confidentiality and Archives Administration Provisions, which also became effective on March 31, 2023. According to the Confidentiality and Archives Administration Provisions, domestic companies that seek overseas offering and listing (either in direct or indirect means) and the securities companies and securities service (either incorporated domestically or overseas) providers that undertake relevant businesses shall institute a sound confidentiality and archives administration system, and take necessary measures to fulfill confidentiality and archives administration obligations. They shall not leak any state secret or working secret of government agencies, or harm national security and public interests. Therefore, a domestic company that plans to, either directly or through its overseas listed entity, publicly disclose or provide to relevant individuals or entities including securities companies, securities service providers and overseas regulators, any documents and materials that contain state secrets or working secrets of government agencies, shall first obtain approval from competent authorities according to law, and file with the secrecy administrative department at the same level. The above-mentioned documents and materials that, if leaked, will be detrimental to national security or public interest, therefore, the domestic company shall strictly fulfill relevant procedures stipulated by applicable regulations. Furthermore, a domestic company that provides accounting archives or copies of accounting archives to any entities, including securities companies, securities service providers and overseas regulators and individuals, shall fulfill due procedures in compliance with applicable regulations. Working papers produced in mainland China by securities companies and securities service providers in the process of undertaking businesses related to overseas offering and listing by domestic companies shall be retained in mainland China. Where such documents need to be transferred or transmitted to areas outside of mainland China, relevant approval procedures stipulated by regulations shall be followed. We believe that this offering does not involve the leaking of any state secret
20
Table of Contents
or working secret of government agencies, or the harming of national security and public interests. However, we may be required to perform additional procedures in connection with the provision of accounting archives. The specific requirements of the relevant procedures are currently unclear and we cannot be certain whether we will be able to perform the relevant procedures.
You may experience difficulties in effecting service of legal process, enforcing foreign judgments or bringing actions in China against us or our directors and officers named in the prospectus based on foreign laws.
We are an exempted company incorporated under the laws of the Cayman Islands. Our operations are conducted by the PRC subsidiaries in China, and most of our assets are located in China. In addition, most of our directors and senior executive officers reside within China for a significant portion of the time and are PRC nationals. As a result, it may be difficult for our shareholders to effect service of process upon us or those persons inside China. In addition, China does not have treaties providing for the reciprocal recognition and enforcement of judgments of courts with the Cayman Islands and many other countries and regions. Therefore, recognition and enforcement in China of judgments of a court in any of these non-PRC jurisdictions in relation to any matter not subject to a binding arbitration provision may be difficult or impossible.
Shareholder claims that are common in the United States, including securities law class actions and fraud claims, generally are difficult to pursue as a matter of law or practicality in China. For example, in China, there are significant legal and other obstacles to obtaining information needed for shareholder investigations or litigation outside China or otherwise with respect to foreign entities. Although the local authorities in China may establish a regulatory cooperation mechanism with the securities regulatory authorities of another country or region to implement cross-border supervision and administration, such regulatory cooperation with the securities regulatory authorities in the Unities States have not been efficient in the absence of mutual and practical cooperation mechanism. According to Article 177 of the PRC Securities Law which took effect in March 2020, overseas securities regulatory authorities may not carry out investigations and evidence collection directly within the territory of mainland China, and that no Chinese entity or individual is allowed to provide any documents or materials related to securities business activities to overseas agencies without prior consent of the securities regulatory authority of the State Council and the competent departments of the State Council.
You may incur additional costs and face procedural obstacles in effecting service of legal process, enforcing foreign judgments or bringing actions in Hong Kong against us or our management named in this prospectus based on Hong Kong laws.
You may incur additional costs and face procedural obstacles in effecting service of legal process, enforcing foreign judgments or bringing actions in Hong Kong against us or our management, as judgments entered in the United States can be enforced in Hong Kong only at common law. To enforce a judgment of the United States in Hong Kong, it must be a final judgment rendered conclusively upon the merits of the claim, for a liquidated amount in a civil matter and not in respect of taxes, fines, penalties, or similar charges, the proceedings in which the judgment was obtained were not contrary to natural justice, and the enforcement of the judgment is not contrary to public policy of Hong Kong. Such a judgment must be for a fixed sum and must also come from a “competent” court, as determined by the private international law rules applied by the Hong Kong courts.
Furthermore, foreign judgments of United States courts will not be directly enforced in Hong Kong. There are currently no treaties or other arrangements providing for reciprocal enforcement of foreign judgments between Hong Kong and the United States. However, the common law of Hong Kong permits an action to be brought upon a foreign judgment. A foreign judgment itself may form the basis of a cause of action, since the judgment may be regarded as creating a debt between the parties to such action. The defenses that are available to a defendant in a common law action brought on the basis of a foreign judgment include lack of jurisdiction, breach of natural justice, fraud, and contrary to public policy. However, a separate legal action for debt must be commenced in Hong Kong in order to recover such debt from the judgment debtor. A foreign judgment of the United States predicated solely upon the federal securities laws of the United States or the securities laws of any State or territory within the United States may be enforceable in Hong Kong, but will be subject to the conditions with regard to enforcement of such judgments being met, including but not limited to the foregoing requirements. See “Enforceability of Civil Liabilities” beginning on page 58.
21
Table of Contents
Any requirement to obtain prior approval under the M&A Rules and/or any other regulations promulgated by relevant PRC regulatory agencies in the future could delay this offering and failure to obtain any such approvals, if required, could have a material adverse effect on the PRC subsidiaries’ and our business, operating results and reputation, as well as the trading price of our Ordinary Shares, and could also create uncertainties for this offering and affect our ability to offer or continue to offer securities to investors outside China.
On August 8, 2006, six PRC regulatory agencies, including the Ministry of Commerce (the “MOFCOM”), the State-Owned Assets Supervision and Administration Commission (“SASAC”), the State Administration of Taxation (“SAT”), the State Administration of Industry and Commerce (“SAIC”), the CSRC, and the State Administration of Foreign Exchange (“SAFE”), jointly adopted the M&A Rules, which came into effect on September 8, 2006 and were amended on June 22, 2009. The M&A Rules include, among other things, provisions that purport to require that offshore special purpose vehicle formed for the purpose of an overseas listing of securities in a PRC entity obtain the approval of the CSRC prior to the listing and trading of any such special purpose vehicle’s securities on an overseas stock exchange. On September 21, 2006, the CSRC published on its official website procedures regarding its approval of overseas listings by special purpose vehicles. However, substantial uncertainty remains regarding the scope and applicability of the M&A Rules to offshore special purpose vehicles.
While the application of the M&A Rules remains unclear, we believe, based on the advice of our PRC counsel, AllBright Law Offices (Fuzhou), that (i) since the Company is neither controlled by PRC entities or individuals, nor formed for overseas listing purposes through acquisitions of PRC domestic interests held by such PRC entities or individuals, we believe that the Company is not an SPV, and therefore, the M&A Rules do not apply to us, and we do not need to obtain the approval from the CSRC under the M&A Rules; and (ii) there are substantial uncertainties regarding the interpretation and application of PRC laws and regulations and future PRC laws and regulations, and there can be no assurance that the relevant government agencies will take a view that is contrary to, or otherwise different from, the conclusion stated above. Substantial uncertainty remains regarding the scope and applicability of the M&A Rules to offshore special purpose vehicles. If the CSRC or other PRC regulatory body subsequently determines that we need to obtain the CSRC’s approval for this offering or if the CSRC or any other PRC government authorities promulgates any interpretation or implements rules before our listing that would require us to obtain CSRC or other governmental approvals for this offering under the M&A Rules, we may face adverse actions or sanctions by the CSRC or other PRC regulatory agencies. In any such event, these regulatory agencies may impose fines and penalties on the PRC subsidiaries’ operations in China, limit the PRC subsidiaries operating privileges in China, delay or restrict the repatriation of the proceeds from this offering into the PRC or take other actions that could have a material adverse effect on the PRC subsidiaries’ and our business, financial condition, results of operations, reputation and prospects, as well as our ability to complete this offering. The CSRC or other PRC regulatory agencies may also take actions requiring us, or making it advisable for us, to halt this offering before settlement and delivery of the Ordinary Shares offered by this prospectus. Consequently, if you engage in market trading or other activities in anticipation of and prior to settlement and delivery, you do so at the risk that such settlement and delivery may not occur. See “Regulations — M&A Rules and Regulation on Overseas Listings” on page 120.
In addition, the security review rules issued by the MOFCOM that took effect in September 2011 specify that if a merger and acquisition of domestic enterprise by a foreign investor falls within the M&A safety review scope, the foreign investor shall file an application for M&A safety review to the Ministry of Commerce. The M&A safety review scope is as follows: foreign investors’ M&A of domestic military industry enterprises and military industry support enterprises, enterprises around key and sensitive military facilities, and other units which have impact on national defense security; and foreign investors’ M&A of domestic enterprises, which have impact on the national security, in fields of important agricultural products, important energy and resources, important infrastructure, important transport service, key technology and major equipment manufacturing, etc. and such M&A may result in foreign investors’ acquirement of actual control over the enterprises. The rules prohibit any activities attempting to bypass a security review, including by holding on agency basis, trust, multi-tier reinvestment, leasing, loan, control by agreement, overseas transactions, etc.
The PRC subsidiaries are not operating in an industry that prohibits or limits foreign investment. As a result, the PRC subsidiaries are not required to obtain any permission from Chinese authorities to operate, other than those permissions a domestic company in China will need to engage in businesses similar to the PRC subsidiaries’. Such licenses and permissions include a Business License, Medical Device Registration Certificate, Medical Device Production Certificate, Medical Device Selling Certificate, Record Form of Medical Device Products, Certificate of Class I medical device production recordation, Certificate of Class II medical device selling recordation, Record Registration
22
Table of Contents
Form for Foreign Trade Business Operators, and Certificate of the Customs of the People’s Republic of China on Registration of a Customs Declaration Entity. The PRC subsidiaries have obtained the above licenses and permissions to conduct their business in China. However, the PRC government may take actions to exert more oversight and control over offerings by China-based issuers conducted overseas and/or foreign investment in such companies, which could significantly limit or completely hinder our ability to offer or continue to offer securities to investors outside China and cause the value of our securities to significantly decline or become worthless.
In the future, the PRC subsidiaries may grow their business by acquiring complementary businesses. Complying with the requirements of the above-mentioned regulations and other relevant rules to complete such transactions, if required, could be time-consuming, and any required approval processes, including obtaining approval from the MOFCOM or its local counterparts may delay or inhibit the PRC subsidiaries’ ability to complete such transactions. It is unclear whether the PRC subsidiaries’ business would be deemed to be in an industry that raises “national defense security” or “national security” concerns. However, the MOFCOM or other government agencies may publish explanations in the future determining that the PRC subsidiaries’ business is in an industry subject to the security review, in which case the PRC subsidiaries’ future acquisitions in the PRC, may be closely scrutinized or prohibited. The PRC subsidiaries’ abilities to expand the business or maintain or expand market share through future acquisitions would as such be materially and adversely affected. Furthermore, according to the M&A Rules, if a PRC entity or individual plans to merge or acquire its related PRC entity through an overseas company legitimately incorporated or controlled by such entity or individual, such a merger and acquisition will be subject to examination and approval by the MOFCOM. There is a possibility that the PRC regulators may promulgate new rules or explanations requiring that the PRC subsidiaries obtain the approval of the MOFCOM or other PRC governmental authorities for the PRC subsidiaries’ completed or ongoing mergers and acquisitions. There is no assurance that, if the PRC subsidiaries plan to make an acquisition, they can obtain such approval from the MOFCOM or any other relevant PRC governmental authorities for their mergers and acquisitions, and if the PRC subsidiaries fail to obtain those approvals, they may be required to suspend their acquisition and be subject to penalties. Any uncertainties regarding such approval requirements could have a material adverse effect on the PRC subsidiaries’ and our business, results of operations and corporate structure.
In addition, on July 6, 2021, the relevant PRC government authorities made public the Opinions on Strictly Cracking Down Illegal Securities Activities in Accordance with the Law. These opinions emphasized the need to strengthen the administration over illegal securities activities and the supervision on overseas listings by China-based companies and proposed to take effective measures, such as promoting the construction of relevant regulatory systems to deal with the risks and incidents faced by China-based overseas-listed companies. Pursuant to the Opinions, Chinese regulators are required to accelerate rulemaking related to the overseas issuance and listing of securities, and update the existing laws and regulations related to data security, cross-border data flow, and management of confidential information. Numerous regulations, guidelines and other measures are expected to be adopted under the umbrella of or in addition to the Cybersecurity Law and Data Security Law.
On July 10, 2021, the Cyberspace Administration of China issued the Measures for Cybersecurity Review (Revision Draft for Comments) for public comments, which proposes to authorize the relevant government authorities to conduct cybersecurity review on a range of activities that affect or may affect national security, including listings in foreign countries by an operator that possess the personal data of more than one million users. The operators refer to operators of critical information infrastructure and data processors. On November 14, 2021, the Cyberspace Administration of China issued the Regulations on Network Data Security (draft for public comments) which set forth cyber data security compliance requirements in greater detail. On December 28, 2021, the Measures for Cybersecurity Review (2021 version) were promulgated and took effect on February 15, 2022, which provide that any “online platform operators” controlling personal information of more than one million users which seeks to list in a foreign stock exchange should also be subject to cybersecurity review. Neither we nor the PRC subsidiaries do expect to be subject to cybersecurity review, because the PRC subsidiaries currently engage in the manufacture and sale of medical devices and neither we nor the PRC subsidiaries possess personal information of over one million users. As of the date of this prospectus, neither we nor the PRC subsidiaries have been involved in any investigations on cybersecurity review initiated by the Cyberspace Administration of China, and neither we nor the PRC subsidiaries have received any warning, sanction, or penalty in such respect. However, the Measures for Cybersecurity Review were recently adopted and the Regulations on Network Data Security (draft for public comments) are in the process of being formulated and the Opinions remain unclear on how they will be interpreted, amended and implemented by the relevant PRC governmental authorities. On February 17, 2023, the CSRC issued the New Administrative Rules Regarding Overseas Listings, which came into force on March 31, 2023. According to the New Administrative Rules Regarding Overseas Listings, among other things, a domestic company in the PRC that seeks to offer and list securities on overseas markets shall
23
Table of Contents
fulfill the filing procedures with the CSRC as per requirement of the Trial Administrative Measures. Where a domestic company seeks to directly offer and list securities on overseas markets, the issuer shall file with the CSRC. Where a domestic company seeks to indirectly offer and list securities on overseas markets, the issuer shall designate a major domestic operating entity, which shall, as the domestic responsible entity, file with the CSRC. Initial public offerings or listings on overseas markets shall be filed with the CSRC within 3 working days after the relevant application is submitted overseas. See “— Additional compliance procedures may be required in connection with this offering, due to the promulgation of the new filing-based administrative rules for overseas offering and listing by domestic companies in China, which could significantly limit or completely hinder our ability to offer or continue to offer our Ordinary Shares to investors and could cause the value of our Ordinary Shares to significantly decline or become worthless” on page 20. Furthermore, if the CSRC or other regulatory agencies later promulgate new rules or explanations requiring that we or the PRC subsidiaries obtain their prior approvals or ex-post record-filing for this offering and any follow-on offering, we or the PRC subsidiaries may be unable to obtain such approvals or record-filing which could significantly limit or completely hinder our ability to offer or continue to offer securities to our investors.
Except for the filing procedures with the CSRC, as of the date of this prospectus, we do not believe we or the PRC subsidiaries are required to obtain any permission from any PRC governmental authorities to offer securities to foreign investors. We have been closely monitoring regulatory developments in China regarding any necessary approvals from the CSRC or other PRC governmental authorities required for overseas listings, including this offering and offering securities to foreign investors. As of the date of this prospectus, neither we nor the PRC subsidiaries have received any inquiry, notice, warning, sanctions or regulatory objection to this offering from the CSRC or other PRC governmental authorities. However, there remains significant uncertainty regarding the enactment, interpretation and implementation of regulatory requirements related to overseas securities offerings and other capital markets activities. If it is determined in the future that the approval of the CSRC, the Cyberspace Administration of China or any other regulatory authority is required for this offering, we or the PRC subsidiaries may face sanctions by the CSRC, the Cyberspace Administration of China or other PRC regulatory agencies. These regulatory agencies may impose fines and penalties on the PRC subsidiaries’ operations in China, limit the PRC subsidiaries ability to pay dividends outside of China, limit the PRC subsidiaries’ operations in China, delay or restrict the repatriation of the proceeds from this offering into China or take other actions that could have a material adverse effect on the PRC subsidiaries’ and our business, financial condition, results of operations and prospects, as well as the trading price of our securities. The CSRC, the Cyberspace Administration of China or other PRC regulatory agencies also may take actions requiring us, or making it advisable for us, to halt this offering before settlement and delivery of our Ordinary Shares. Consequently, if you engage in market trading or other activities in anticipation of and prior to settlement and delivery, you do so at the risk that settlement and delivery may not occur. In addition, if the Cyberspace Administration of China or other PRC regulatory agencies later promulgate new rules requiring that we or the PRC subsidiaries obtain their approvals for this offering, we or the PRC subsidiaries may be unable to obtain such approval or a waiver of such approval requirements, if and when procedures are established to obtain such a waiver. Any uncertainties and/or negative publicity regarding such an approval requirement could have a material adverse effect on the trading price of our securities.
Hong Kong does not have a comprehensive cyber or data security law. The Personal Data (Privacy) Ordinance (Chapter 486 of the Laws of Hong Kong) (“PDPO”) is the main legislation in Hong Kong which aims to protect the privacy of individuals in relation to personal data, and to regulate the collection, holding, processing, or use of personal data based on a set of data protection principles. The PDPO regulates the conduct of a data user, i.e. any person who, either alone or jointly or in common with other persons, controls the collection, holding, processing or use of personal data.
Pursuant to Section 33 of the PDPO, the PDPO is applicable to the collection and processing of personal data if such activities take place in Hong Kong, or if the personal data is collected by a data user whose principal place of business is in Hong Kong. Contravention with the data protection principals set out in PDPO may entitle the Privacy Commissioner for Personal Data to issue a written notice directing the data user to remedy and prevent recurrence of any contravention. As of the date of this prospectus, we have neither collected nor processed any personal data in Hong Kong, and our Hong Kong subsidiary, Work Medical Technology, has not collected or processed any personal data. Therefore, we believe the PDPO does not currently have any material impact on our Hong Kong subsidiary’s business, or our business, financial condition or this offering. However, if (i) we or our Hong Kong subsidiary do not comply with any privacy laws or regulations, or any compromise of security that results in the unauthorized release or transfer of personally identifiable information or users’ personal data, or (ii) applicable laws, regulations, or interpretations thereof change and we or our Hong Kong subsidiary become subject to any requirements for additional
24
Table of Contents
permissions or approvals in the future, we or our Hong Kong subsidiary may have to expend significant time and costs to procure them. If we or our Hong Kong subsidiary is unable to do so, on commercially reasonable terms, in a timely manner or otherwise, we or our Hong Kong subsidiary may become subject to governmental enforcement actions, litigation or public statements against us or our Hong Kong subsidiary by customers or others, and our and our Hong Kong subsidiary’s and business, reputation, financial condition, and results of operations may be materially and adversely affected.
In addition, although Work Medical Technology, our Hong Kong subsidiary, is an investment holding company, the legal and operational risks associated with operating in mainland China may also apply to the future limited activities (if any) in Hong Kong of Work Medical Technology, to the extent that they are made applicable to such entity and its anticipated operations. Work Medical Technology, as of the date of this prospectus, has yet to commence operations, and is expected to be limited to operating as an investment holding company in the future without any substantive or data-related operations in Hong Kong. However, such operations may be affected if Hong Kong adopts rules, regulations or policy guidance with respect to currency exchange control. Furthermore, as of the date of this prospectus, we do not expect that any regulatory actions related to data security or anti-monopoly concerns in Hong Kong will impact the Company’s ability to conduct its business, accept foreign investments, or list on a U.S. or foreign exchange, because we have never had and do not plan to have any material operations in Hong Kong.
PRC regulations regarding acquisitions impose significant regulatory approval and review requirements, which could make it more difficult for the PRC subsidiaries to pursue growth through acquisitions.
Under the Anti-Monopoly Law, companies undertaking acquisitions relating to businesses in China must notify the State Council’s anti-monopoly law enforcement authority, in advance of any transaction where the parties’ revenue in the China market exceed certain thresholds and the buyer would obtain control of, or decisive influence over, the target, while under the M&A Rules, the approval of MOFCOM must be obtained in circumstances where overseas companies established or controlled by PRC entities or residents acquire domestic companies affiliated with such PRC entities or residents. Applicable PRC laws, rules and regulations also require certain merger and acquisition transactions to be subject to security review. Due to the level of our revenue, the PRC subsidiaries’ proposed acquisition of control of, or decisive influence over, any company with revenue within China of more than RMB400 million in the year prior to any proposed acquisition would be subject to the State Council’s anti-monopoly law enforcement authority’s merger control review. As a result, many of the transactions the PRC subsidiaries may undertake could be subject to the State Council’s anti-monopoly law enforcement authority’s merger review. Complying with the requirements of the relevant regulations to complete such transactions could be time-consuming, and any required approval processes, including approval from the State Council’s anti-monopoly law enforcement authority, may delay or inhibit the PRC subsidiaries’ ability to complete such transactions, which could affect the PRC subsidiaries’ ability to expand their business or maintain market share, and which may adversely affect the Group as a whole. If the practice of the State Council’s anti-monopoly law enforcement authority and MOFCOM remains unchanged, the PRC subsidiaries’ ability to carry out their investment and acquisition strategy may be materially and adversely affected and there may be significant uncertainty as to whether the PRC subsidiaries will be able to complete large acquisitions in the future in a timely manner or at all, which could adversely affect the expansion goals of the Group.
PRC regulations relating to investments in offshore companies by PRC residents may subject our PRC-resident beneficial owners or the PRC subsidiaries to liability or penalties, limit our ability to inject capital into the PRC subsidiaries or limit the PRC subsidiaries’ ability to increase their registered capital or distribute profits.
SAFE promulgated the Circular on Relevant Issues Concerning Foreign Exchange Control on Domestic Residents’ Offshore Investment and Financing and Roundtrip Investment through Special Purpose Vehicles, or the SAFE Circular 37, on July 4, 2014, which replaced the former circular commonly known as “SAFE Circular 75” promulgated by SAFE on October 21, 2005. SAFE Circular 37 requires PRC residents to register with local branches of SAFE in connection with their direct establishment or indirect control of an offshore entity, for the purpose of overseas investment and financing, with such PRC residents’ legally owned assets or equity interests in domestic enterprises or offshore assets or interests, referred to in SAFE Circular 37 as a “special purpose vehicle.” SAFE Circular 37 further requires amendment to the registration in the event of any significant changes with respect to the special purpose vehicle, such as increase or decrease of capital contributed by PRC individuals, share transfer or exchange, merger, division or other material event. In the event that a Chinese mainland shareholder-holding interests in a special purpose vehicle fails to fulfill the required SAFE registration, the PRC subsidiaries of that special purpose vehicle may be
25
Table of Contents
prohibited from making profit distributions to the offshore parent and from carrying out subsequent cross-border foreign exchange activities, and the special purpose vehicle may be restricted in its ability to contribute additional capital into its PRC subsidiary. Moreover, failure to comply with the various SAFE registration requirements described above could result in liability under PRC law for evasion of foreign exchange controls.
We have notified substantial beneficial owners of Ordinary Shares who we know are PRC residents of their filing obligation, and are aware that all substantial beneficial owners have completed the necessary registration with the local SAFE branch or qualified banks as required by SAFE Circular 37. However, we may not at all times be aware of the identities of all of our beneficial owners who are PRC residents. We do not have control over our beneficial owners and cannot assure you that all of our PRC-resident beneficial owners will comply with SAFE Circular 37 and subsequent implementation rules. The failure of our beneficial owners who are PRC residents to register or amend their SAFE registrations in a timely manner pursuant to SAFE Circular 37 and subsequent implementation rules, or the failure of future beneficial owners of our company who are PRC residents to comply with the registration procedures set forth in SAFE Circular 37 and subsequent implementation rules, may subject such beneficial owners or the PRC subsidiaries to fines and legal sanctions. Furthermore, since it is unclear how SAFE Circular 37, and any future regulation concerning offshore or cross-border transactions, will be interpreted, amended and implemented by the relevant PRC government authorities, we cannot predict how these regulations will affect the PRC subsidiaries’ business operations or future strategy. Failure to register or comply with relevant requirements may also limit our ability to contribute additional capital to the PRC subsidiaries and limit the PRC subsidiaries’ ability to distribute dividends to our company. These risks may have a material adverse effect on our business, financial condition and results of operations.
PRC regulation of loans to and direct investment in PRC entities by offshore holding companies and governmental control of currency conversion may delay us from using the proceeds of this offering to make loans or additional capital contributions to the PRC subsidiaries, which could materially and adversely affect our liquidity and our ability to fund and expand the PRC subsidiaries’ business.
We are an offshore holding company conducting our operations in China through the PRC subsidiaries. We may make loans to PRC subsidiaries subject to the approval from governmental authorities and limitation of amount, or we may make additional capital contributions to our subsidiaries in China.
Any loans to our WFOE in China, which is treated as a foreign-invested enterprise under PRC law, are subject to PRC regulations and foreign exchange loan registrations. For example, loans by us to our WFOE in China to finance its activities cannot exceed statutory limits and must be registered with the local counterpart of SAFE. In addition, a foreign invested enterprise shall use its capital pursuant to the principle of authenticity and self-use within its business scope. The capital of a foreign invested enterprise shall not be used for the following purposes: (i) directly or indirectly used for payment beyond the business scope of the enterprise or the payment prohibited by relevant laws and regulations; (ii) directly or indirectly used for investment in securities investments other than banks’ principal-secured products unless otherwise provided by relevant laws and regulations; (iii) the granting of loans to non-affiliated enterprises, except where it is expressly permitted in the business license; and (iv) paying the expenses related to the purchase of real estate that is not for self-use (except for the foreign-invested real estate enterprises).
SAFE promulgated the Notice of the State Administration of Foreign Exchange on Reforming the Administration of Foreign Exchange Settlement of Capital of Foreign-invested Enterprises, or SAFE Circular 19, effective on June 2015, in replacement of the Circular on the Relevant Operating Issues Concerning the Improvement of the Administration of the Payment and Settlement of Foreign Currency Capital of Foreign-Invested Enterprises, the Notice from the State Administration of Foreign Exchange on Relevant Issues Concerning Strengthening the Administration of Foreign Exchange Businesses, and the Circular on Further Clarification and Regulation of the Issues Concerning the Administration of Certain Capital Account Foreign Exchange Businesses. Although SAFE Circular 19 allows RMB capital converted from foreign currency-denominated registered capital of a foreign-invested enterprise to be used for equity investments within China, it also reiterates the principle that RMB converted from the foreign currency-denominated capital of a foreign-invested company may not be directly or indirectly used for purposes beyond its business scope. Thus, it is unclear whether SAFE will permit such capital to be used for equity investments in China in actual practice. SAFE promulgated the Notice of the State Administration of Foreign Exchange on Reforming and Standardizing the Foreign Exchange Settlement Management Policy of Capital Account, or SAFE Circular 16, effective on June 9, 2016, which reiterates some of the rules set forth in SAFE Circular 19, but changes the prohibition against using RMB capital converted from foreign currency-denominated registered capital of a foreign-invested company to issue RMB entrusted loans to a prohibition against using such capital to issue loans to non-associated enterprises.
26
Table of Contents
Violations of SAFE Circular 19 and SAFE Circular 16 could result in administrative penalties. SAFE Circular 19 and SAFE Circular 16 may significantly limit our ability to transfer any foreign currency we hold, including the net proceeds from this offering, to our WFOE, which may adversely affect our liquidity and our ability to fund and expand the PRC subsidiaries’ business in China.
On October 23, 2019, SAFE issued the Circular on Further Promoting Cross-border Trade and Investment Facilitation, or SAFE Circular 28, which took effect on the same day. SAFE Circular 28, subject to certain conditions, allows foreign-invested enterprises whose business scope does not include investment, or non-investment foreign-invested enterprises, to use their capital funds to make equity investments in China. On December 4, 2023, SAFE issued the Circular of the State Administration of Foreign Exchange on Further Deepening Reforms to Facilitate Cross-Border Trade and Investment, which stipulated that domestic equity transferors (including institutions and individuals) can directly remit to the capital project settlement account the equity transfer consideration funds paid in foreign currency by domestic entities, as well as the foreign exchange funds raised by domestic enterprises listed overseas. The funds in the capital project settlement account can be independently settled and utilized. Since SAFE Circular 28 was issued only recently, its interpretation and implementation in practice are still subject to substantial uncertainties.
In light of the various requirements imposed by PRC regulations on loans to and direct investment in PRC entities by offshore holding companies, and the fact that the PRC government may at its discretion restrict access to foreign currencies for current account transactions in the future, we cannot assure you that we will be able to complete the necessary government registrations or obtain the necessary government approvals on a timely basis, if at all, with respect to future loans to PRC subsidiaries in or future capital contributions by us to our WFOE in China. As a result, uncertainties exist as to our ability to provide prompt financial support to the PRC subsidiaries when needed. If we fail to complete such registrations or obtain such approvals, our ability to use the proceeds we expect to receive from this offering and to capitalize or otherwise fund the PRC operations may be negatively affected, which could materially and adversely affect our liquidity and our ability to fund and expand the PRC subsidiaries’ business.
We rely to a significant extent on dividends and other distributions on equity paid by our subsidiaries to fund offshore cash and financing requirements and any limitation on the ability of the PRC subsidiaries to make remittance to pay dividends to us could limit our ability to access cash generated by the operations of those entities.
We are a holding company and rely to a significant extent on dividends and other distributions on equity paid by our subsidiaries for our offshore cash and financing requirements, including the funds necessary to pay dividends and other cash distributions to our shareholders, fund inter-company loans, service any debt we may incur outside of China and pay our expenses. The laws, rules and regulations applicable to the PRC subsidiaries permit payments of dividends only out of their retained earnings, if any, determined in accordance with applicable accounting standards and regulations.
Under PRC laws, rules and regulations, each of our subsidiaries incorporated in mainland China is required to set aside at least 10% of its after-tax profits each year, after making up for previous years’ accumulated losses, if any, to fund certain statutory reserves, until the aggregate amount of such fund reaches 50% of its registered capital. As a result of these laws, rules and regulations, our subsidiaries incorporated in mainland China are restricted in their ability to transfer a portion of their respective net assets to their shareholders as dividends. As of March 31, 2024, September 30, 2023, and September 30, 2022, these restricted assets were $972,494, $972,494 and $935,702, respectively.
Limitations on the ability of the PRC subsidiaries to make remittance to pay dividends to us could limit our ability to access cash generated by the operations of those entities, including to make investments or acquisitions that could be beneficial to our businesses, pay dividends to our shareholders or otherwise fund and conduct our business.
We may be treated as a resident enterprise for PRC tax purposes under the PRC Enterprise Income Tax Law, and we may therefore be subject to PRC income tax on our global income.
Under the PRC Enterprise Income Tax Law and its implementing rules, both of which came into effect on January 1, 2008 and were last amended on December 29, 2018, enterprises established under the laws of jurisdictions outside of China with “de facto management bodies” located in China may be considered PRC tax resident enterprises for tax purposes and may be subject to the PRC enterprise income tax at the rate of 25% on their global income. “De facto management body” refers to a managing body that exercises substantive and overall management and control over the production and business, personnel, accounting books and assets of an enterprise. The SAT issued the Notice Regarding the Determination of Chinese-Controlled Offshore-Incorporated Enterprises as PRC Tax Resident
27
Table of Contents
Enterprises on the Basis of De Facto Management Bodies, or the SAT Circular 82, on April 22, 2009. SAT Circular 82 provides certain specific criteria for determining whether the “de facto management body” of a Chinese-controlled offshore-incorporated enterprise is located in China. Although Circular 82 only applies to offshore enterprises controlled by PRC enterprises, not those controlled by individuals or foreign enterprises, the determining criteria set forth in SAT Circular 82 may reflect the SAT’s general position on how the “de facto management body” test should be applied in determining the tax resident status of offshore enterprises, regardless of whether they are controlled by PRC enterprises. If we were to be considered a PRC resident enterprise, we would be subject to PRC enterprise income tax at the rate of 25% on our global income, and our profitability and cash flow may be materially reduced as a result of our global income being taxed under the Enterprise Income Tax Law. We believe that none of our entities outside of mainland China is a PRC resident enterprise for PRC tax purposes. However, the tax resident status of an enterprise is subject to determination by the PRC tax authorities and uncertainties remain with respect to the interpretation of the term “de facto management body.”
Dividends payable to our foreign investors and gains on the sale of our Ordinary Shares by our foreign investors may be subject to PRC tax.
Under the Enterprise Income Tax Law and its implementation regulations issued by the State Council, a 10% PRC withholding tax is applicable to dividends payable to investors that are non-resident enterprises, which do not have an establishment or place of business in mainland China or which have such establishment or place of business but the dividends are not effectively connected with such establishment or place of business, to the extent such dividends are derived from sources within mainland China. Any gain realized on the transfer of Ordinary Shares by such investors is also subject to PRC tax at a current rate of 10% which in the case of dividends will be withheld at source if such gain is regarded as income derived from sources within mainland China. If we are deemed a PRC resident enterprise, dividends paid on our Ordinary Shares, and any gain realized from the transfer of our Ordinary Shares, may be treated as income derived from sources within mainland China and may as a result be subject to PRC taxation. See “Regulations — Regulations Relating to Taxation.” Furthermore, if we are deemed a PRC resident enterprise, dividends payable to individual investors who are non-PRC residents and any gain realized on the transfer of Ordinary Shares by such investors may be subject to PRC tax at a current rate of 20%. Any PRC tax liability may be reduced under applicable tax treaties. However, it is unclear whether holders of our Ordinary Shares would be able to claim the benefit of income tax treaties or agreements entered into between China and other countries or areas if we are considered a PRC resident enterprise. If dividends payable to our non-PRC investors, or gains from the transfer of our Ordinary Shares by such investors are subject to PRC tax, the value of your investment in our Ordinary Shares may decline significantly.
We and our shareholders face uncertainties with respect to indirect transfers of equity interests in PRC resident enterprises by their non-PRC holding companies.
On February 3, 2015, the SAT issued the Announcement on Several Issues Concerning the Enterprise Income Tax on Indirect Transfer of Assets by Non-Resident Enterprises, or the SAT Circular 7. The SAT Circular 7 extends its tax jurisdiction to transactions involving the transfer of taxable assets through offshore transfer of a foreign intermediate holding company. In addition, SAT Circular 7 has introduced safe harbors for internal group restructurings and the purchase and sale of equity through a public securities market. SAT Circular 7 also brings challenges to both foreign transferor and transferee (or other person who is obligated to pay for the transfer) of taxable assets. On October 17, 2017, the SAT issued the Announcement on Issues Relating to Withholding at Source of Income Tax of Non-resident Enterprises, or the SAT Circular 37, which came into effect on December 1, 2017. The SAT Circular 37 further clarifies the practice and procedures of the withholding of non-resident enterprise income tax.
Where a non-resident enterprise transfers taxable assets indirectly by disposing of the equity interests of an overseas holding company, which is an Indirect Transfer, the non-resident enterprise as either transferor or transferee, or the PRC entity that directly owns the taxable assets, may report such Indirect Transfer to the relevant tax authority. Using a “substance over form” principle, the PRC tax authority may disregard the existence of the overseas holding company if it lacks a reasonable commercial purpose and was established for the purpose of reducing, avoiding or deferring PRC tax. As a result, gains derived from such Indirect Transfer may be subject to PRC enterprise income tax, and the transferee or other person who is obligated to pay for the transfer is obligated to withhold the applicable taxes, currently at a rate of 10% for the transfer of equity interests in a PRC resident enterprise. Both the transferor and the transferee may be subject to penalties under PRC tax laws if the transferee fails to withhold the taxes and the transferor fails to pay the taxes.
28
Table of Contents
We face uncertainties as to the reporting and other implications of certain past and future transactions where PRC taxable assets are involved, such as offshore restructuring, sale of the shares in our offshore subsidiaries and investments. Our company may be subject to filing obligations or taxed if our company is transferor in such transactions, and may be subject to withholding obligations if our company is transferee in such transactions, under SAT Circular 7 and/or SAT Circular 37. For transfer of shares in our company that do not qualify for the public securities market safe harbor by investors who are non-PRC resident enterprises, the PRC subsidiaries may be requested to assist in the filing under SAT Circular 7 and/or SAT Circular 37. As a result, we may be required to expend valuable resources to comply with SAT Circular 7 and/or SAT Circular 37 or to request the relevant transferors from whom we purchase taxable assets to comply with these circulars, or to establish that our company should not be taxed under these circulars, which may have a material adverse effect on our financial condition and results of operations.
Restrictions on currency exchange may limit our ability to utilize our revenue effectively.
All of our revenue is denominated in Renminbi. The Renminbi is currently convertible under the “current account,” which includes dividends, trade and service-related foreign exchange transactions, but not under the “capital account,” which includes foreign direct investment and loans, including loans we may secure from our onshore subsidiaries. Currently, PRC subsidiaries may purchase foreign currency for settlement of “current account transactions,” including payment of dividends to us, without the approval of SAFE by complying with certain procedural requirements. However, the relevant PRC governmental authorities may limit or eliminate our ability to purchase foreign currencies in the future for current account transactions. Since we expect a significant portion of our future revenue will be denominated in Renminbi, any existing and future restrictions on currency exchange may limit our ability to utilize revenue generated in Renminbi to fund our business activities outside of the PRC or pay dividends in foreign currencies to our shareholders. Foreign exchange transactions under the capital account remain subject to limitations and require approvals from, or registration with, SAFE and other relevant PRC governmental authorities. This could affect our ability to obtain foreign currency through debt or equity financing for our subsidiaries.
Fluctuations in exchange rates could result in foreign currency exchange losses to us and may reduce the value of, and amount in U.S. Dollars of dividends payable on, our shares in foreign currency terms.
The value of the RMB and the Hong Kong dollar against the U.S. dollar and other currencies may fluctuate and is affected by, among other things, changes in political and economic conditions and the foreign exchange policy adopted by the PRC government. In August 2015, the People’s Bank of China, or the PBOC, changed the way it calculates the mid-point price of RMB against the U.S. dollar, requiring the market-makers who submit for reference rates to consider the previous day’s closing spot rate, foreign-exchange demand and supply as well as changes in major currency rates. In 2017, the value of the Renminbi appreciated by approximately 6.3% against the U.S. dollar; and in 2018, the Renminbi depreciated by approximately 5.7% against the U.S. dollar. From the end of 2018 through the end of December 2020, the value of the Renminbi appreciated by approximately 5.10% against the U.S. dollar. It is difficult to predict how market forces or PRC or U.S. government policy, including any interest rate increases by the Federal Reserve, may impact the exchange rate between the RMB and the U.S. dollar in the future. There remains significant international pressure on the PRC government to adopt a more flexible currency policy, including from the U.S. government, which has threatened to label China as a “currency manipulator,” which could result in greater fluctuation of the RMB against the U.S. dollar. However, the PRC government may still at its discretion restrict access to foreign currencies for current account transactions in the future. Therefore, it is difficult to predict how market forces or government policies may impact the exchange rate between the RMB and the U.S. dollar or other currencies in the future. In addition, the PBOC regularly intervenes in the foreign exchange market to limit fluctuations in RMB exchange rates and achieve policy goals. If the exchange rate between RMB and U.S. dollar fluctuates in unanticipated manners, our results of operations and financial condition, and the value of, and dividends payable on, our shares in foreign currency terms may be adversely affected. We may not be able to pay dividends in foreign currencies to our shareholders. Appreciation of RMB to U.S dollar will result in exchange loss, while depreciation of RMB to U.S dollar will result in exchange gain.
Failure to make adequate contributions to various employee benefit plans and withhold individual income tax on employees’ salaries as required by PRC regulations may subject the PRC subsidiaries to penalties.
Companies operating in China are required to participate in various government-mandated employee benefit contribution plans, including certain social insurance, housing funds and other welfare-oriented payment obligations, and contribute to the plans in amounts equal to certain percentages of salaries, including bonuses and allowances,
29
Table of Contents
of the PRC subsidiaries’ employees up to a maximum amount specified by the local government from time to time at locations where the PRC subsidiaries operate their businesses. The requirement of employee benefit contribution plans has not been implemented consistently by the local governments in China, given the different levels of economic development in different locations. Companies operating in China are also required to withhold individual income tax on employees’ salaries based on the actual salary of each employee upon payment. The PRC subsidiaries may be subject to late fees and fines in relation to the underpaid employee benefits and under-withheld individual income tax, which may adversely affect the PRC subsidiaries’ and the Group’s financial condition and results of operations.
As of the date of this prospectus, (i) some of the PRC subsidiaries have not completed the social insurance registration and the housing fund registration; (ii) the PRC subsidiaries did not make contributions in the full amount for the social insurance fund and the housing provident fund for their employees, as required under the relevant PRC laws and regulations; and (iii) the PRC subsidiaries did not make contributions in the housing fund for some employees. For the six months ended March 31, 2024 and the fiscal years of 2023 and 2022, $141,340, $287,629, and $295,009 of contributions for the social insurance fund, respectively, should have been made but were not. For the six months ended March 31, 2024 and the fiscal years of 2023 and 2022, $44,530, $90,620, and $92,945 of contributions for the housing provident fund, respectively, should have been made but were not.
Although the PRC subsidiaries have not received any order or notice from the local authorities, nor any claims or complaints from their current and former employees regarding such non-compliance, we cannot assure you that the PRC subsidiaries will not be subject to any order to rectify non-compliance in the future, nor can we assure you that there will not be any employee complaints regarding social insurance payments or housing provident fund contributions against the PRC subsidiaries, or that the PRC subsidiaries will not receive any claims in respect of social insurance payments or housing provident fund contributions under the PRC laws and regulations. In addition, the PRC subsidiaries may incur additional costs to comply with such laws and regulations by the PRC government or relevant local authorities. Any such development could materially and adversely affect the PRC subsidiaries’ and our business, financial condition and results of operations.
Our Ordinary Shares may be delisted under the Holding Foreign Companies Accountable Act if the PCAOB is unable to inspect our auditors. The delisting of our Ordinary Shares, or the threat of their being delisted, may materially and adversely affect the value of your investment. Furthermore, on June 22, 2021, the U.S. Senate passed the Accelerating Holding Foreign Companies Accountable Act, and on December 29, 2022, the Consolidated Appropriations Act was signed into law by President Biden. The Consolidated Appropriations Act contained, among other things, an identical provision to the Accelerating Holding Foreign Companies Accountable Act, which reduces the number of consecutive non-inspection years required for triggering the prohibitions under the HFCA Act from three years to two.
The Holding Foreign Companies Accountable Act, or the HFCA Act, was enacted on December 18, 2020. The HFCA Act states if the SEC determines that a company has filed audit reports issued by a registered public accounting firm that has not been subject to inspection by the PCAOB for two consecutive years, the SEC shall prohibit such Ordinary Shares from being traded on a national securities exchange or in the over-the-counter trading market in the U.S.
On March 24, 2021, the SEC adopted interim final rules relating to the implementation of certain disclosure and documentation requirements of the HFCA Act. A company will be required to comply with these rules if the SEC identifies it as having a “non-inspection” year under a process to be subsequently established by the SEC. The SEC is assessing how to implement other requirements of the HFCA Act, including the listing and trading prohibition requirements described above. Furthermore, on June 22, 2021, the U.S. Senate passed the Accelerating Holding Foreign Companies Accountable Act, and on December 29, 2022, the Consolidated Appropriations Act was signed into law by President Biden. The Consolidated Appropriations Act contained, among other things, an identical provision to the Accelerating Holding Foreign Companies Accountable Act, which reduces the number of consecutive non-inspection years required for triggering the prohibitions under the HFCA Act from three years, as was formerly required under the HFCA Act before such amendment, to two consecutive years. According to the Consolidated Appropriations Act, any foreign jurisdiction could be the reason why the PCAOB does not have complete access to inspect or investigate a company’s auditor. As it was originally enacted, the HFCA Act applied only if the PCAOB’s inability to inspect or investigate was due to a position taken by an authority in the foreign jurisdiction where the relevant public accounting firm is located. As a result of the Consolidated Appropriations Act, the HFCA Act now also applies if the PCAOB’s inability to inspect or investigate the relevant accounting firm is due to a position taken by an authority in any foreign jurisdiction. The denying jurisdiction does not need to be where the accounting firm is located.
30
Table of Contents
On September 22, 2021, the PCAOB adopted a final rule implementing the HFCA Act, which provides a framework for the PCAOB to use when determining, as contemplated under the HFCA Act, whether the PCAOB is unable to inspect or investigate completely registered public accounting firms located in a foreign jurisdiction because of a position taken by one or more authorities in that jurisdiction. On December 2, 2021, the SEC issued amendments to finalize rules implementing the submission and disclosure requirements in the HFCA Act. The rules apply to registrants that the SEC identifies as having filed an annual report with an audit report issued by a registered public accounting firm that is located in a foreign jurisdiction and that PCAOB is unable to inspect or investigate completely because of a position taken by an authority in foreign jurisdictions. On December 16, 2021, the PCAOB issued a Determination Report which found that the PCAOB is unable to inspect or investigate completely registered public accounting firms headquartered in: (i) mainland China, and (ii) Hong Kong. Our auditor is not headquartered in mainland China or Hong Kong and was not identified in this report as a firm subject to the PCAOB’s determination. On August 26, 2022, CSRC, the MOF, and the PCAOB signed a Protocol, which sets out specific arrangements on conducting inspections and investigations over relevant audit firms within the jurisdiction of both the PRC and the U.S., including the audit firms based in mainland China and Hong Kong, taking the first step toward opening access for the PCAOB to inspect and investigate registered public accounting firms headquartered in mainland China and Hong Kong. On December 15, 2022, the PCAOB Board determined that the PCAOB was able to secure complete access to inspect and investigate registered public accounting firms headquartered in mainland China and Hong Kong and voted to vacate its previous determinations to the contrary. However, should PRC authorities obstruct or otherwise fail to facilitate the PCAOB’s access in the future, the PCAOB Board will consider the need to issue a new determination. Notwithstanding the foregoing, in the event it is later determined that the PCAOB is unable to inspect or investigate completely our auditor, then such lack of inspection could cause our securities to be delisted from the stock exchange.
Furthermore, various equity-based research organizations have recently published reports on China-based companies after examining their corporate governance practices, related party transactions, sales practices and financial statements, and these reports have led to special investigations and listing suspensions on U.S. national exchanges. Any similar scrutiny on us, regardless of its lack of merit, could cause the market price of our Ordinary Shares to fall, divert management resources and energy, cause us to incur expenses in defending ourselves against rumors, and increase the premiums we pay for director and officer insurance.
Our auditor, the independent registered public accounting firm that issues the audit report included elsewhere in this prospectus, as an auditor of companies that are traded publicly in the United States and a firm registered with the PCAOB, is subject to laws in the United States pursuant to which the PCAOB conducts regular inspections to assess its compliance with the applicable professional standards. Our auditor’s registration with the PCAOB took effect in San Mateo, California and it is currently subject to PCAOB inspections. The PCAOB currently has access to inspect the working papers of our auditor and its latest inspection was completed in December 2021. However, the recent developments would add uncertainties to our offering, and we cannot assure you whether Nasdaq or regulatory authorities would apply additional and more stringent criteria to us after considering the effectiveness of our auditor’s audit procedures and quality control procedures, adequacy of personnel and training, or sufficiency of resources, geographic reach or experience as it relates to the audit of our financial statements.
The SEC may propose additional rules or guidance that could impact us if our auditor is not subject to PCAOB inspection. For example, on August 6, 2020, the President’s Working Group on Financial Markets, or the PWG, issued the Report on Protecting United States Investors from Significant Risks from Chinese Companies to the then President of the United States. This report recommended the SEC implement five recommendations to address companies from jurisdictions that do not provide the PCAOB with sufficient access to fulfil its statutory mandate. Some of the concepts of these recommendations were implemented with the enactment of the HFCA Act. However, some of the recommendations were more stringent than the HFCA Act. For example, if a company’s auditor was not subject to PCAOB inspection, the report recommended that the transition period before a company would be delisted would end on January 1, 2022.
The SEC has announced that the SEC staff is preparing a consolidated proposal for the rules regarding the implementation of the HFCA Act and to address the recommendations in the PWG report. It is unclear when the SEC will complete its rulemaking and when such rules will become effective and what, if any, of the PWG recommendations will be adopted. The implications of this possible regulation in addition to the requirements of the HFCA Act are uncertain. While we understand that there has been dialogue among the CSRC, the SEC and the PCAOB regarding the inspection of PCAOB-registered accounting firms in China, there can be no assurance that we will be able to comply with requirements imposed by U.S. regulators. Such uncertainty could cause the
31
Table of Contents
market price of our Ordinary Shares to be materially and adversely affected, and our securities could be delisted and prohibited from being traded on the national securities exchange earlier than would be required by the HFCA Act. If our securities are unable to be listed on another securities exchange by then, such a delisting would substantially impair your ability to sell or purchase our Ordinary Shares when you wish to do so, and the risk and uncertainty associated with a potential delisting would have a negative impact on the price of our Ordinary Shares. The delisting of our Ordinary Shares, or the threat of their being delisted, may materially and adversely affect the value of your investment, even making it worthless.
Further, new laws and regulations or changes in laws and regulations in both the United States and China could affect our ability to maintain the listing of our Ordinary Shares on Nasdaq, which could materially impair the market for and market price of our Ordinary Shares.
The enactment of Law of the PRC on Safeguarding National Security in the Hong Kong Special Administrative Region and Hong Kong Autonomy Act of U.S. could impact our Hong Kong holding subsidiary.
On June 30, 2020, the Standing Committee of the PRC National People’s Congress adopted the Law of the PRC on Safeguarding National Security in the Hong Kong Special Administrative Region (Hong Kong National Security Law). This law defines the duties and government bodies of the Hong Kong National Security Law for safeguarding national security and four categories of offences — secession, subversion, terrorist activities, and collusion with a foreign country or external elements to endanger national security — and their corresponding penalties. On July 14, 2020, U.S. President Donald Trump signed the Hong Kong Autonomy Act (“HKAA”), into law, authorizing the U.S. administration to impose blocking sanctions against individuals and entities who are determined to have materially contributed to the erosion of Hong Kong’s autonomy. On August 7, 2020, the U.S. government imposed HKAA-authorized sanctions on eleven individuals, including former chief executive of Hong Kong Special Administrative Region Carrie Lam. On October 14, 2020, the U.S. State Department submitted to relevant committees of Congress the report required under HKAA, identifying persons materially contributing to “the failure of the Government of China to meet its obligations under the Joint Declaration or the Basic Law.” The HKAA further authorizes secondary sanctions, including the imposition of blocking sanctions, against foreign financial institutions that knowingly conduct a significant transaction with foreign persons sanctioned under this authority. The imposition of sanctions may directly affect the foreign financial institutions as well as any third parties or customers dealing with any foreign financial institution that is targeted. It is difficult to predict the full impact of the Hong Kong National Security Law and HKAA on Hong Kong and companies located in Hong Kong. If our Hong Kong subsidiary is determined to be in violation of the Hong Kong National Security Law or the HKAA by competent authorities, our business operations, financial position and results of operations could be materially and adversely affected.
Risks Related to the PRC Subsidiaries’ Business and Industry
The PRC subsidiaries’ operating history may not be indicative of our future growth or financial results and the PRC subsidiaries may not be able to sustain their historical growth rates.
The PRC subsidiaries’ operating history may not be indicative of their future growth or financial results. There is no assurance that the PRC subsidiaries will be able to grow their revenue in future periods. Their growth rates may decline for any number of possible reasons, and some of them are beyond their control, including decreasing customer demand, increasing competition, declining growth of the medical device industry in general, emergence of alternative business models, or changes in government policies or general economic conditions. The PRC subsidiaries expect to continue to expand their sales network and product offerings to bring greater convenience to their customers and to increase their customer base and number of transactions. However, the execution of their expansion plan is subject to uncertainty and the total number of items sold and number of transacting customers may not grow at the rate they expect for the reasons stated above. If their growth rates decline, investors’ perceptions of their business and prospects may be adversely affected and the market price of our Ordinary Shares could decline.
Failure to maintain the quality and safety of the PRC subsidiaries’ products could have a material and adverse effect on the PRC subsidiaries’ and our reputation, financial condition and results of operations.
The quality and safety of the PRC subsidiaries’ products, whether self-manufactured or out-sourced, are critical to our success. As a medical device manufacturer with a history of over 20 years, quality and safety are always the core values, as medical devices are directly used for the human body and thus essential to the human health. The PRC
32
Table of Contents
subsidiaries pay close attention to quality control, monitoring each step in the process from procurement to production and from warehouse to delivery. Yet, maintaining consistent product quality depends significantly on the effectiveness of their quality control system, which in turn depends on a number of factors, including, but not limited to the design of a quality control system, employee training to ensure that employees adhere to and implement the quality control policies and procedures and the effectiveness of monitoring any potential violation of their quality control policies and procedures. Despite such quality control management system, the PRC subsidiaries cannot eliminate the risks of errors, defects or failures. They may fail to detect or cure defects as a result of a number of factors, many of which are outside their control. Please refer to “Business — Quality Control” for more information.
In addition, the quality of the products or services provided by the PRC subsidiaries’ suppliers or business partners is subject to factors beyond the PRC subsidiaries’ control, including the effectiveness and the efficiency of their quality control system, among others. There can be no assurance that the PRC subsidiaries’ suppliers or business partners may always be able to adopt appropriate quality control systems and meet the PRC subsidiaries’ stringent quality control requirements in respect of the products or services they provide. Any failure of suppliers or business partners to provide satisfactory products or services could harm the PRC subsidiaries’ reputation and adversely impact the PRC subsidiaries’ operations. In addition, the PRC subsidiaries may be unable to receive sufficient compensation from suppliers and business partners for the losses caused by them.
As of the date of this prospectus, the PRC subsidiaries are unaware of material quality accidents.
The PRC subsidiaries may experience significant liability claims or complaints from customers, doctors and patients, litigation and regulatory investigations and proceedings, such as claiming in relation to medical device safety, or adverse publicity involving their products, which could adversely affect the PRC subsidiaries’ and our financial condition and results of operations.
The PRC subsidiaries face an inherent risk of liability claims or complaints from customers, doctors and patients. The PRC subsidiaries take those complaints and claims seriously and endeavor to reduce such complaints by implementing various remedial measures. Nevertheless, we cannot assure you that they can successfully prevent or address all complaints.
Any complaints or claims against the PRC subsidiaries, even if meritless and unsuccessful, may divert management attention and other resources from the PRC subsidiaries’ business and adversely affect their business and operations. Customers may lose confidence in the PRC subsidiaries and their brands, which may adversely affect their business and results of operations. Furthermore, negative publicity including but not limited to negative online reviews on social media and crowd-sourced review platforms, industry findings or media reports related to medical device quality and safety, public health concerns, illness, injuries, whether or not accurate, and whether or not concerning the PRC subsidiaries’ products, can adversely affect the PRC subsidiaries’ business, results of operations and reputation.
The PRC subsidiaries face potential liability, expenses for legal claims and harm due to the nature of their business. For example, customers could assert legal claims against them in connection with personal injuries or illness related to the use of medical devices they sell. The PRC government, media outlets and public advocacy groups have been increasingly focused on customer protection in recent years. Selling of defective products may expose the PRC subsidiaries to liabilities associated with customer protection laws. The PRC subsidiaries may be responsible for compensation on a customer’s loss even if personal injuries or illness are not caused by them. Thus, they may also be held liable if their suppliers or other business partners fail to comply with applicable product quality and safety related rules and regulations. Though they can ask the responsible parties for indemnity after that, their reputation could still be adversely affected.
The PRC subsidiaries may face additional exposure to claims and lawsuits. These claims could divert management time and attention away from their business and result in significant costs to investigate and defend, regardless of the merits of the claims. In some instances, they may elect or be forced to pay substantial damages if they are unsuccessful in their efforts to defend against these claims, which could harm their business, financial condition and results of operations. In addition, the PRC subsidiaries’ directors, management and employees may from time to time be subject to litigation and regulatory investigations and proceedings or otherwise face potential liability and expense in relation to medical device quality and safety, commercial, labor, employment, securities or other matters, which could adversely affect their and respective reputations and the Group’s financial condition and results of operations.
33
Table of Contents
Moreover, the PRC subsidiaries’ products may be subject to recall by competent authorities if they fail to comply with certain quality requirements. Recalls may be accompanied by claims or lawsuits for breaches of contract, for the inability to provide products of a certain quality on time, for which the PRC subsidiaries may elect or be forced to pay substantial damages if they are unsuccessful in their efforts to defend against these claims or lawsuits, and thus, could harm their business, and the Group’s financial condition and results of operations.
On November 16, 2020, Hangzhou Shanyou was required to recall 20,000 disposable medical masks by Heilongjiang Provincial Drug Administration, because the mask strings failed to comply with an elastic requirement.
The PRC subsidiaries have not, as of the date of this prospectus, been notified that they will be subject to any punishment, nor are they aware of any risk, currently or in the foreseeable future, regarding these recalls by the relevant government authorities, however, there is no assurance that the relevant government authorities will not later determine that a basis does exist for punishment with respect to recalled products.
As of the date of this prospectus, the PRC subsidiaries are not aware of any warning, investigations, prosecutions, disputes, claims or other proceedings in respect of customer protection rights or breaches of contract, nor have they been punished or can foresee any punishment to be made by any government authorities of the PRC or any in any overseas jurisdiction. However, there is no assurance that such entities will not find a basis in the future for investigations, prosecutions, disputes, claims or other proceedings with respect to customer protection rights or breaches of contract with respect to the PRC subsidiaries’ products, which could adversely harm their business, and the Group’s financial condition and results of operations.
The decreased demand for masks and in the unit price of masks could reduce our revenue, which could adversely affect the PRC subsidiaries’ and our results of operations.
Since China lifted travel restrictions and quarantine requirements in December 2022, the demand for masks and the sales volume and unit price of masks substantially decreased. With the reduced demand for masks and unit price of masks, our net revenue from sales of masks decreased from $10,619,035, which accounted for approximately 53.87% of our total net revenue, for the fiscal year ended September 30, 2022, to $5,091,331, or approximately 37.53%, for the fiscal year ended September 30, 2023. Additionally, the net revenue from sales of masks decreased from $4,752,892, or approximately 52.74%, for the six months ended March 31, 2023, to approximately $566,549, or approximately 10.67%, for the six months ended March 31, 2024.
While we do not expect this trend to continue in future financial periods, we estimate that the demand and unit price of masks have since stabilized. To mitigate these adverse effects, we have been strengthening our marketing efforts, focusing on medical devices other than masks. Nevertheless, if such trend continues, it could further reduce our revenue from sales of masks, thereby decreasing our total revenue, which could adversely affect the PRC subsidiaries’, the Group’s financial condition and our results of operations.
The PRC subsidiaries face the risk of fluctuations in the cost, availability and quality of their raw materials, which could adversely affect their results of operations, and thus, adversely affect the Group as a whole.
The cost, availability and quality of the PRC subsidiaries’ principal raw materials, such as meltblown, non-woven fabrics, steel spring, OPP membrane, and methyl silicone oil, are important to their operations. If the cost of raw materials increases due to policy changes, large market price fluctuation or any other reason, the business and results of operations of the PRC subsidiaries could be adversely affected, as well as the Group’s financial condition and results of operations.
Lack of availability of raw materials, whether due to shortages in supply, delays or interruptions in processing, failure of timely delivery or otherwise, could interrupt the PRC subsidiaries’ operations and adversely affect their financial results, which could adversely affect the Group as a whole.
Defective raw materials could subject the PRC subsidiaries to product liability claims or legal actions, which could adversely affect their and the Group’s financial condition and results of operations.
34
Table of Contents
A significant interruption in the operations of the PRC subsidiaries’ third-party suppliers and other business partners could potentially disrupt their operations.
The PRC subsidiaries have limited control over the operations of their third-party suppliers and other business partners and any significant interruption in their operations may have an adverse impact on the PRC subsidiaries’ operations. For example, a significant interruption in the operations of their supplier’s manufacturing facilities could cause delay or termination of shipment of the raw materials to them, which may cause delay or termination of shipment of ordered products to their customers, resulting in damage to their customer relationships. If the PRC subsidiaries are unable to adequately address the impact of the interruptions of operations of their third-party suppliers, their business operations and financial results may be materially and adversely affected, and the Group’s financial condition and results of operations may be materially and adversely affected accordingly.
Although the PRC subsidiaries believe that they could establish alternate sources from other suppliers for most of their raw materials, any delay in locating and establishing relationships with other sources could result in shortages or back orders for such raw materials. There can be no assurance that such replacement suppliers will provide the raw materials that are needed by the PRC subsidiaries in the quantities that they request or at the prices that they are willing to pay. Any shortage in quantities or increase in prices could adversely affect the PRC subsidiaries’ and the Group’s financial condition and results of operations.
Moreover, in February 2022, the Russian Federation and Belarus commenced a military action with the country of Ukraine. As a result of this action, various nations, including the United States, have instituted economic sanctions against the Russian Federation and Belarus. Additionally, on October 7, 2023, Hamas, a U.S. designated terrorist organization, launched a series of coordinated attacks from the Gaza Strip onto Israel. On October 8, 2023, Israel formally declared war on Hamas, and the armed conflict is ongoing as of the date of this prospectus. Since the PRC subsidiaries’ raw and auxiliary materials are purchased from certified and qualified suppliers in China, their supply has been very stable for many years and are easily sourced due to the PRC subsidiaries’ geographical locations. The prices of raw materials used in the PRC subsidiaries’ operations are not volatile and they, along with the PRC subsidiaries’ supply chain, have not been impacted by the recent conflict between Russia and Ukraine or the war between Isarel and Hamas. However, the impact of these actions and related sanctions on the world economy and the specific impact on the Company’s financial condition, results of operations and cash flows are not determinable as of the date of this prospectus.
The PRC subsidiaries do not have long term contracts with their suppliers and the suppliers can reduce order quantities or terminate sales to the PRC subsidiaries at any time.
The PRC subsidiaries do not have long term contracts with their suppliers. At any time, their suppliers can reduce the quantities of products that sell to the PRC subsidiaries, or cease selling products to the PRC subsidiaries altogether. Such reductions or terminations could have a material adverse impact on the PRC subsidiaries’ revenue, profits and financial condition, which may also adversely affect the Group as a whole.
Overall tightening of the labor market, increases in labor costs or any possible labor unrest may adversely affect the PRC subsidiaries’ business and results of operations, which may also adversely affect the Group as a whole.
The PRC subsidiaries’ business requires a substantial number of personnel. Any failure to retain stable and dedicated labor by them may lead to disruption to their business operations. Although they have not experienced any labor shortages as of the date of this prospectus, they have observed an overall tightening and increasingly competitive labor market. The PRC subsidiaries have experienced, and expect to continue to experience, increases in labor costs due to increases in salary, social benefits and employee headcount. The PRC subsidiaries compete with other companies in their industry and other labor-intensive industries for labor, and they may not be able to offer competitive remuneration and benefits compared to their competitors. If they are unable to manage and control their labor costs, their business, financial condition and results of operations, along with the Group’s financial condition and results of operations may be materially and adversely affected.
The PRC subsidiaries’ industry is intensely competitive. The PRC subsidiaries may face competition from, and they may be unable to compete successfully against, new entrants and established companies with greater resources.
The medical device industry is intensely competitive and includes thousands of companies, both domestically and internationally. As more medical device companies seek to outsource more of the design, prototyping and manufacturing of their products, the PRC subsidiaries will face increasing competitive pressures to grow their business in order to
35
Table of Contents
maintain their competitive position, and they may encounter competition from, and lose customers to, other companies with design, technological and manufacturing capabilities similar to theirs. Some of their potential competitors may have greater name recognition, greater operating revenue, larger customer bases, longer customer relationships and greater financial, technical, personnel and marketing resources than they have. If they are unsuccessful competing with their competitors for their existing and prospective customers’ business, their financial condition and results of operation may be adversely affected, and the Group’s financial condition and results of operation may be accordingly adversely affected.
Furthermore, increased competition may reduce the PRC subsidiaries’ market share and profitability and require them to increase their sales and marketing efforts and capital commitment in the future, which could negatively affect their results of operations or force them to incur further losses, which could also adversely affect the Group as a whole. Although the PRC subsidiaries have continuously grown their customer base, there is no assurance that they will be able to continue to do so in the future against current or future competitors, and such competitive pressures may have a material adverse effect on their and the Group’s business, financial condition and results of operations.
The continuing development of the PRC subsidiaries’ products depends upon the PRC subsidiaries’ maintaining strong working relationships with their customers, distributors and sales agents.
The research, development, marketing and sale of the PRC subsidiaries’ current products and potential new and improved products or future product indications for which we receive regulatory clearance or approval depend upon the PRC subsidiaries’ maintaining working relationships with their customers, distributors and sales agents. See “Business — Research and Development.” The PRC subsidiaries rely on those professionals to provide them with considerable knowledge and experience regarding the research, development, marketing and sale of their products. Distributors and sales agents assist them in marketing and sales, as well as collecting customers’ feedback and advice related to their products. Researchers at hospital and medical institution customers keep them informed of the latest requirements and research and development (“R&D”) results. If the PRC subsidiaries cannot maintain strong working relationships with these professionals and continue to receive their advice and input, the development, improvement and marketing of the PRC subsidiaries’ products could suffer, which could have a material adverse effect on the PRC subsidiaries’ and our business, financial condition and results of operations.
Technological changes may adversely affect sales of the PRC subsidiaries’ products and may cause their products to become obsolete.
The medical device market is characterized by extensive research and development and rapid technological changes. Technological progress or new developments in the industry could adversely affect sales of the PRC subsidiaries’ products. Products could be rendered obsolete because of future innovations by competitors or others, which would have a material adverse effect on the business, financial condition and results of operations of the PRC subsidiaries and the Group.
Consolidation in the medical device industry could have an adverse effect on the PRC subsidiaries’ revenue and results of operations.
Many medical device companies are consolidating to create new companies with greater market power. As the medical device industry consolidates, competition to provide goods and services to industry participants will become more intense. These industry participants may try to use their market power to negotiate price concessions or reductions for the PRC subsidiaries’ products. If the PRC subsidiaries reduce their prices because of consolidation in the healthcare industry, their revenue would decrease, which could have a material adverse effect on their and our business, financial condition and results of operations.
If the PRC subsidiaries fail to identify, acquire and develop other products, they may be unable to grow their business.
As a significant part of their growth strategy, the PRC subsidiaries intend to develop and commercialize additional products through their research and development program or by acquiring additional technologies and patents from third parties. The success of this strategy depends upon their ability to identify, select and acquire the technologies and patents on terms that are acceptable to them.
36
Table of Contents
Any patents and technology the PRC subsidiaries identify or acquire may require additional development efforts prior to commercial manufacturing and sale, including approval or clearance by the applicable regulatory authorities. All products are prone to the risks of failure inherent in medical device product development, including the possibility that the product will not be shown to be sufficiently safe and effective for approval or clearance by regulatory authorities. In addition, we cannot assure you that any such products that are approved or cleared will be manufactured or produced economically, successfully commercialized or widely accepted in the marketplace.
If the PRC subsidiaries are unable to develop suitable potential products through internal research programs or by obtaining patents or technologies from third parties, it could have a material adverse effect on the PRC subsidiaries and our business, financial condition and results of operations.
If the PRC subsidiaries are not able to implement their strategies to achieve their business objectives, their business operations and financial performance will be adversely affected.
The PRC subsidiaries’ business plan and growth strategy are based on currently prevailing circumstances and the assumption that certain circumstances will or will not occur, as well as the inherent risks and uncertainties involved in various stages of development. However, there is no assurance that the PRC subsidiaries will be successful in implementing their strategies or that their strategies, even if implemented, will lead to the successful achievement of their objectives. If they are not able to successfully implement their strategies, their business operations and financial performance will be adversely affected, which will adversely affect the Group as a whole.
If the PRC subsidiaries fail to adopt new technologies to meet evolving customer needs or emerging industry standards, their business may be materially and adversely affected.
To remain competitive, the PRC subsidiaries must continue to stay abreast of the constantly evolving industry trends and to enhance and improve their technology accordingly. Success will depend, in part, on the ability of the PRC subsidiaries to identify, develop or acquire leading technologies useful in their business. There can be no assurance that they will be able to use new technologies effectively or meet customer’s requirements. If they are unable to adapt in a cost-effective and timely manner in response to changing market conditions or customer preferences, whether for technical, legal, financial or other reasons, their business may be materially and adversely affected, and may adversely affect the Group’s financial condition and results of operations.
If the PRC subsidiaries sell medical devices to non-profit medical institutions through public bid invitation, their sales prices will be limited based on the results of the bidding.
In China, the procurement of medical devices by non-profit medical institutions is subject to specific procurement procedures. According to relevant laws and regulations, all non-profit medical institutions under all levels of government and state-owned enterprises from different industries shall participate in the centralized procurement of medical devices. The centralized procurement of medical devices must follow the basic principles of openness, fairness, impartiality and good faith, and the procurement method is mainly based on public bid invitation. Therefore, if the PRC subsidiaries sell medical devices to non-profit medical institutions through public bid invitation, their sales prices will be limited based on the results of the bidding, which may reduce their profitability, which result would adversely impact the profitability of the Group.
Changes to the PRC subsidiaries’ payment terms with both customers and suppliers may materially and adversely affect their operating cash flows.
The PRC subsidiaries may experience significant pressure from their suppliers to reduce the number of days of their accounts payable. At the same time, the PRC subsidiaries may experience pressure from their customers to extend the number of days before paying their accounts receivable. Any failure to manage their accounts payable and accounts receivable may have a material adverse effect on their and our business, financial condition and results of operations.
Our success depends on our management team and other key personnel, the loss of any of whom could disrupt our operations.
Our future success will depend in substantial part on the continued service of the members of our senior management, in particular, those identified under the section titled “Management.” The loss of the services of one or more of our key personnel could impede implementation of our business plan and result in reduced profitability. We do not
37
Table of Contents
carry key person life insurance on any of our officers or employees. Our future success will also depend on the PRC subsidiaries’ continued ability to attract, retain and motivate highly qualified technical sales and marketing customer support. Because of the rapid growth of the economy in China, competition for qualified personnel is intense. We cannot assure you that we will be able to retain our key personnel or that we will be able to attract, assimilate or retain qualified personnel in the future. Any loss of key personnel or failure to attract, assimilate or retain qualified personnel could disrupt our operations.
The PRC subsidiaries may not be able to adequately protect and maintain their intellectual property.
Our success will depend on the PRC subsidiaries’ ability to continue to develop and market their products. The PRC subsidiaries have been granted 29 patents in mainland China relating to their products and have 14 pending patent applications. No assurance can be given that such patents will not be challenged, invalidated, infringed or circumvented, or that such intellectual property rights will provide a competitive advantage to the PRC subsidiaries. Also, litigation may be necessary to enforce their intellectual property rights or determine the validity and scope of the proprietary rights of others.
The outcome of such potential litigation may not be favorable and any success in litigation may not be able to adequately protect their rights. Such litigation may be costly and divert management attention away from the PRC subsidiaries’ business. An adverse determination in any such litigation would impair their intellectual property rights and may harm their business, prospects and reputation, as well as the business and prospects of the Group. Enforcement of judgments in China is uncertain and even if they are successful in such litigation, it may not provide them with an effective remedy.
The PRC subsidiaries’ introduction of new technologies and products may increase the likelihood that third parties will assert claims that the PRC subsidiaries’ products infringe upon their proprietary rights.
The rapid technological changes that characterize our industry require that the PRC subsidiaries quickly implement new processes and components with respect to their products. Often with respect to recently developed processes and components, a degree of uncertainty exists as to who may rightfully claim ownership rights in such processes and components. Uncertainty of this type increases the risk that claims alleging that such components or processes infringe upon third party rights may be brought against the PRC subsidiaries. Although Hangzhou Shanyou takes, and will continue to take, steps to ensure that its new products do not infringe upon third party rights, if its products or manufacturing processes are found to infringe upon third party rights, it may be subject to significant liabilities and be required to change its manufacturing processes or be prohibited from manufacturing certain products, which could have a material adverse effect on its and our operations and financial condition, as well as the Group as a whole.
The PRC subsidiaries may be required to defend against charges of infringement of patent or other proprietary rights of third parties. Although patent and other intellectual property disputes in our industry have often been settled through licensing or similar arrangements, such defense could require the PRC subsidiaries to incur substantial expense and to divert significant resources of their technical and management personnel and could result in their loss of rights to develop or make certain products or require them to pay monetary damages or royalties to license proprietary rights from third parties. Furthermore, we cannot be certain that the necessary licenses would be available to the PRC subsidiaries on acceptable terms, if at all. Accordingly, an adverse determination in a judicial or administrative proceeding or failure to obtain necessary licenses could prevent the PRC subsidiaries from manufacturing and selling certain of their products. Any such litigation, whether successful or unsuccessful, could result in substantial costs to the PRC subsidiaries and diversions of their resources, either of which could adversely affect their business, and the financial condition and results of operations of the Group.
The PRC subsidiaries may not be able to prevent others from unauthorized use of their intellectual property, which could harm their business and competitive position and adversely affect the Group as a whole.
The PRC subsidiaries rely on a combination of trademark, fair trade practice, patent, copyright and trade secret protection laws in China, as well as confidentiality procedures, to protect their intellectual property rights. The PRC subsidiaries are careful to remain current in their annual patent fee payments. They regard their trademark, patents, know-how, proprietary technologies, and similar intellectual property as critical to our success. The PRC subsidiaries may become an attractive target to intellectual property attacks in the future with the increasing recognition of their brands. Any of their intellectual property rights could be challenged, invalidated, circumvented or misappropriated, or
38
Table of Contents
such intellectual property may not be sufficient to provide them with competitive advantages. In addition, there can be no assurance that (i) all of their intellectual property rights will be adequately protected, or (ii) their intellectual property rights will not be challenged by third parties or found by a judicial authority to be invalid or unenforceable. Intellectual property protection may not be sufficient in China. Accordingly, the PRC subsidiaries may not be able to effectively protect their intellectual property rights or to enforce their contractual rights in China. In addition, policing any unauthorized use of their intellectual property is difficult, time-consuming and costly and the steps taken may be inadequate to prevent the misappropriation of their intellectual property. In the event that the PRC subsidiaries resort to litigation to enforce their intellectual property rights, such litigation could result in substantial costs and a diversion of their managerial and financial resources. We can provide no assurance that the PRC subsidiaries will prevail in such litigation. In addition, their trade secrets may be leaked or otherwise become available to, or be independently discovered by, their competitors. Any failure in protecting or enforcing their intellectual property rights could have a material adverse effect on their and our business, financial condition and results of operations.
Economic recessions could have a significant, adverse impact on the PRC subsidiaries’ business.
Our revenue is generated from sales of medical devices, both domestically and internationally, and we anticipate that revenue from such sales will continue to represent the substantial portion of our total revenue in the near future. The PRC subsidiaries’ sales and earnings can also be affected by changes in the general economy.
The medical device industry historically has experienced cyclical fluctuations in financial results due to economic recessions, downturns in business cycles of the PRC subsidiaries’ customers, interest rate fluctuations, and other economic factors beyond the PRC subsidiaries’ control. Deterioration in the economic environment subjects their business to various risks, which may have a material and adverse impact on their operating results, causes them not to reach their long-term growth goals, and, thus, adversely affects the Group as a whole. For example, a downturn in the economy could directly affect the discretionary spending power of their customers and in turn, depress the number of orders for their products.
Changes in U.S. and international trade policies, particularly with regard to China, may adversely impact the PRC subsidiaries’ business and operating results, which may adversely affect the Group as a whole.
The U.S. government has recently made statements and taken certain actions that may lead to potential changes to the U.S. and international trade policies, including recently-imposed tariffs affecting certain products manufactured in China. It is unknown whether and to what extent new tariffs (or other new laws or regulations) will be adopted, or the effect that any such actions would have on the PRC subsidiaries or the industry and customers. Although cross-border business currently contributes a small portion of the PRC subsidiaries’ business, as they continue to sell their products internationally in the future, any unfavorable government policies on international trade, such as capital controls or tariffs, may affect the demand for their products and services, impact the competitive position of their products or prevent them from being able to sell products in certain countries. If any new tariffs, legislation and/or regulations are implemented, or if existing trade agreements are renegotiated or, in particular, if the U.S. government takes retaliatory trade actions due to the recent U.S.-China trade tension, such changes could have an adverse effect on the PRC subsidiaries’ and the Group’s financial condition and results of operations.
The PRC subsidiaries do not have any product liability, business interruption, or property insurance and they may incur liabilities that are not covered by insurance, which could expose them to significant costs and business disruption.
The PRC subsidiaries do not maintain product liability, business interruption or property insurance. Moreover, they do not carry any key-man life insurance of any variety. The PRC subsidiaries provide social security insurance, including pension, medical insurance, unemployment insurance, maternity insurance, on-the-job injury insurance and housing fund plans through a PRC government-mandated benefit contribution plan for their employees.
In the event the PRC subsidiaries were to incur substantial losses or liabilities due to fire, explosions, floods, other natural disasters or accidents, or any product liability claims or business interruption, their results of operations would be materially and adversely affected. Any such occurrences could have an adverse effect on the PRC subsidiaries and the Group’s financial condition and results of operations.
39
Table of Contents
If we do not obtain substantial additional financing, including the financing sought in this offering, our ability to execute on our business plan as outlined in this prospectus will be impaired.
Our plans for business expansion and development are dependent upon our raising significant additional capital, including the capital sought in this offering. Our plans call for significant new investments in research and development, marketing, expanded productions capacity, and working capital for raw materials and other items. Management estimates that our capital needs for expansion will be approximately $30 million. Although we expect the proceeds of this offering and our net earnings to substantially fund our planned growth and development, our management will be required to properly and carefully administer and allocate these funds. Should our capital needs be higher than estimated, or should additional capital be required after the close of this offering, we will be required to seek additional investments, loans or debt financing to fully pursue our business plans. Such additional investment may not be available to us on terms which are favorable or acceptable. Should we be unable to meet our full capital needs, our ability to fully implement our business plan will be impaired.
Pandemics and epidemics, natural disasters, terrorist activities, political unrest, and other outbreaks could disrupt the PRC subsidiaries’ delivery and operations, which could materially and adversely affect the PRC subsidiaries’ and our business, financial condition, and results of operations.
Global pandemics, epidemics in China or elsewhere in the world, or fear of spread of contagious diseases, such as Ebola virus disease (EVD), coronavirus disease 2019 (COVID-19), Middle East respiratory syndrome (MERS), severe acute respiratory syndrome (SARS), H1N1 flu, H7N9 flu, and avian flu, as well as hurricanes, earthquakes, tsunamis, or other natural disasters could disrupt the PRC subsidiaries’ business operations, reduce or restrict their supply of products and services, incur significant costs to protect their employees and facilities, or result in regional or global economic distress, which may materially and adversely affect the PRC subsidiaries’ and our business, financial condition, and results of operations. Actual or threatened war, terrorist activities, political unrest, civil strife, and other geopolitical uncertainty could have a similar adverse effect on the PRC subsidiaries’ and our business, financial condition, and results of operations. Any one or more of these events may impede the PRC subsidiaries’ production and delivery efforts and adversely affect their sales results, or even for a prolonged period of time, which could materially and adversely affect the PRC subsidiaries’ and our business, financial condition, and results of operations.
Beginning in 2020, the COVID-19 pandemic resulted in quarantines, travel restrictions, and temporary closure of stores and facilities in China and elsewhere. China lifted its travel restrictions and quarantine requirements in December 2022. The extent of the impact of COVID-19 on the Company’s future financial results will be dependent on future developments, such as the length and severity of any resurgence, future government actions in response to COVID-19 on the global economy and capital markets, among many other factors, all of which remain highly uncertain and unpredictable. Given this uncertainty, the Company is currently unable to quantify the expected impact of COVID-19 on its future operations, financial condition, liquidity, and results of operations.
The PRC subsidiaries are also vulnerable to natural disasters and other calamities. We cannot assure you that the PRC subsidiaries are adequately protected from the effects of fire, floods, typhoons, earthquakes, power loss, telecommunications failures, break-ins, war, riots, terrorist attacks, or similar events. Any of the foregoing events may give rise to interruptions, damage to the PRC subsidiaries’ property, delays in production, breakdowns, system failures, technology platform failures, or internet failures, which could cause the loss or corruption of data or malfunctions of our manufacturing facilities, as well as adversely affect the PRC subsidiaries’ and our business, financial condition, and results of operations.
The PRC subsidiaries’ international sales are subject to a variety of risks that could adversely affect their profitability and operating results, which may adversely affect the profitability and operating results of the Group.
The PRC subsidiaries sell disposable medical devices internationally. For the six months ended March 31, 2024 and the fiscal years ended September 30, 2023 and 2022, their international sales accounted for 13%, 7%, and 7%, respectively, of our revenue. Although they take measures to minimize risks inherent to their international sales, the following risks may have a negative effect on their profitability and operating results, impair the performance of their foreign sales or otherwise disrupt their business, which may adversely affect the profitability and operating results of the Group:
• Fluctuations in the value of currencies could cause exchange rates to change and impact their profitability;
• Greater difficulty in collecting accounts receivable and longer payment cycles, which can be more common in their international sales, could adversely impact their operating results over a particular fiscal period;
40
Table of Contents
• According to the PRC regulations, enterprises that export medical devices are required to ensure that the exported medical devices meet the legal requirements of the importing country. The PRC subsidiaries strive to meet the legal requirements of medical devices of foreign jurisdictions, including with respect to product quality, however, changes in foreign regulations, export duties, taxation and limitations on imports or exports could increase their operational costs, impose fines or restrictions on their ability to carry on their business or expand their international sales.
• Loss of goods caused by long-distance sea transportation, extreme weather, third-party logistics companies, which could increase their operational costs; and
• No product liability insurance has been bought for their products, and thus, they may incur considerable damages if their costumers sue them for product defects and obtain favorable judgments.
The PRC subsidiaries are subject to a variety of environmental laws that could be costly for them to comply with, and they could incur liability if they fail to comply with such laws or if they are responsible for releases of contaminants to the environment, which could adversely affect the Group as a whole.
Our operating subsidiaries are all located in the PRC. Chinese laws impose various environmental controls on the management, handling, generation, manufacturing, transportation, storage, use and disposal of wastewater, solid waste, and other materials used or generated in the manufacturing of Hangzhou Shanyou’s products. If the PRC subsidiaries fail to comply with any present or future environmental laws, they could be subject to fines, corrective actions, other liabilities or the suspension of production, which could adversely affect the Group.
The PRC subsidiaries endeavor to adhere to environmental protection and governance requirements and have formulated a systematic environmental protection management system in the treatment of wastewater and solid waste in accordance with national requirements. However, changes in environmental laws may result in costly compliance requirements or otherwise subject them to future liabilities.
In particular, with the further expansion of Hangzhou Shanyou’s production capacity, its pollutant emissions will increase, resulting in the increase of the environmental protection spending and the difficulty of environmental protection management, which could have an adverse effect on the PRC subsidiaries’ and the Group’s financial condition and results of operations.
With respect to one construction project, Hangzhou Shanyou failed to file the required Environmental Impact Registration Form for the records. According to the PRC laws and regulations, Hangzhou Shanyou is required to file the Environmental Impact Registration Form of construction project for the records. Its failure to meet such requirement may result in a penalty of up to RMB50,000 imposed by local authorities, which it does not expect will have a material impact on its and our business, financial condition and results of operations.
Moreover, Hangzhou Shanyou failed to obtain the relevant authorities’ approval of an Environmental Impact Statement before construction of facilities for its production lines, and failed to inspect the production lines to determine if they complied with relevant regulations of environmental protection and did not make the Inspection and Acceptance Reports of Environmental Protection upon completion of such construction. According to PRC laws and regulations, before construction, Hangzhou Shanyou was required to obtain the approval of the relevant authorities after reviewing its Environmental Impact Statement. Upon the completion of construction, it is required to inspect the production lines to determine if they comply with relevant regulations of environmental protection and make Inspection and Acceptance Reports of Environmental Protection. Hangzhou Shanyou’s failure to inspect the production lines to determine if they comply with relevant regulations of environmental protection and make Inspection and Acceptance Reports of Environmental Protection may result in an order to complete relevant procedures, and a penalty of between RMB0.2 million and RMB1 million, which may be imposed by local authorities. If Hangzhou Shanyou fails to complete relevant procedures within the stipulated period, the local authorities may impose a penalty of between RMB1 million and RMB2 million. Furthermore, if material environmental pollution or ecological damage is caused by such failure, Hangzhou Shanyou may be ordered to stop production or stop using the affected production lines by the PRC regulatory authorities. Moreover, its non-compliance in failing to inspect its production lines and making Inspection and Acceptance Reports of Environmental Protection may be recorded in Credibility Archive, a list that record companies’ non-compliance with environmental matters that can be accessed by the public, according to Interim Measures for Environmental Protection Acceptance of Completion of Construction Projects. Being recorded in Credibility Archive results in disclosure of such non-compliance to the public, which may adversely affect the
41
Table of Contents
reputation of Hangzhou Shanyou, and adversely affect the Group as a whole. As of the date of the prospectus, Hangzhou Shanyou believes it has obtained the approval for its Environmental Impact Statement by the relevant authorities, and it has completed the procedures for the inspection of its production lines; however, despite the completion of the required procedures, there is no assurance that Hangzhou Shanyou will not be penalized for not completing the above procedures in a timely manner, and any penalty could adversely affect the reputation of Hangzhou Shanyou, which could adversely affect the business, financial condition and results of operations of the Group.
The PRC subsidiaries are subject to a variety of fire protection laws that could be costly for them to comply with, and they could incur liability if they fail to comply with such laws, which could adversely affect the Group as a whole.
Companies operating in China are required to comply with a variety of fire protection laws, such as having an examination of fire protection design, inspection and acceptance of fire protection, record-filing and random inspection of construction projects. According to relevant laws and regulations with respect to construction projects, construction projects are subject to inspection and acceptance for fire protection. For certain construction projects, such as Hangzhou Shanyou’s production lines, a project facility owner shall provide fire protection design drawings to the relevant authorities before commencing construction and prepare the Inspection and Acceptance Reports of Fire Protection for the records of the competent authorities, and such authorities shall conduct a random inspection thereof. In case of failure to provide fire protection design drawings that meet the requirements of construction, the relevant authorities shall not issue a construction license. If a project facility owner places into service a construction project which was required to undergo a fire protection inspection and acceptance and the preparation of an Inspection and Acceptance Report of Fire Protection, but has not undergone such inspection and acceptance and failed to prepare an Inspection and Acceptance Report of Fire Protection, or has failed the inspection and acceptance, or failed to suspend the use of such construction project for which such project facility owner prepared the Inspection and Acceptance Report of Fire Protection for the records of the competent authorities but is later found to be unqualified in a random inspection, such project facility owner will be ordered to stop using the affected construction project, stop production or business operations and a fine of between RMB30,000 and RMB300,000 may be imposed by competent departments. If a project facility owner has undergone a fire protection inspection and acceptance and has already prepared an Inspection and Acceptance Report of Fire Protection but only fails to submit the report to the competent authorities for the records, such project facility owner will be subject to a fine of RMB5,000. Please see “Regulation — Fire Prevention Management” on page 114.
As of the date of this prospectus, Hangzhou Shanyou, the PRC subsidiary that manages all production lines, has not provided fire protection design drawings before construction nor has it prepared the Inspection and Acceptance Reports of Fire Protection for the records of the competent authorities, which may result in it being ordered to stop the use of its production lines and it being subject to a fine of between RMB30,000 and RMB300,000. If it makes up the inspection and acceptance and prepares Inspection and Acceptance Reports of Fire Protection but does not submit them to the competent authorities for the records, it will be subject to a fine of RMB5,000. Since all of its products are manufactured by operation of such production lines, any such development could materially and adversely affect the Group’s business, financial condition and results of operations.
The PRC subsidiaries are subject to a variety of construction laws, and they could incur liability if they fail to comply with such laws, which could adversely affect their operations.
According to relevant laws and regulations, any company engaged in the construction and decoration of various types of buildings and their attached facilities, including the installation of their supporting lines, pipelines and equipment, prior to the commencement of construction, should apply to the competent department of the local government for a construction license. Those who have not obtained the construction license shall be ordered by the license-issuing authority that has jurisdiction to stop the construction, to correct such lapse within a time limit, and pay a fine of between 1% to 2% of the contract price of the construction project. Furthermore, the project facility owner shall, within 15 days from the date of the completion and acceptance inspection of the construction project, submit the inspection and acceptance report to the competent administrative department of construction or other relevant departments for the record. If it fails to do so, it shall be ordered to make corrections and pay a fine of between RMB0.2 million to RMB0.5 million. Where the project facility is placed into service without being subject to completion inspection and acceptance, the project facility owner shall be ordered to make corrections and pay a fine of between 2% to 4% of the contract price of the project. If any loss is caused as a consequence of the failure to obtain the construction license, it shall also be liable for compensation.
42
Table of Contents
As of the date of this prospectus, as to the production lines of Hangzhou Shanyou, which were placed into service without having been subject to completion inspection and acceptance, it did not obtain a construction license prior to the commencement of construction nor undergone the completion inspection and acceptance nor did it prepare the Inspection and Acceptance Reports of Construction for the records of the competent authorities, therefore, Hangzhou Shanyou could be fined (i) an amount equal to 1% to 2% of the contract price of the production lines construction (approximately $16,500 to $33,000), for its failure to obtain a construction license prior to the commencement of construction, (ii) plus a fine of between RMB0.2 million to RMB0.5 million (approximately $28,000 to $70,000) for its failure to prepare the Inspection and Acceptance Reports of Construction for the records of the competent authorities and make adequate rectification, and (iii) a fine of between 2% to 4% of the contract price of the production lines construction (approximately $33,000 to $66,100), for its failure to complete inspection and acceptance before placing the production lines into service. As a result, the aggregate amount of potential fine could be between $77,500 to $169,100. While we anticipate that the possibility of being fined is relatively low, since Hangzhou Shanyou has not been subject to such fine historically, and the estimated maximum fine amount is relatively immaterial, being less than 1% of our consolidated revenue in the fiscal year ended September 30, 2022, we cannot assure you that the production lines will not be ordered to be suspended and rectified, which could affect Hangzhou Shanyou’s production, and therefore could materially and adversely affect both its and our business, financial condition and results of operations. Moreover, we cannot assure that the safety of the production lines has been adequately addressed, since they have not been subject to completion inspection and acceptance. If any personal injury or property loss is caused by the production lines, Hangzhou Shanyou would be liable for compensation, which could materially and adversely affect both its and our reputation, business, financial condition and results of operations.
The PRC subsidiaries’ rights to use their leased driveways, warehouses, and a parking area could be challenged by governmental authorities, due to the lessor’s failure to obtain the property ownership certificate as required by law, and their rights to use the leased collectively managed construction land could be challenged by governmental authorities, due to the lessor’s failure to comply with legal procedures related to land lease. Failure to comply with administrative or regulatory requirements with respect to property leased by the PRC subsidiaries may disrupt their usage and occupancy rights and could result in penalties and dispossession from such properties, which may disrupt their operations.
The PRC subsidiaries lease several properties for various operational purposes. Non-compliance with administrative or regulatory requirements by the lessors or our PRC subsidiaries regarding these rental properties could lead to disruptions in usage and occupancy rights, potential penalties, and dispossession from the rental properties, which may disrupt the PRC subsidiaries’ operations.
As of the date of this prospectus, the PRC subsidiaries’ have leased three offices, two driveways, two warehouses, and a parking area. However, the property ownership certificates for the two driveways, two warehouses, and the parking area have not been provided by the lessors of such properties to the PRC subsidiaries. This poses a risk of these sites being identified as illegally constructed, or the PRC subsidiaries lacking the legal right to use these properties. If these properties are determined to have been illegally constructed and are ordered to be demolished, or the PRC subsidiaries are determined to lack the legal right to use these properties, the PRC subsidiaries may be at risk of not being able to continue to use such properties. Moreover, for those properties that the PRC subsidiaries are determined to lack the legal right to use, the leases of these properties may be invalidated or terminated due to challenges by entitled third parties, which may extinguish our PRC subsidiaries’ rights to continued usage and occupancy of these properties, and their operations may be disrupted.
Moreover, the land leased by the PRC subsidiaries for warehouses is classified as collectively managed construction land. Under PRC laws and regulations, the lease of such land, which is duly registered according to law, can be granted by the landowner to an entity, but requires approval from over two-thirds of the collective economic organization’s members or villagers. If this requirement is not met, competent authorities may mandate rectification within a prescribed time-frame, which would result in the termination of the lease and tenant’s dispossession from the leased property. As of the date of this prospectus, the lessors of our PRC subsidiaries’ leased land have not furnished valid property ownership certificates or obtained the necessary consent from the required percentage of collective members or villagers. This non-compliance poses a risk that the lessors may be ordered to rectify the situation, which would terminate the lease and extinguish the PRC subsidiaries’ rights to continued usage of the leased land and their operations may be disrupted.
43
Table of Contents
In addition, lease agreements of some of the PRC subsidiaries’ leased properties are not registered and filed with the competent PRC government authorities as required by applicable PRC laws and regulations. Pursuant to the PRC laws, such registration and filing shall be accompanied by valid property ownership certificates provided by the lessors of such properties, which have yet to be provided, as stated above. The PRC subsidiaries could face fines of up to RMB10,000 for each such unregistered property, should the PRC subsidiaries fail to rectify the non-compliance within the time-frame prescribed by the relevant authorities.
Although the PRC subsidiaries have not received any notices from the lessors or competent departments, we cannot assure you that the lessors will provide valid property ownership certificates, nor can we assure you that the properties mentioned above will not be determined to have been illegally constructed and be ordered to be demolished.
Additionally, we cannot guarantee that the lessors of such land will not be required to rectify any violations, nor can we assure that these lessors possess the legal right to enter into the lease agreements. If the PRC subsidiaries’ expectations are wrong, they may need to find new facilities for one or more of their needs, and bear additional costs. Any consequential loss of any of the leased properties mentioned above would require additional expense and divert management’s time and attention to find an alternative location, and would disrupt the PRC subsidiaries’ business operations, which could materially and adversely impact our business, financial condition, and results of operations.
If the PRC subsidiaries fail to timely renew their medical device licenses or registration certificates, it could adversely affect the PRC subsidiaries’ and our reputation, financial condition and results of operations.
As a medical device manufacturer with all of our operating subsidiaries located in the PRC, all of the PRC subsidiaries’ manufacturing and sales activities must comply with relevant laws and regulations of China and other countries. Pursuant to the Administrative Measures for the Registration and Record-filing of Medical Devices promulgated on August 26, 2021 and effective on October 1, 2021, as amended from time to time, Class I medical devices are subject to recordation administration with Class II and Class III medical devices subject to registration administration. The U.S. Food, Drug and Cosmetic Act stipulates that manufacturer from foreign country which intend to sell its products in the U.S. should register with the FDA and list its medical devices with FDA. The PRC subsidiaries are in the business of manufacturing and sales of Class I and II medical devices and sales of III medical devices. If they fail to timely record or register their medical devices, both their and our financial condition and results of operations will be adversely affected.
The PRC subsidiaries have entered into a number of related party transactions in the ordinary course of their business, and may continue to enter into related party transactions in the future.
In the ordinary course of the PRC subsidiaries’ business, they have entered into transactions with related parties. For the six months ended March 31, 2024 and the fiscal years ended September 30, 2023 and 2022, the total revenues of the Group were $5,309,095, $13,565,951, and $19,711,290, respectively, while the revenues generated by sales to related parties were $233,109, $1,064,336, and $212,975, respectively, which accounted for 4%, 8%, and 0.04% of the total revenues, respectively. For the six months ended March 31, 2024 and the fiscal years ended September 30, 2023 and 2022, the total borrowed money of the Group was $6,660,746, $9,107,082, and $7,248,367, respectively, while the money borrowed from related parties was $416,297, $nil, and $nil, respectively, which accounted for 6%, 0%, and 0%, respectively.
Based on the foregoing analysis, we conclude that the PRC subsidiaries have not relied on a large number of related party transactions to conduct their ordinary business transactions. Nevertheless, past experience may not reflect the future. We cannot guarantee that the PRC subsidiaries will not continue to enter into related party transactions in the future. In addition, there can be no assurance that they have achieved and will achieve the most favorable terms with related parties for each related party transaction. Furthermore, there can be no assurance that the above-mentioned transactions or any future related party transactions that they may enter into, individually or in the aggregate, will not have an adverse effect on their business, and their and the Group’s financial condition and results of operations. Further, the transactions with the related parties may potentially involve conflicts of interests. Additionally, there can be no assurance that any disputes that may arise among the PRC subsidiaries and related parties will be resolved in the PRC subsidiaries’ favor. For details, see “Related Party Transactions.”
44
Table of Contents
If our PRC subsidiaries cannot collect timely payments from their customers, their business and operations, and the Group’s financial condition and results of operations may be materially and adversely affected.
Our PRC subsidiaries could not collect timely payments from some of their customers due to such customers’ lack of working capital, and, as a result, the Group made a significantly greater amount of bad debt provision in the fiscal year ended September 30, 2022, based on credit-worthiness and financial condition of the distributor customers. Despite the recovering economy since late 2022 and management’s increased efforts in payment collections, we cannot assure you that our PRC subsidiaries will be able to collect timely payments as expected. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations — Liquidity and Capital Resources.” Our PRC subsidiaries’ failure to collect timely payments in future financial periods could have a material and adverse effect on their business, and their and the Group’s financial condition and results of operations.
Our PRC subsidiaries rely in part on third-party distributors to place their products into the market and they may not be able to control their distributors.
Our PRC subsidiaries rely in part on third-party distributors to sell their products. For the six months ended March 31, 2024, the PRC subsidiaries had approximately 849 domestic distributors and 22 exporting distributors. See “Business — Marketing and Sales — Distribution Network.”
As our PRC subsidiaries sell and distribute their products through their distributors, and any one of the following events could cause fluctuations or reductions in our revenue that could have an adverse effect on our PRC subsidiaries’ and our financial condition and results of operations:
• reduction, delay or cancelation of orders from one or more of the distributors;
• selection or increased sales by the distributors of our PRC subsidiaries’ competitors’ products;
• failure to renew distribution agreements and maintain relationships with the existing distributors;
• failure to establish relationships with new distributors on favorable terms; or
• inability to timely identify and appoint additional or replacement distributors upon the loss of one or more of the distributors.
Our PRC subsidiaries may not be able to compete successfully against larger and better-funded sales and marketing campaigns of some of their current or future competitors, especially if these competitors provide their distributors with more favorable arrangements. We cannot assure you that our PRC subsidiaries will not lose any of their distributors to their competitors, which could cause them to lose some or all of their favorable arrangements with such distributors and may result in the termination of their relationships with other distributors. In addition, our PRC subsidiaries may not be able to successfully manage their distributors and the cost of any consolidation or further expansion of their distribution and sales network may exceed the revenue generated from these efforts. There can be no assurance that our PRC subsidiaries will be successful in detecting any non-compliance by their distributors with the provisions of their distribution agreements. Non-compliance by the distributors could, among other things, negatively affect our brand, demand for our PRC subsidiaries’ products, and our PRC subsidiaries’ relationships with other distributors. Furthermore, if the sales volumes of our PRC subsidiaries’ products to consumers are not maintained at a satisfactory level or if distributor orders fail to track consumers’ demand, our PRC subsidiaries’ distributors may not place orders for new products from our PRC subsidiaries, or may decrease the quantity of the distributors’ usual orders. The occurrence of any of these factors could result in a significant decrease in the sales volume of our PRC subsidiaries’ products, and therefore, adversely affect both our PRC subsidiaries’ and our financial condition and results of operations.
Our success will be dependent upon our PRC subsidiaries’ ability to maintain relationships with existing suppliers who are critical and necessary to the production of our PRC subsidiaries’ products and our PRC subsidiaries’ ability to create relationships with new suppliers.
As of the date of this prospectus, the PRC subsidiaries have a total of 130 suppliers. For the six months ended March 31, 2024, the top three significant suppliers represented approximately 15%, 11%, and 8% of the total supplies purchased, respectively. For the fiscal year ended September 30, 2023, the top three significant suppliers represented approximately 13%, 11%, and 9% of the total supplies purchased, respectively. For the year ended September 30,
45
Table of Contents
2022, the top three significant suppliers represented approximately 16%, 11%, and 7% of the total supplies purchased, respectively. For the year ended September 30, 2021, the top three significant suppliers represented approximately 18%, 16%, and 5% of the total supplies purchased, respectively. See “Business — Supplier.”
Our success will be dependent upon our PRC subsidiaries’ ability to maintain relationships with existing suppliers and enter into new supplier agreements. Our PRC subsidiaries rely on suppliers to provide raw materials for the products. If our PRC subsidiaries’ suppliers experience substantial financial difficulties, cease operations, or otherwise face business disruptions, our PRC subsidiaries would be required to take measures to ensure raw materials remain available. Any supply chain disruption could affect our PRC subsidiaries’ ability to provide medical devices, and could increase the production costs and negatively affect both our PRC subsidiaries’ and our financial condition and results of operations.
Risks Relating to this Offering and the Trading Market
There is no public market for the Units or the Warrants.
There is no established public trading market for the Units or the Warrants, and we do not expect a market to develop. In addition, we do not intend to apply to list the Warrants on any national securities exchange or other nationally recognized trading system, including Nasdaq. Without an active market, the liquidity of the Warrants will be limited.
The Warrants in this offering are speculative in nature.
Following this offering, the market value of the Warrants, if any, is uncertain and there can be no assurance that the market value of the Warrants will equal or exceed their imputed offering price. The Warrants will be not listed or quoted for trading on any market or exchange. In addition, each Series A Warrant will expire five years from its date of issuance and each Series B Warrant will expire five years from the date of issuance.
Holders of the Warrants will not have rights of holders of our Ordinary Shares until such Warrants are exercised.
Until holders of the Warrants acquire Ordinary Shares upon exercise of the Warrants, holders of the Warrants will have no rights with respect to the Ordinary Shares underlying such Warrants.
The Issuance of our Ordinary Shares in the public market as a result of this offering is likely to cause the market price of our Ordinary Shares to fall.
We are registering a maximum of 12,500,000 Ordinary Shares (including the Ordinary Shares Underlying the Warrants) offered under this prospectus. Sales of substantial amounts of our Ordinary Shares in the public market, or the perception that such sales might occur, is likely to adversely affect the market price of our Ordinary Shares. The issuance of new Ordinary Shares is likely to result in resales of our Ordinary Shares by our current shareholders concerned about the potential ownership dilution of their holdings. Any such issuance is likely to result in substantial dilution to our existing shareholders and will likely cause our share price to decline.
The trading price of the Ordinary Shares is likely to be volatile, which could result in substantial losses to investors.
Recently, there have been instances of extreme stock price run-ups followed by rapid price declines and strong stock price volatility with a number of recent initial public offerings, especially among companies with relatively smaller public floats. As a relatively small-capitalized company with relatively small public float after this offering, we may experience greater stock price volatility, lower trading volume and less liquidity than large-capitalized companies. In particular, our Ordinary Shares may be subject to rapid and substantial price volatility, low volumes of trades and large spreads in bid and ask prices due to factors beyond our control. Such volatility, including any stock-run up, may be unrelated to our actual or expected operating performance and financial condition or prospects, making it difficult for prospective investors to assess the rapidly changing value of our Ordinary Shares. This may happen because of broad
46
Table of Contents
market and industry factors, including the performance and fluctuation of the market prices of other companies with business operations located mainly in China that have listed their securities in the United States. In addition to market and industry factors, the price and trading volume for the ordinary shares may be highly volatile for factors specific to our own operations, including the following:
• variations in our revenues, earnings, cash flow;
• fluctuations in operating metrics;
• announcements of new investments, acquisitions, strategic partnerships or joint ventures by us or our competitors;
• announcements of new solutions and services and expansions by us or our competitors;
• termination or non-renewal of contracts or any other material adverse change in our relationship with our key customers or strategic investors;
• changes in financial estimates by securities analysts;
• detrimental negative publicity about us, our competitors or our industry;
• additions or departures of key personnel;
• release of lockup or other transfer restrictions on our outstanding equity securities or sales of additional equity securities;
• regulatory developments affecting us or our industry; and
• potential litigation or regulatory investigations.
Any of these factors may result in large and sudden changes in the volume and price at which the Ordinary Shares will trade. Furthermore, the stock market in general experiences price and volume fluctuations that are often unrelated or disproportionate to the operating performance of companies like us. These broad market and industry fluctuations may adversely affect the market price of our Ordinary Shares.
In addition, if the trading volumes of our Ordinary Shares are low, persons buying or selling in relatively small quantities may easily influence prices of our Ordinary Shares. This low volume of trades could also cause the price of our Ordinary Shares to fluctuate greatly, with large percentage changes in price occurring in any trading day session. Holders of our Ordinary Shares may also not be able to readily liquidate their investment or may be forced to sell at depressed prices due to low volume trading. If high spreads between the bid and ask prices of our Ordinary Shares exist at the time of a purchase, the stock would have to appreciate substantially on a relative percentage basis for an investor to recoup their investment. Broad market fluctuations and general economic and political conditions may also adversely affect the market price of our Ordinary Shares. As a result of this volatility, investors may experience losses on their investment in our Ordinary Shares. A decline in the market price of our Ordinary Shares also could adversely affect our ability to issue additional Ordinary Shares or other of our securities and our ability to obtain additional financing in the future. No assurance can be given that an active market in our Ordinary Shares will develop or be sustained. If an active market does not develop, holders of our Ordinary Shares may be unable to readily sell the shares they hold or may not be able to sell their shares at all.
In the past, shareholders of public companies have often brought securities class action suits against companies following periods of instability in the market price of their securities. If we were involved in a class action suit, it could divert a significant amount of our management’s attention and other resources from our business and operations and require us to incur significant expenses to defend the suit, which could harm our results of operations. Any such class action suit, whether or not successful, could harm our reputation and restrict our ability to raise capital in the future. In addition, if a claim is successfully made against us, we may be required to pay significant damages, which could have a material adverse effect on our financial condition and results of operations.
47
Table of Contents
You will experience immediate and substantial dilution in the net tangible book value of Ordinary Shares included in the Units and may experience additional dilution of your investment in the future. The existing shareholders will likely experience substantial dilution when the Warrants issued in this offering are exercised.
The offering price of per Ordinary Share included in the Units is higher than the net tangible book value per Ordinary Share outstanding prior to this offering. Consequently, when you purchase Units in the offering, at an assumed public offering price of $6.00 per Ordinary Unit, upon completion of the offering, you will incur immediate dilution of $4.26 per share, with respect to the net tangible book value of the Ordinary Shares as of March 31, 2024. See “Dilution.” In addition, you will likely experience further dilution upon the exercise of the Warrants issued in connection with this offering.
In addition, the Series B Warrants may be exercised in whole by means of a one-time only “alternative cashless exercise” in which the holder shall be entitled to receive a number of Ordinary Shares equal to the product of (a) the aggregate number of Ordinary Shares that would be issuable upon exercise of the Series B Warrants if such exercise were by means of a cash exercise rather than a cashless exercise; multiplied by (b) the quotient obtained by dividing (i) the exercise price minus the lowest VWAP of the Ordinary Shares over the 10 trading days immediately prior to the exercise date by (ii) 50% of the lowest VWAP of the Ordinary Shares over the 10 trading days immediately prior to the exercise date. Such alternative cashless exercise is subject to the Beneficial Ownership Limitation (as defined below), and shall only be available at a time when the applicable VWAP is lower than the then applicable exercise price. The maximum number of Ordinary Shares issuable pursuant to such alternative cashless exercise shall be equal to the quotient obtained by dividing (a) the aggregate exercise price of the Series B Warrant as of its issuance date, by (b) the Series B Floor Price. The Company may reduce the Series B Floor Price to any amounts set forth in a written notice to the holder of the Series B Warrants; provided that such reduction shall be in compliance with applicable Nasdaq rules, irrevocable and not be subject to increase thereafter. This is likely to result in substantial dilution to our existing shareholders and is likely to cause the market price of our Ordinary Shares to decline.
Our Chief Operating Officer and Liwei Zhang have substantial influence over our company. Their interests may not be aligned with the interests of our other shareholders, and they could prevent or cause a change of control or other transactions.
As of the date of this prospectus, Baiming Yu, our Chief Operating Officer (“COO”), beneficially owns an aggregate of 42.83% of our outstanding Ordinary Shares. Upon the completion of this offering, Mr. Yu will beneficially own 6,250,000 Ordinary Shares, or approximately 36.57% of our outstanding Ordinary Shares. Liwei Zhang, the spouse of Baiming Yu, beneficially owns an aggregate of 4.28% of our outstanding Ordinary Shares. Upon the completion of this offering, Ms. Zhang will beneficially own approximately 625,000 Ordinary Shares, or approximately 3.66% of our outstanding Ordinary Shares. Baiming Yu and Liwei Zhang beneficially own an aggregate of 47.11% of our outstanding Ordinary Shares, and will beneficially own approximately 6,875,000 Ordinary Shares, or approximately 40.22% of our outstanding Ordinary Shares upon the completion of this offering. Moreover, Baiming Yu owns 3.35% of shares and Liwei Zhang owns 1.65% of shares of Hangzhou Shanyou. Because share percentage is aligned with voting power, Baiming Yu and Liwei Zhang do not have substantial influence over Hangzhou Shanyou.
Accordingly, as to Work Cayman, Mr. Yu and Ms. Zhang could have significant influence in determining the outcome of any corporate transaction or other matter submitted to the shareholders for approval, including mergers, consolidations, the appointment of directors and other significant corporate actions. They will also have the power to prevent or cause a change in control. Without the consent of Mr. Yu and Ms. Zhang, we may be prevented from entering into transactions that could be beneficial to us or our minority shareholders. In addition, they could violate their fiduciary duties by diverting business opportunities from us to themselves or others. The interests of Mr. Yu and Ms. Zhang may differ from the interests of our other shareholders. The concentration in the ownership of our Ordinary Shares may cause a material decline in the value of our Ordinary Shares. For more information regarding Mr. Yu, Ms. Zhang, and their affiliated entity, see “Principal Shareholders.”
48
Table of Contents
If we fail to implement and maintain an effective system of internal controls or fail to remediate the material weaknesses in our internal control over financial reporting that have been identified, we may fail to meet our reporting obligations or be unable to accurately report our results of operations or prevent fraud, and investor confidence and the market price of our Ordinary Shares may be materially and adversely affected.
In preparing our CFS as of and for the fiscal years ended September 30, 2023 and 2022, we and our independent registered public accounting firm have identified material weaknesses in our internal control over financial reporting (“ICFR”), as defined in the standards established by the PCAOB, and other control deficiencies.
According to the PCAOB, a “material weakness” is a deficiency, or combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the annual or interim financial statements will not be prevented or detected on a timely basis. The material weaknesses identified in our ICFR included (i) a lack of staff sufficiently experienced with generally accepted accounting principles in the United States of America (“U.S. GAAP”) and the SEC reporting experiences in the accounting department to provide accurate information on a timely manner; (ii) a lack of the key monitoring mechanisms, such as an internal audit department to oversee and monitor the Company’s risk management, business strategies, and financial reporting procedures; and (iii) a lack of adequately designed and documented management review controls to properly detect and prevent certain accounting errors and omitted disclosures in the footnotes to the CFS.
Following the identification of the material weaknesses and control deficiencies, we have taken remedial measures, including (a) hiring an experienced Chief Financial Officer with adequate experience with U.S. GAAP and the SEC reporting and compliance requirements; (b) providing ongoing training courses in U.S. GAAP to existing personnel, including our Chief Financial Officer; (c) setting up the internal audit department to enhance the effectiveness of the internal control system; and (d) implementing necessary review and controls at related levels, so all important documents and contracts (including those of all of our subsidiaries) will be submitted to the office of our chief administrative officer for retention. We expect that we will incur significant costs in the implementation of such measures. However, the implementation of these measures may not fully address the material weaknesses in our internal control over financial reporting. Our failure to correct the material weaknesses or our failure to discover and address any other material weaknesses or control deficiencies could result in inaccuracies in our financial statements and could also impair our ability to comply with applicable financial reporting requirements and related regulatory filings on a timely basis. As a result, our business, financial condition, results of operations and prospects, and the trading price of our Ordinary Shares, may be materially and adversely affected. Moreover, ineffective internal control over financial reporting significantly hinders our ability to prevent fraud.
We are subject to the reporting requirements of the Exchange Act, the Sarbanes-Oxley Act of 2002 as well as rules and regulations of The Nasdaq Stock Market LLC. Section 404 of the Sarbanes-Oxley Act of 2002 requires that we include a report of management on our internal control over financial reporting in our annual report on Form 20-F beginning with our annual report for the fiscal year ending September 30, 2025. In addition, once we cease to be an “emerging growth company,” as such term is defined in the JOBS Act, our independent registered public accounting firm must attest to and report on the effectiveness of our ICFR. Our management may conclude that our ICFR is not effective. Moreover, even if our management concludes that our internal control over financial reporting is effective, our independent registered public accounting firm, after conducting its own independent testing, may issue a report that is qualified if it is not satisfied with our internal controls or the level at which our controls are documented, designed, operated, or reviewed, or if it interprets the relevant requirements differently from us. In addition, since we have become a public company, our reporting obligations may place a significant strain on our management, operational, and financial resources and systems for the foreseeable future. We may be unable to complete our evaluation testing and any required remediation in a timely manner.
The requirements of being a public company may strain our resources and divert management’s attention.
As a public company, we are subject to the reporting requirements of the Exchange Act, the Sarbanes-Oxley Act, the Dodd-Frank Wall Street Reform and Consumer Protection Act, the listing requirements of the securities exchange on which we list, and other applicable securities rules and regulations. Despite recent reforms made possible by the JOBS Act, compliance with these rules and regulations will nonetheless increase our legal, accounting, and financial compliance costs and investor relations and public relations costs, make some activities more difficult, time-consuming or costly and increase demand on our systems and resources, particularly after we are no longer an “emerging growth company.” The Exchange Act requires, among other things, that we file annual, quarterly, and current reports with respect to our business and operating results as well as proxy statements.
49
Table of Contents
As a result of disclosure of information in this prospectus and in filings required of a public company, our business and financial condition is more visible, which we believe may result in threatened or actual litigation, including by competitors and other third parties. If such claims are successful, our business and operating results could be harmed, and even if the claims do not result in litigation or are resolved in our favor, these claims, and the time and resources necessary to resolve them, could divert the resources of our management and adversely affect our business, brand and reputation and results of operations.
On August 23, 2024, the Company purchased a directors and officers liability insurance policy from China Pacific Property Insurance Co., Ltd., covering all directors and officers for the period from August 23, 2024 to August 22, 2025, with a premium of $59,850. We may be required to accept reduced coverage or incur substantially higher costs in the future. These factors could also make it more difficult for us to attract and retain qualified members of our board of directors, particularly to serve on our audit committee and compensation committee, and qualified executive officers.
We will likely not receive any additional funds upon the exercise of the Series B Warrants.
Each of the Warrants may be exercised by way of a cashless exercise provision, and the Series B Warrants may be exercised by way of an alternative cashless exercise provision, meaning that the holders thereof may not pay a cash purchase price upon exercise, but instead would receive upon such exercise a number of Ordinary Shares determined according to the applicable formula set forth in the Series B Warrants. If the Series B Warrants are exercised pursuant to such alternative cashless exercise provision, such exercising holder will receive a number of Ordinary Shares for each Series B Warrant exercised, without any cash payment to us, in accordance with the formular set forth in the Series B Warrants. Accordingly, we will likely not receive any additional funds upon the exercise of such Series B Warrants. See Description of Share Capital — Warrants” for more information.
Substantial future issuance and sales of our Ordinary Shares, including as a result of certain provisions contained in the Series A Warrants and Series B Warrants, or the anticipation of future sales of our Ordinary Shares in the public market could cause the price of our Ordinary Shares to decline.
Sales of substantial amounts of our Ordinary Shares in the public market after this offering, or the perception that these sales could occur, could cause the market price of our Ordinary Shares to decline. All of the Ordinary Shares sold in the offering will be freely transferable without restriction or further registration under the Securities Act. Some of the remaining Ordinary Shares are “restricted securities” as defined in Rule 144. These Ordinary Shares may be sold without registration under the Securities Act to the extent permitted by Rule 144 or other exemptions under the Securities Act. An aggregate of 14,591,942 Ordinary Shares are outstanding before the consummation of this offering and 17,091,942 Ordinary Shares will be outstanding immediately after the consummation of this offering, assuming no sale of the Pre-Funded Warrants and no exercise of the Warrants.
The Series A Warrants will have five-year terms, will be immediately exercisable upon issuance and have an initial exercise price equal to the actual public offering price of the Ordinary Units. The exercise price will be reset on the Reset Date to a price that is equal to 105% of the arithmetic average of the sum of the three lowest per share VWAPs of the Ordinary Shares on the trading market for the twenty (20) trading days immediately prior to the Reset Date; provided that the exercise price shall not be lower than the Series A Floor Price. The Company may reduce the Series A Floor Price to any amounts set forth in a written notice to the holder of the Series A Warrants; provided that such reduction shall be in compliance with applicable Nasdaq rules, irrevocable and not be subject to increase thereafter. In the event that the Series A Warrants are subsequently exercised, such issuances would result in substantial dilution to shareholders.
The Series B Warrants will have five-years terms and will be immediately exercisable upon issuance at an exercise price equal to the actual public offering price of the Ordinary Units. The Series B Warrants may also be exercised in whole by means of a one-time only “alternative cashless exercise” in which the holder shall be entitled to receive a number of Ordinary Shares equal to the product of (a) the aggregate number of Ordinary Shares that would be issuable upon exercise of the Series B Warrants if such exercise were by means of a cash exercise rather than a cashless exercise; multiplied by (b) the quotient obtained by dividing (i) the exercise price minus the lowest VWAP of the Ordinary Shares over the 10 trading days immediately prior to the exercise date by (ii) 50% of the lowest VWAP of the Ordinary Shares over the 10 trading days immediately prior to the exercise date. Such alternative cashless exercise is subject to the Beneficial Ownership Limitation, and shall only be available at a time when the applicable VWAP is lower than the then applicable exercise price. The maximum number of Ordinary Shares issuable pursuant to such alternative cashless exercise shall be equal to the quotient obtained by dividing (a) the aggregate exercise price of the Series B Warrant as
50
Table of Contents
of its issuance date, by (b) the Series B Floor Price. The Company may reduce the Series B Floor Price to any amounts set forth in a written notice to the holder of the Series B Warrants; provided that such reduction shall be in compliance with applicable Nasdaq rules, irrevocable and not be subject to increase thereafter. Sales of these shares into the market could cause the market price of our Ordinary Shares to decline. In the event that the Series B Warrants are subsequently exercised, such issuances would result in substantial dilution to shareholders.
We do not intend to pay dividends for the foreseeable future.
We currently intend to retain any future earnings to finance the operation and expansion of our business, and we do not expect to declare or pay any dividends in the foreseeable future. As a result, you may only receive a return on your investment in our Ordinary Shares if the market price of our Ordinary Shares increases.
If securities or industry analysts do not publish research or reports about our business, or if the publish a negative report regarding our Ordinary Shares, the price of our Ordinary Shares and trading volume could decline.
Any trading market for our Ordinary Shares may depend in part on the research and reports that industry or securities analysts publish about us or our business. We do not have any control over these analysts. If one or more of the analysts who cover us downgrade us, the price of our Ordinary Shares would likely decline. If one or more of these analysts cease coverage of our Company or fail to regularly publish reports on us, we could lose visibility in the financial markets, which could cause the price of our Ordinary Shares and the trading volume to decline.
Our management has broad discretion to determine how to use the funds raised in the offering and may use them in ways that may not enhance our results of operations or the price of our Ordinary Shares.
We anticipate that we will use the net proceeds from this offering for (i) upgrading production equipment and investing in the PRC subsidiaries’ R&D; (ii) hiring experienced employees to improve our systems of internal control and compliance with U.S. GAAP and the Sarbanes-Oxley Act of 2002; and (iii) working capital and general corporate purpose. Our management will have significant discretion as to the use of the net proceeds from this offering and could spend the proceeds in ways that do not improve our results of operations or enhance the market price of our Ordinary Shares.
If we cease to qualify as a foreign private issuer, we would be required to comply fully with the reporting requirements of the Exchange Act applicable to U.S. domestic issuers, and we would incur significant additional legal, accounting and other expenses that we would not incur as a foreign private issuer.
We qualify as a foreign private issuer. As a foreign private issuer, we are exempt from the rules under the Exchange Act prescribing the furnishing and content of proxy statements, and our officers, directors and principal shareholders are exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act. In addition, we are not required under the Exchange Act to file periodic reports and financial statements with the SEC as frequently or as promptly as United States domestic issuers, and we are not required to disclose in our periodic reports all of the information that United States domestic issuers are required to disclose. While we currently qualify as a foreign private issuer, we may cease to qualify as a foreign private issuer in the future, in which case we would incur significant additional expenses that could have a material adverse effect on our results of operations.
Because we are a foreign private issuer and are exempt from certain Nasdaq corporate governance standards applicable to U.S. issuers, you will have less protection than you would have if we were a domestic issuer.
Nasdaq listing rules require listed companies to have, among other things, a majority of its board members be independent. As a foreign private issuer, however, we are permitted to, and we may follow home country practice in lieu of the above requirements, or we may choose to comply with the above requirement within one year of listing. The corporate governance practice in our home country, the Cayman Islands, does not require a majority of our board to consist of independent directors. Thus, although a director must act in the best interests of the Company, it is possible that fewer board members will be exercising independent judgment and the level of board oversight on the management of our company may decrease as a result. In addition, Nasdaq listing rules also require U.S. domestic issuers to have a compensation committee, a nominating and corporate governance committee composed entirely of independent directors, and an audit committee with a minimum of three members. We, as a foreign private issuer, are not subject to these requirements.
51
Table of Contents
Nasdaq Listing Rule 5635 generally provides that shareholder approval is required of U.S. domestic companies listed on Nasdaq prior to issuance (or potential issuance) of securities (i) the acquisition of the stock or assets of another company; (ii) equity-based compensation of officers, directors, employees or consultants; (iii) a change of control; and (iv) transactions other than public offerings. In addition, Nasdaq Listing Rule 5640 provides that voting rights of existing shareholders of publicly traded common stock registered under Section 12 of the Exchange Act cannot be disparately reduced or restricted through any corporate action or issuance. Notwithstanding this general requirement, Nasdaq Listing Rule 5615(a)(3)(A) permits foreign private issuers to follow their home country practice rather than these requirements. The Cayman Islands do not require shareholder approval prior to any of the foregoing types of issuances, nor are existing shareholders’ voting rights disparately reduced or restricted through any corporate action or issuance. We, therefore, are not required to obtain such shareholder approval prior to entering into a transaction with the potential to issue securities as described above, and our existing shareholders’ voting rights may be disparately reduced or restricted through corporate action or issuance. Specifically, our board of directors has elected to follow our home country rules and be exempt from the prohibition of disparately reducing or restricting our existing shareholders’ voting rights, and the requirements to obtain shareholder approval for (i) the acquisition of the stock or assets of another company; (ii) equity-based compensation of officers, directors, employees or consultants; (iii) a change of control; and (iv) transactions other than public offerings.
If we cannot continue to satisfy the continued listing requirements and other rules of the Nasdaq Capital Market, our securities may be delisted, which could negatively impact the price of our securities and your ability to sell them.
Our Ordinary Shares are listed on the Nasdaq Capital Market. In order to maintain our listing on the Nasdaq Capital Market, we are required to comply with certain rules of the Nasdaq Capital Market, including those regarding minimum stockholders’ equity, minimum share price, minimum market value of publicly held shares, and various additional requirements. We may not be able to continue to satisfy these requirements and applicable rules. If we are unable to satisfy the Nasdaq Capital Market criteria for maintaining our listing, our securities could be subject to delisting.
If The Nasdaq Stock Market LLC subsequently delists our securities from trading, we could face significant consequences, including:
• a limited availability for market quotations for our securities;
• reduced liquidity with respect to our securities;
• a determination that our Ordinary Shares are a “penny stock,” which will require brokers trading in our Ordinary Shares to adhere to more stringent rules and possibly result in a reduced level of trading activity in the secondary trading market for our Ordinary Shares;
• limited amount of news and analyst coverage; and
• a decreased ability to issue additional securities or obtain additional financing in the future.
Anti-takeover provisions in our amended and restated articles of association may discourage, delay, or prevent a change in control.
Some provisions of our amended and restated articles of association may discourage, delay or prevent a change in control of our Company or management that shareholders may consider favorable, including, among other things, the following:
• provisions that authorize our board of directors to issue shares with preferred, deferred or other special rights or restrictions without any further vote or action by our shareholders; and
• provisions that restrict the ability of our shareholders to call shareholder meetings.
Our board of directors may decline to register transfers of Ordinary Shares in certain circumstances.
Our board of directors may, in its sole discretion, decline to register any transfer of any Ordinary Share which is not fully paid up or on which we have a lien. Our directors may also decline to register any transfer of any Ordinary Share unless (i) the instrument of transfer is lodged with us, accompanied by the certificate for the shares to which it relates and such other evidence as our board of directors may reasonably require to show the right of the transferor to make
52
Table of Contents
the transfer; (ii) the instrument of transfer is in respect of only one class of shares; (iii) the instrument of transfer is properly stamped, if required; (iv) in the case of a transfer to joint holders, the number of joint holders to whom the share is to be transferred does not exceed four; (v) the shares transferred are free of any lien in favor of us; or (vi) a fee of such maximum sum as the Nasdaq Capital Market may determine to be payable, or such lesser sum as our board of directors may from time to time require, is paid to us in respect thereof.
If our directors refuse to register a transfer they shall, within three months after the date on which the instrument of transfer was lodged, send to each of the transferor and the transferee notice of such refusal. The registration of transfers may, on 14 days’ notice being given by advertisement in one or more newspapers or by electronic means, be suspended and the register closed at such times and for such periods as our board of directors may from time to time determine, provided, however, that the registration of transfers shall not be suspended nor the register closed for more than 30 days in any year.
This, however, is unlikely to affect market transactions of the Ordinary Shares purchased by investors in the public offering. Once the Ordinary Shares have been listed, the legal title to such Ordinary Shares and the registration details of those Ordinary Shares in the Company’s register of members will remain with the Depository Trust Company. All market transactions with respect to those Ordinary Shares will then be carried out without the need for any kind of registration by the directors, as the market transactions will all be conducted through the Depository Trust Company systems.
We are an “emerging growth company” within the meaning of the Securities Act, and if we take advantage of certain exemptions from disclosure requirements available to emerging growth companies, this will make it more difficult to compare our performance with other public companies. In addition, we may not be subject to requirements that other public companies are subject to, which could affect investor confidence in us and our Ordinary Shares.
We are an “emerging growth company” within the meaning of the Securities Act, as modified by the JOBS Act. Section 102(b)(1) of the JOBS Act exempts emerging growth companies from being required to comply with new or revised financial accounting standards until private companies (that is, those that have not had a Securities Act registration statement declared effective or do not have a class of securities registered under the Exchange Act) are required to comply with the new or revised financial accounting standards. The JOBS Act provides that a company can elect to opt out of the extended transition period and comply with the requirements that apply to non-emerging growth companies but any such an election to opt out is irrevocable. We have elected not to opt out of such extended transition period, which means that when a standard is issued or revised and it has different application dates for public or private companies, we, as an emerging growth company, can adopt the new or revised standard at the time private companies adopt the new or revised standard. This will make comparison of our financial statements with another public company which is neither an emerging growth company nor an emerging growth company which has opted out of using the extended transition period difficult or impossible because of the potential differences in accounting standards used.
In addition, for as long as we remain an “emerging growth company,” we will elect to take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not “emerging growth companies,” including not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act, reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements, and exemptions from the requirements of shareholder approval of any golden parachute payments not previously approved. Because of these lessened regulatory requirements, our shareholders would be left without information or rights available to shareholders of more mature companies. If some investors find our Ordinary Shares less attractive as a result, there may be a less active trading market for our Ordinary Shares and our share price may be more volatile. See “Prospectus Summary — Implications of Being an Emerging Growth Company.”
The laws of the Cayman Islands may not provide our shareholders with benefits comparable to those provided to shareholders of corporations incorporated in the United States.
We are an exempted company incorporated under the laws of the Cayman Islands. Our corporate affairs are governed by our amended and restated memorandum of association and articles of association, the Cayman Companies Act, and the common law of the Cayman Islands. The rights of shareholders to take action against the directors, actions by minority shareholders and the fiduciary duties of our directors to us under Cayman Islands law are to a large extent governed by the common law of the Cayman Islands. The common law of the Cayman Islands is derived in part from comparatively limited judicial precedent in the Cayman Islands, as well as from the common law of England, the decisions of whose courts are of persuasive authority, but are not binding, on a court in the Cayman Islands. The rights
53
Table of Contents
of our shareholders and the fiduciary duties of our directors under Cayman Islands law are not as clearly established as they would be under statutes or judicial precedent in some jurisdictions in the United States. It may be difficult or impossible for you to bring an action against us or against these individuals in the United States in the event that you believe that your rights have been infringed under the U.S. federal securities laws or otherwise. Even if you are successful in bringing an action of this kind, the laws of the Cayman Islands and of the PRC may render you unable to enforce a judgment against our assets or the assets of our directors and officers. In particular, the Cayman Islands has a different body of securities laws than the United States. Some U.S. states, such as Delaware, have more fully developed and judicially interpreted bodies of corporate law than the Cayman Islands. In addition, Cayman Islands companies may not have standing to initiate a shareholder derivative action in a federal court of the United States. There is no statutory recognition in the Cayman Islands of judgments obtained in the United States, although the courts of the Cayman Islands will in certain circumstances recognize and enforce a non-penal judgment of a foreign court of competent jurisdiction without retrial on the merits.
Shareholders of Cayman Islands exempted companies like us have no general rights under Cayman Islands law to inspect corporate records or to obtain copies of lists of shareholders of these companies. Our directors have discretion under our amended and restated articles of association to determine whether or not, and under what conditions, our corporate records may be inspected by our shareholders, but are not obliged to make them available to our shareholders. This may make it more difficult for you to obtain the information needed to establish any facts necessary for a shareholder motion or to solicit proxies from other shareholders in connection with a proxy contest.
Certain corporate governance practices in the Cayman Islands, which is our home country, differ significantly from requirements for companies incorporated in other jurisdictions such as the United States. Nasdaq Listing Rule 5635 generally provides that shareholder approval is required of U.S. domestic companies listed on Nasdaq prior to issuance (or potential issuance) of securities (i) the acquisition of the stock or assets of another company; (ii) equity-based compensation of officers, directors, employees or consultants; (iii) a change of control; and (iv) transactions other than public offerings. In addition, Nasdaq Listing Rule 5640 provides that voting rights of existing shareholders of publicly traded common stock registered under Section 12 of the Exchange Act cannot be disparately reduced or restricted through any corporate action or issuance. Notwithstanding this general requirement, Nasdaq Listing Rule 5615(a)(3)(A) permits foreign private issuers to follow their home country practice rather than these requirements. The Cayman Islands do not require shareholder approval prior to any of the foregoing types of issuances, nor are existing shareholders’ voting rights disparately reduced or restricted through any corporate action or issuance. We, therefore, are not required to obtain such shareholder approval prior to entering into a transaction with the potential to issue securities as described above, and our existing shareholders’ voting rights may be disparately reduced or restricted through corporate action or issuance. Specifically, our board of directors has elected to follow our home country rules and be exempt from the prohibition of disparately reducing or restricting our existing shareholders’ voting rights, and the requirements to obtain shareholder approval for (i) the acquisition of the stock or assets of another company; (ii) equity-based compensation of officers, directors, employees or consultants; (iii) a change of control; and (iv) transactions other than public offerings. Therefore, our shareholders may be afforded less protection than they otherwise would under rules and regulations applicable to U.S. domestic issuers.
As a result of all of the above, our public shareholders may have more difficulty in protecting their interests in the face of actions taken by management, members of the board of directors or controlling shareholders than they would as public shareholders of a company incorporated in the United States.
You may be unable to present proposals before annual general meetings or extraordinary general meetings not called by shareholders.
Cayman Islands law provides shareholders with only limited rights to requisition a general meeting, and does not provide shareholders with any right to put any proposal before a general meeting. These rights, however, may be provided in a company’s articles of association. Our amended and restated articles of association allow our shareholders holding shares which carry in aggregate not less than 10% of all votes attaching to all of our issued and outstanding shares, to requisition a general meeting of our shareholders, in which case our directors are obliged to call such meeting. Advance notice of at least five calendar days is required for the convening of any general meeting of our shareholders. A quorum required for a meeting of shareholders consists of at least one shareholder, present in person or by proxy, holding not less than one third of the issued shares of the class of the Company.
54
Table of Contents
Certain judgments obtained against us by our shareholders may not be enforceable.
We are an exempted company limited by shares incorporated under the laws of the Cayman Islands. We conduct our operations outside the United States and substantially all of our assets are located outside the United States. In addition, most of our directors and executive officers named in this prospectus reside outside the United States, and most of their assets are located outside the United States. As a result, it may be difficult or impossible for you to bring an action against us or against them in the United States in the event that you believe that your rights have been infringed under the U.S. federal securities laws or otherwise. Even if you are successful in bringing an action of this kind, the laws of the Cayman Islands or other relevant jurisdictions may render you unable to enforce a judgment against our assets or the assets of our directors and officers.
If we are classified as a passive foreign investment company, United States taxpayers who own our Ordinary Shares or Warrants may have adverse United States federal income tax consequences.
A non-U.S. corporation such as ourselves will be classified as a passive foreign investment company, which is known as a PFIC, for any taxable year if, for such year, either:
• at least 75% of our gross income for the year is passive income; or
• the average percentage of our assets (determined at the end of each quarter) during the taxable year which produce passive income or which are held for the production of passive income is at least 50%.
Passive income generally includes dividends, interest, rents and royalties (other than rents or royalties derived from the active conduct of a trade or business), and gains from the disposition of passive assets.
If we are determined to be a PFIC for any taxable year (or portion thereof) that is included in the holding period of a U.S. taxpayer who holds our Ordinary Shares and Warrants, the U.S. taxpayer may be subject to increased U.S. federal income tax liability and may be subject to additional reporting requirements.
Depending on the amount of cash we raise in this offering, together with any other assets held for the production of passive income, it is possible that, for our 2024 taxable year or for any subsequent year, more than 50% of our assets may be assets which produce passive income, in which case we would be deemed a PFIC, which could have adverse U.S. federal income tax consequences for U.S. taxpayers who are shareholders. We will make this determination following the end of any particular tax year.
For purposes of the PFIC analysis, in general, a non-U.S. corporation is deemed to own its pro rata share of the gross income and assets of any entity in which it is considered to own at least 25% of the equity by value.
For a more detailed discussion of the application of the PFIC rules to us and the consequences to U.S. taxpayers if we were or are determined to be a PFIC, see “Material Income Tax Considerations — United States Federal Income Taxation — Passive Foreign Investment Company.”
Our shareholders may be held liable for claims by third parties against us to the extent of distributions received by them upon redemption of their shares.
If we are forced to enter into an insolvency liquidation, any distributions received by shareholders could be viewed as an unlawful payment if it was proved that immediately following the date on which the distribution was made, we were unable to pay our debts as they fall due in the ordinary course of business. As a result, a liquidator could seek to recover some or all amounts received by our shareholders. Furthermore, our directors may be viewed as having breached their fiduciary duties to us or our creditors and/or may have acted in bad faith, thereby exposing themselves and our Company to claims, by paying public shareholders from the trust account prior to addressing the claims of creditors. We cannot assure you that claims will not be brought against us for these reasons. We and our directors and officers who knowingly and willfully authorized or permitted any distribution to be paid out of our share premium account* in violation of the Cayman Companies Act, while we were unable to pay our debts as they fall due in the ordinary course of business would be guilty of an offence and may be liable for a monetary fine and to imprisonment for five years in the Cayman Islands.
55
Table of Contents
DISCLOSURE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains forward-looking statements that reflect our current expectations and views of future events. The forward-looking statements are contained principally in the sections entitled “Prospectus Summary,” “Risk Factors,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” “Business,” and “Regulation.” Known and unknown risks, uncertainties and other factors, including those listed under “Risk Factors,” may cause our actual results, performance or achievements to be materially different from those expressed or implied by the forward-looking statements.
You can identify some of these forward-looking statements by words or phrases such as “may,” “will,” “expect,” “anticipate,” “aim,” “estimate,” “intend,” “plan,” “believe,” “is/are likely to,” “potential,” “continue” or other similar expressions. We have based these forward-looking statements largely on our current expectations and projections about future events that we believe may affect our financial condition, results of operations, business strategy and financial needs. These forward-looking statements include statements relating to:
• the expected or potential impact of COVID-19 or any resurgence, and the related responses of the government, consumers, and the Company, on the PRC subsidiaries’ and our business, financial condition and results of operations;
• our dependence on introducing new products on a timely basis;
• our dependence on growth in the demand for the PRC subsidiaries’ products;
• the PRC subsidiaries ability to effectively manage inventories;
• the PRC subsidiaries’ ability to compete effectively;
• the PRC subsidiaries’ ability to successfully manage the capacity expansion and allocation in response to changing industry and market conditions;
• implementation of our expansion plans and our ability to obtain capital resources for our planned growth;
• the PRC subsidiaries’ ability to acquire sufficient raw materials and key components and obtain equipment and services from their suppliers in suitable quantity and quality;
• our dependence on key personnel;
• the PRC subsidiaries’ ability to expand into new businesses, industries, or geographic locations and to undertake mergers, acquisitions, investments, or divestments;
• changes in technology and competing products;
• general economic and political conditions, including those related to the display panel industry;
• possible disruptions in commercial activities caused by events such as natural disasters and terrorist activity;
• fluctuations in foreign currency exchange rates; and
• other factors in the “Risk Factors” section in this prospectus.
These forward-looking statements are subject to various and significant risks and uncertainties, including those which are beyond our or the PRC subsidiaries’ control. Although we believe that our expectations expressed in these forward-looking statements are reasonable, our expectations may later be found to be incorrect. The forward-looking statements made in this prospectus relate only to events or information as of the date on which the statements are made in this prospectus. Except as required by law, we undertake no obligation to update or revise publicly any forward-looking statements, whether as a result of new information, future events or otherwise, after the date on which the statements are made or to reflect the occurrence of unanticipated events. You should thoroughly read this prospectus and the documents that we refer to herein with the understanding that our actual future results may be materially different from and worse than what we expect. We qualify all of our forward-looking statements by these cautionary statements. We disclaim any obligation to update our forward-looking statements, except as required by law.
56
Table of Contents
This prospectus contains certain data and information that we obtained from various Chinese government and private publications, including industry data and information from publicly available information, market research reports, and industry publications and surveys. Although we believe such information to be accurate, we have not independently verified the data from these sources. Statistical data in these publications also include projections based on a number of assumptions.
In addition, the new and rapidly changing nature of the medical device industry results in significant uncertainties for any projections or estimates relating to the growth prospects or future condition of our industry. Furthermore, if any one or more of the assumptions underlying the market data are later found to be incorrect, actual results may differ from the projections based on these assumptions. You should not place undue reliance on these forward-looking statements.
57
Table of Contents
ENFORCEABILITY OF CIVIL LIABILITIES
We were incorporated in the Cayman Islands in order to enjoy the following benefits:
• political and economic stability;
• an effective judicial system;
• a favorable tax system;
• the absence of exchange control or currency restrictions; and
• the availability of professional and support services.
However, certain disadvantages accompany incorporation in the Cayman Islands. These disadvantages include, but are not limited to, the following:
• the Cayman Islands has a less developed body of securities laws as compared to the United States and these securities laws provide significantly less protection to investors; and
• Cayman Islands companies may not have the standing to sue before the federal courts of the United States.
Our constitutional documents do not contain provisions requiring that disputes, including those arising under the securities laws of the United States, between us, our officers, directors and shareholders, be arbitrated. Currently, all of the operations are conducted outside the United States, and substantially all of our assets are located outside the United States. Most of our officers are nationals or residents of jurisdictions other than the United States and a substantial portion of their assets are located outside the United States. As a result, it may be difficult for a shareholder to effect service of process within the United States upon these persons, or to enforce against us or them judgments obtained in United States courts, including judgments predicated upon the civil liability provisions of the securities laws of the United States or any state in the United States.
We have appointed Cogency Global Inc., as our agent upon whom process may be served in any action brought against us under the securities laws of the United States.
Ogier (Cayman) LLP, our counsel as to Cayman Islands law, and AllBright Law Offices (Fuzhou), our counsel as to PRC law, have advised us, respectively, that there is uncertainty as to whether the courts of the Cayman Islands and China, respectively, would:
• recognize or enforce judgments of United States courts obtained against us or our directors or officers predicated upon the civil liability provisions of the securities laws of the United States or any state in the United States; or
• in original actions brought in each respective jurisdiction against us or our directors or officers predicated upon the civil liability provisions of the federal securities laws of the United States or any state in the United States so far as the liabilities imposed by those provisions are penal in nature.
We have been advised by our Cayman Islands legal counsel, Ogier (Cayman) LLP, that the courts of the Cayman Islands are unlikely (i) to recognize or enforce against us judgments of courts of the United States predicated upon the civil liability provisions of the securities laws of the United States or any State; and (ii) in original actions brought in the Cayman Islands, to impose liabilities against us predicated upon the civil liability provisions of the securities laws of the United States or any State, so far as the liabilities imposed by those provisions are penal in nature. In those circumstances, although there is no statutory enforcement in the Cayman Islands of judgments obtained in the United States, the courts of the Cayman Islands will recognize and enforce a foreign money judgment of a foreign court of competent jurisdiction without retrial on the merits based on the principle that a judgment of a competent foreign court imposes upon the judgment debtor an obligation to pay the sum for which judgment has been given provided certain conditions are met. For a foreign judgment to be enforced in the Cayman Islands, such judgment must be final and conclusive and for a liquidated sum, and must not be in respect of taxes or a fine or penalty, inconsistent with a Cayman Islands judgment in respect of the same matter, impeachable on the grounds of fraud or obtained in a manner, and or be of a kind the enforcement of which is, contrary to natural justice or the public policy of the Cayman Islands (awards of punitive or multiple damages may well be held to be contrary to public policy). A Cayman Islands Court may stay enforcement proceedings if concurrent proceedings are being brought elsewhere.
58
Table of Contents
We have been advised by our PRC counsel, AllBright Law Offices (Fuzhou), that the recognition and enforcement of foreign judgments are provided for under the PRC Civil Procedure Law. PRC courts may recognize and enforce foreign judgments in accordance with the requirements of the PRC Civil Procedure Law based either on treaties between mainland China and the country where the judgment is made or on reciprocity between different jurisdictions, and PRC courts will not recognize or enforce these foreign judgments if PRC courts believe the foreign judgments violate the basic principles of PRC laws or national sovereignty, security or public interest after review. However, currently, mainland China does not have treaties or reciprocity arrangement providing for recognition and enforcement of foreign judgments ruled by courts in the United States or the Cayman Islands. Thus, it is uncertain whether a PRC court would enforce a judgment ruled by a court in the United States or the Cayman Islands.
In addition, our investors may incur additional costs and face procedural obstacles in effecting service of legal process, enforcing foreign judgments or bringing actions in Hong Kong against us or our management, as judgments entered in the United States can be enforced in Hong Kong only at common law. To enforce a judgment of the United States in Hong Kong, it must be a final judgment rendered conclusive upon the merits of the claim, for a liquidated amount in a civil matter and not in respect of taxes, fines, penalties, or similar charges, the proceedings in which the judgment was obtained were not contrary to natural justice, and the enforcement of the judgment is not contrary to public policy of Hong Kong. Such a judgment must be for a fixed sum and must also come from a “competent” court, as determined by the private international law rules applied by the Hong Kong courts.
Furthermore, foreign judgments of United States courts will not be directly enforced in Hong Kong. There are currently no treaties or other arrangements providing for reciprocal enforcement of foreign judgments between Hong Kong and the United States. However, the common law of Hong Kong permits an action to be brought upon a foreign judgment. A foreign judgment itself may form the basis of a cause of action, since the judgment may be regarded as creating a debt between the parties to such action. The defenses that are available to a defendant in a common law action brought on the basis of a foreign judgment include lack of jurisdiction, breach of natural justice, fraud, and contrary to public policy. However, a separate legal action for debt must be commenced in Hong Kong in order to recover such debt from the judgment debtor. A foreign judgment of the United States predicated solely upon the federal securities laws of the United States or the securities laws of any State or territory within the United States could be enforceable in Hong Kong, but will be subject to the conditions with regard to enforcement of such judgments being met, including but not limited to the foregoing requirements.
59
Table of Contents
USE OF PROCEEDS
We estimate that we will receive net proceeds from this offering of approximately $13,712,279, based on an assumed public offering price of $6.00 per Ordinary Unit, assuming no sale of the Pre-Funded Warrants and no exercise of the Warrants included in the Units, after deducting underwriting discounts and commissions, non-accountable expense allowance and estimated offering expenses payable by us.
We will only receive additional proceeds from the exercise of the Warrants issuable in connection with this offering if the Warrants are exercised for cash. The Series B Warrants may be exercised by way of an alternative cashless exercise, meaning that the holder thereof may not pay a cash purchase price upon exercise, but instead would receive upon such exercise a number of Ordinary Shares every Series B Warrant they exercise in accordance with the formula contained in the Series B Warrants. Accordingly, we will likely not receive any additional funds upon the exercise of the Series B Warrants.
We plan to use the net proceeds we receive from this offering for the following purposes:
• approximately 30% for upgrading production equipment and investing in the PRC subsidiaries’ R&D;
• approximately 10% for hiring experienced employees to improve our systems of internal control and compliance with U.S. GAAP and the Sarbanes-Oxley Act of 2002; and
• approximately 60% for working capital and general corporate purposes.
The foregoing represents our current intentions based upon our present plans and business conditions to use and allocate the net proceeds of this offering. Our management, however, will have significant flexibility and discretion to apply the net proceeds of this offering. If an unforeseen event occurs or business conditions change, we may use the proceeds of this offering differently than as described in this prospectus. See “Risk Factors — Risks Relating to this offering and the Trading Market — Our management has broad discretion to determine how to use the funds raised in the offering and may use them in ways that may not enhance our results of operations or the price of our Ordinary Shares.” To the extent that the net proceeds we receive from this offering are not immediately used for the above purposes, we intend to invest our net proceeds in short-term, interest-bearing bank deposits or debt instruments.
In using the proceeds of this offering, we are permitted under PRC laws and regulations to utilize the proceeds from this offering to fund the PRC subsidiaries by making loans or additional capital contributions, subject to applicable government registration and approval requirements. We cannot assure you that we will be able to obtain these government registrations or approvals on a timely basis, if at all. See “Risk Factors — Risks Related to Doing Business in China — PRC regulation of loans to and direct investment in PRC entities by offshore holding companies and governmental control of currency conversion may delay us from using the proceeds of this offering to make loans or additional capital contributions to the PRC subsidiaries, which could materially and adversely affect our liquidity and our ability to fund and expand the PRC subsidiaries’ business.”
Each $1.00 increase (decrease) in the assumed public offering price of $6.00 per Ordinary Unit would increase (decrease) the net proceeds to us from this offering, after deducting the underwriting discounts and commissions and estimated offering expenses payable by us, by approximately $2,300,000, assuming that the number of Units offered by us, as set forth on the cover page of this prospectus, remains the same. We may also increase or decrease the number of Units we are offering. An increase (decrease) of 100,000 in the number of Units we are offering would increase (decrease) the net proceeds to us from this offering, after deducting the underwriting discounts and commissions and estimated offering expenses payable by us, by approximately $552,000, assuming the public offering price stays the same. An increase of 100,000 in the number of Units we are offering, together with a $1.00 increase in the public offering price of $6.00 per Ordinary Unit, would increase the net proceeds to us from this offering, after deducting the underwriting discounts and commissions and estimated offering expenses payable by us, by approximately $2,944,000. A decrease of 100,000 in the number of Units we are offering, together with a $1.00 decrease in the assumed public offering price of $6.00 per Ordinary Unit, would decrease the net proceeds to us from this offering, after deducting the underwriting discounts and commissions and estimated offering expenses payable by us, by approximately $2,760,000. We do not expect that a change in the offering price or the number of Units by these amounts would have a material effect on our intended uses of the net proceeds from this offering, although it may impact the amount of time prior to which we may need to seek additional capital.
60
Table of Contents
DIVIDEND POLICY
Our board of directors has discretion regarding whether to declare or pay dividends. In addition, our shareholders may by ordinary resolution declare a dividend, but no dividend may exceed the amount recommended by our board of directors. All dividends are subject to certain restrictions under Cayman Islands law, namely that our Company may only pay dividends out of profits or share premium, and provided always that we are able to pay our debts as they fall due in the ordinary course of business. Even if our board of directors decides to pay dividends, the form, frequency and amount will depend upon our future operations and earnings, capital requirements and surplus, general financial condition, contractual restrictions and other factors that the board of directors may deem relevant.
We have never declared or paid cash dividends on our Ordinary Shares. We currently do not have any plans to pay cash dividends. Rather, we currently intend to retain all of our available funds and any future earnings to operate and grow our business.
If we determine to pay dividends on any of our Ordinary Shares in the future, as a holding company, we will depend upon receipt of funds from our British Virgin Islands subsidiary, Work BVI.
Current PRC regulations permit the PRC subsidiaries to pay dividends to Work Medical Technology only out of their accumulated profits, if any, determined in accordance with Chinese accounting standards and regulations. In addition, each of our subsidiaries in mainland China is required to set aside at least 10% of its after-tax profits each year, if any, to fund a statutory reserve until such reserve reaches 50% of its registered capital. Each of such entities in mainland China is also required to further set aside a portion of its after-tax profits to fund the employee welfare fund. Although the statutory reserves can be used, among other ways, to increase the registered capital and eliminate future losses in excess of retained earnings of the respective companies, the reserve funds are not distributable as cash dividends except in the event of liquidation.
The PRC government also imposes controls on the conversion of RMB into foreign currencies and the remittance of currencies out of mainland China. Therefore, we may experience difficulties in complying with the administrative requirements necessary to obtain and remit foreign currency for the payment of dividends from our profits, if any. Furthermore, if our subsidiaries and affiliates in mainland China incur debt on their own in the future, the instruments governing the debt may restrict their ability to pay dividends or make other payments. If we or our subsidiaries are unable to receive all of the revenue from our operations, we may be unable to pay dividends on our Ordinary Shares.
Cash dividends, if any, on our Ordinary Shares will be paid in U.S. dollars. Work Medical Technology or any of our subsidiaries outside of China may be considered a non-resident enterprise for tax purposes, so that any dividends the PRC subsidiaries pay to Work Medical Technology or any of our subsidiaries outside of China may be regarded as China-sourced income and as a result may be subject to PRC withholding tax at a rate of up to 10%. See “Taxation — People’s Republic of China Taxation.”
In order for us to pay dividends to our shareholders, we will rely on payments made from Work Hangzhou’s subsidiaries to Work Hangzhou and from Work Hangzhou to WFOE, and the distribution of such payments to Work Medical Technology, and from Work Medical Technology to Work BVI and then to our Company. According to the PRC Enterprise Income Tax Law (the “EIT Law”), income obtained by enterprises shall pay enterprise income tax including income from equity investment such as dividends and bonuses. In addition, if Work Hangzhou or its subsidiaries or branches incur debt on their own behalf in the future, the instruments governing the debt may restrict its ability to pay dividends or make other distributions to us.
61
Table of Contents
EXCHANGE RATE INFORMATION
Our business is conducted by our PRC subsidiaries, in PRC denominated in RMB. Capital accounts of our financial statements are translated into U.S. dollars from RMB at their historical exchange rates when the capital transactions occurred. No representation is made that the RMB amounts could have been, or could be, converted into U.S. dollars at the rates used in translation. The following table sets forth information concerning exchange rates between RMB and the U.S. dollar for the periods indicated.
Assets and liabilities are translated at the exchange rates as of the balance sheet date as provided in the table below.
| | September 30, |
Balance sheet items, except for equity accounts | | 2023 | | 2022 |
RMB:1USD | | 7.2960 | | 7.1135 |
| | March 31, |
Balance sheet items, except for equity accounts | | 2024 | | 2023 |
RMB:1USD | | 7.2203 | | 6.8676 |
Items in the statements of operations and comprehensive income (loss), and statements cash flows are translated at the average exchange rate of the period.
| | Six Months Ended
March 31, |
| | | 2024 | | 2023 |
RMB:1USD | | 7.2064 | | 6.9761 |
| | Fiscal Years Ended |
| | | September 30,
2023 | | September 30,
2022 |
RMB:1USD | | 7.0824 | | 6.5532 |
62
Table of Contents
CAPITALIZATION
The following table sets forth our capitalization as of March 31, 2024:
• on an actual basis;
• on a pro forma basis to give effect to the issuance and sales of 2,091,942 Ordinary Shares, including 91,942 Ordinary Shares of the over-allotment shares, by us in the IPO at $4.00 per share on a firm commitment basis, for net proceeds of approximately $5,654,525, after deducting underwriting discounts and other related expenses, which IPO was completed on August 26, 2024; and
• on a pro forma as adjusted basis to give effect to (i) the transactions described above; (ii) the issuance and sale of 2,500,000 Units offered hereby, based on an assumed offering price of $6.00 per Ordinary Unit, each Unit consisting of (i) one Ordinary Share (or one Pre-Funded Warrant), (ii) one Series A Warrant, and (iii) one Series B Warrant, assuming no exercise of the Warrants included in the Units, no issuance of Pre-Funded Ordinary Units and no other change to the number of Units sold by us as set forth on the front cover of this prospectus; and (iii) the application of the net proceeds after deducting estimated offering expenses payable by us.
The information set forth in the table below is illustrative only and will be adjusted based on the actual public offering price and other terms of this offering as determined at pricing. You should read this capitalization table in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and the consolidated financial statements and the related notes appearing elsewhere in this prospectus.
| | As of March 31, 2024 |
Actual
(unaudited) | | Pro Forma
(unaudited) | | Pro Forma
As adjusted
(unaudited) |
| | | $ | | $ | | $ |
Cash, cash equivalents and restricted cash | | 1,080,639 | | | 6,735,164 | | | 20,447,443 | |
Debt | | | | | | | | | |
Short term borrowings | | 14,057,588 | | | 14,057,588 | | | 14,057,588 | |
Shareholders’ Equity: | | | | | | | | | |
Ordinary Shares, $0.0005 par value, 100,000,000 Ordinary Shares authorized, 12,500,000 Ordinary Shares issued and outstanding as of March 31, 2024; 14,591,942 Ordinary Shares issued and outstanding, pro forma; 17,091,942 Ordinary Shares issued and outstanding, pro forma as adjusted based on an assumed public offering price of $6.00 per Ordinary Unit | | 6,250 | | | 7,296 | | | 8,546 | |
Subscription receivable | | (6,250 | ) | | (7,296 | ) | | (8,546 | ) |
Additional paid-in capital | | 85,012 | | | 5,738,491 | | | 19,450,770 | |
Statutory surplus reserves | | 887,482 | | | 887,482 | | | 887,482 | |
Retained earnings | | 9,807,110 | | | 9,807,110 | | | 9,807,110 | |
Accumulated other comprehensive income | | (565,263 | ) | | (565,263 | ) | | (565,263 | ) |
Total equity attributable to Work Cayman | | 10,214,341 | | | 15,867,820 | | | 29,580,099 | |
Equity attributable to non-controlling interests | | 1,121,155 | | | 1,121,155 | | | 1,121,155 | |
Total equity | | 11,335,496 | | | 16,988,975 | | | 30,701,254 | |
Total Capitalization | | 24,271,929 | | | 29,925,408 | | | 43,637,687 | |
The number of Ordinary Shares to be outstanding on a pro forma and pro forma as adjusted basis, is based on 12,500,000 Ordinary Shares outstanding as of March 31, 2024, and excludes:
Each $1.00 increase (decrease) in the assumed public offering price of $6.00 per Ordinary Unit, would increase (decrease) the amount of cash and cash equivalents, additional paid-in capital, total shareholders’ equity, and total capitalization on a pro forma, as adjusted, basis, by approximately $2,300,000, assuming the number of Units offered by us, as set forth on the front cover of this prospectus, remains the same, and after deducting the underwriters’ discounts and commissions, non-accountable expense allowance, and estimated offering expenses payable by us. Similarly,
63
Table of Contents
each increase (decrease) of 100,000 Units offered by us would increase (decrease) cash and cash equivalents, total shareholders’ equity and total capitalization on a pro forma, as adjusted, basis by approximately $552,000, assuming the assumed public offering price of $6.00 per Ordinary Unit remains the same, and after deducting underwriting discounts and commissions and estimated offering expenses payable by us. Each 100,000 Unit increase in the number of Units offered by us together with a concomitant $1.00 increase in the assumed public offering price of $6.00 per Ordinary Unit would increase each of cash and total shareholders’ equity by approximately $2,944,000 after deducting underwriting discounts and commissions and any estimated offering expenses payable by us.
64
Table of Contents
DILUTION
If you invest in the securities being offered in this offering, assuming no value is attributed to any of the Warrants included in the Units offered hereby and assuming no issuance of any Pre-Funded Ordinary Units, your ownership interest will be diluted to the extent of the difference between the public offering price per Ordinary Share included in the Units and our pro forma as adjusted net tangible book value per Ordinary Share immediately after this offering. Dilution results from the fact that the public offering price per Ordinary Share included in Units is substantially in excess of the pro forma as adjusted net tangible book value per Ordinary Share attributable to the existing shareholders for our presently outstanding Ordinary Shares.
Our net tangible book value as of March 31, 2024, was $10,355,812, or $0.83 per Ordinary Share. Net tangible book value is the amount of our total consolidated tangible assets, less the amount of our total consolidated liabilities. Dilution is determined by subtracting the net tangible book value per Ordinary Share (as adjusted for the offering) from the public offering price per Ordinary Share and after deducting the underwriting discounts, non-accountable expense allowance, and the estimated offering expenses payable by us.
Our pro forma net tangible book value was $16,010,337, or $1.10 per Ordinary Share. Pro forma net tangible book value represents the amount of our total tangible assets less our total liabilities, after giving effect to the issuance and sales of Ordinary Shares in our IPO for net proceeds of approximately $5,654,525, after deducting underwriting discounts and other related expenses. Pro forma net tangible book value per share represents pro forma net tangible book value divided by the total number of Ordinary Shares outstanding as of March 31, 2024, after giving effect to the pro forma adjustments described above.
After giving effect to the issuance and sale of 2,500,000 Units offered in this offering based on the assumed public offering price of $6.00 per Ordinary Unit, after deduction of the underwriting discounts, non-accountable expense allowance and the estimated offering expenses payable by us and assuming no issuance of Pre-Funded Ordinary Units and no exercise of the Warrants included in the Units, our pro forma as adjusted net tangible book value as of March 31, 2024, would have been approximately $29,722,616, or $1.74 per outstanding Ordinary Share. This represents an immediate increase in net tangible book value of $0.64 per Ordinary Share to the existing shareholders, and an immediate dilution in net tangible book value of $4.26 per Ordinary Share to investors purchasing Units in this offering.
The dilution information discussed above is illustrative only and may change based on the actual public offering price and other terms of this offering.
The following table illustrates such dilution:
Assumed public offering price per Ordinary Unit | | $ | 6.00 |
Net tangible book value per Ordinary Share as of March 31, 2024 | | $ | 0.83 |
Pro forma net tangible book value per Ordinary Share, as of March 31, 2024, before giving
effect to this offering | | $ | 1.10 |
Increase in net tangible book value per Ordinary Share, before giving effect to this offering | | $ | 0.27 |
Increase in pro forma as adjusted net tangible book value per Ordinary Share attributable to payments by new investors | | $ | 0.64 |
Pro forma as adjusted net tangible book value per Ordinary Share immediately after this offering | | $ | 1.74 |
Amount of dilution in net tangible book value per Ordinary Share to new investors in the offering | | $ | 4.26 |
A $1.00 increase in the assumed public offering price of $6.00 per Ordinary Unit would increase our pro forma as adjusted net tangible book value as of March 31, 2024 after this offering, given the same assumptions described above, by approximately $2,300,000, or $0.13 per Ordinary Share, and would increase dilution to new investors by approximately $6.87 per Ordinary Share, assuming that the number of Units offered by us, as set forth on the cover page of this prospectus, remains the same, and after deducting the underwriter discounts and offering expenses payable by us.
We may also increase or decrease the number of Units we are offering. An increase (decrease) of 100,000 in the number of Units we are offering would increase (decrease) our pro forma as adjusted net tangible book value as of March 31, 2024, giving effect to this offering, by approximately $552,000, or approximately $0.03 per Ordinary Share, and would decrease dilution (increase) to investors in this offering by approximately $5.97 per Ordinary Share, given the same assumptions described above.
65
Table of Contents
Each 100,000 Unit increase (decrease) in the number of Units offered by us together with a concomitant $1.00 increase (decrease) in the assumed public offering price of $6.00 per Ordinary Unit would increase (decrease) the pro forma as adjusted net tangible book value by $0.17 per Ordinary Share and the dilution to new investors by $6.83 per Ordinary Share, given the same assumptions described above.
The pro forma as adjusted information as discussed above is illustrative only. Our net tangible book value following the completion of this offering is subject to adjustment based on the actual public offering price of our Units and other terms of this offering determined at the pricing.
The following table summarizes, on a pro forma as adjusted basis as of March 31, 2024, the differences between the number of Ordinary Shares purchased from us given the assumptions described above, the total cash consideration and the average price per Ordinary Share paid to us by existing shareholders and by new investors purchasing Units in this offering at an assumed public offering price of $6.00 per Ordinary Unit, before deducting estimated underwriting discounts and estimated offering expenses payable by us:
| | Ordinary Shares
Purchased | | Total
Consideration | | Average Price
Per Ordinary
Share |
| | | Number | | Percent | | Amount | | Percent | |
Existing shareholders | | 14,591,942 | | 85 | % | | $ | 8,452,780 | | 36 | % | | $ | 0.58 |
New public investors | | 2,500,000 | | 15 | % | | | 15,000,000 | | 64 | % | | $ | 6.00 |
Total | | 17,091,942 | | 100 | % | | | 23,452,780 | | 100 | % | | $ | 6.58 |
To the extent that warrants, options or other securities are issued, including under our equity incentive plans, or we issue additional Ordinary Shares or preferred stock in the future, there will be further dilution to the persons being issued Ordinary Shares in this offering. In addition, we may choose to raise additional capital because of market conditions or strategic considerations, even if we believe that we have sufficient funds for our current or future operating plans. If we raise additional capital through the sale of equity or convertible debt securities, the issuance of such securities could result in further dilution to our shareholders.
66
Table of Contents
CORPORATE HISTORY AND STRUCTURE
Corporate History
Work Cayman is a Cayman Islands exempted company incorporated on March 1, 2022. Work BVI is our wholly-owned subsidiary formed in the British Virgin Islands on March 15, 2022. Work Medical Technology is Work BVI’s wholly-owned subsidiary formed in Hong Kong on April 19, 2022. WFOE, is Work Medical Technology’s wholly-owned subsidiary formed in Hangzhou on April 28, 2022. Work Hangzhou is WFOE’s wholly-owned subsidiary formed in Hangzhou on November 10, 2021.
Our operations are primarily conducted by Work Hangzhou and its subsidiaries in China.
Work Hangzhou is a limited liability company formed in China on November 10, 2021 with registered capital of RMB5 million.
Hangzhou Woli, a PRC limited liability company formed on July 29, 2022, with registered capital of RMB15 million, is a wholly owned subsidiary of Work Hangzhou.
Shanghai Saitumofei, a PRC limited liability company formed on May 15, 2019, with registered capital of RMB10 million, is a majority owned subsidiary of Work Hangzhou, of which Work Hangzhou owns approximately 44.20% shares, Jun Ma owns approximately 20.80% shares, Jianyuan Lu owns approximately 17.33% shares, Huangshan Tunxi District Leading Industry Incubation Fund Partnership (Limited Partnership) owns approximately 13.33% shares, and Shanghai Aikerui Medical Technology Co., Ltd. owns approximately 4.33% shares. It currently engages in the research and development of Class II and III medical devices, biological and medical technology.
Hunan Saitumofei, a PRC limited liability company formed on April 2, 2021, with registered capital of RMB2 million, is a wholly owned subsidiary of Shanghai Saitumofei.
Hangzhou Shanyou, a PRC limited liability company formed on April 29, 2002, with registered capital of RMB100 million, is a majority owned subsidiary of Work Hangzhou, of which Work Hangzhou owns 95% shares, our COO, Baiming Yu owns 3.35% shares and Liwei Zhang, spouse of Baiming Yu, owns 1.65% shares. It currently engages in the manufacture and sale of Class I and II medical devices.
Hangzhou Hanshi, a PRC limited liability company formed on July 22, 2019, with registered capital of RMB1 million, is a majority owned subsidiary of Hangzhou Shanyou and engages in the sales of Class II medical devices. The rest of Hangzhou Hanshi’s shareholders are Cheng Peng, Xiuwen Zhang and Zhengyan He, who own 25%, 10% and 5% shares, respectively.
Shanghai Chuqiang, a PRC limited liability company formed on March 12, 2018, with registered capital of RMB2 million, is a wholly owned subsidiary of Hangzhou Shanyou and engages in the sales of Class II medical devices.
Hangzhou Youshunhe, a PRC limited liability company formed on February 27, 2023, with registered capital of RMB1 million, is a majority owned subsidiary of Hangzhou Shanyou. The other shareholder of Hangzhou Youshunhe is Hangzhou Shunshunxin Material Technology Co., Ltd. Hangzhou Youshunhe does not have any operations as of the date of this prospectus.
Huangshan Saitumofei, a PRC limited liability company formed on April 30, 2024, with registered capital of RMB1 million, is a fully owned subsidiary of Shanghai Saitumofei. Huangshan Saitumofei does not have any operations as of the date of this prospectus.
67
Table of Contents
Corporate Structure
The chart below summarizes our corporate structure as of the date of this prospectus.

On April 6, 2023, the shareholders of the Company unanimously passed resolutions effecting the subdivision of the Company’s authorized and issued share capital and the adoption of the amended and restated memorandum of association, pursuant to which, (1) the Company effectuated a 1:2000 share subdivision, whereupon the Company’s authorized share capital was amended from US$50,000 divided into 50,000 shares of par value US$1.00 each to US$50,000 divided into 100,000,000 Ordinary Shares of a par value of $0.0005 each; and (2) immediately after the share subdivision, the shareholders voluntarily surrendered, on a pro rata basis, a total of 87,500,000 Ordinary Shares of a par value of $0.0005 each, after which, the Company had an aggregate of 12,500,000 Ordinary Shares issued and outstanding.
68
Table of Contents
For details of our principal shareholders’ ownership, please refer to the beneficial ownership table in the section captioned “Principal Shareholders.”
Neither we nor the PRC subsidiaries are operating in an industry that prohibits or limits foreign investment. As a result, neither we nor the PRC subsidiaries are required to obtain any permission from Chinese authorities to operate other than those requisite that a domestic company in China will need to engage in the businesses similar to the that of the PRC subsidiaries. Such licenses and permissions include Business License, Medical Device Registration Certificate, Medical Device Production Certificate, Medical Device Selling Certificate, Record Form of Medical Device Products, Certificate of Class I medical device production recordation, Certificate of Class II medical device selling recordation, Record Registration Form for Foreign Trade Business Operators, and Certificate of the Customs of the People’s Republic of China on Registration of a Customs Declaration Entity. The PRC subsidiaries have obtained the above licenses and permissions to conduct their business in China. However, the PRC government may take actions to exert more oversight and control over offerings by China based issuers conducted overseas and/or foreign investment in such companies, which could significantly limit or completely hinder our ability to offer or continue to offer securities to investors and cause the value of our securities to significantly decline or become worthless.
We treated Work Hangzhou and its subsidiaries as our consolidated affiliated entities under U.S. GAAP for the six months ended March 31, 2024 and the fiscal years ended September 30, 2023 and 2022. We have consolidated the financial results of Work Hangzhou and its subsidiaries in our financial statements in accordance with U.S. GAAP for the same periods.
Work Hangzhou and its subsidiaries contributed to 100% of our consolidated revenue and accounted for 100% of our consolidated total assets and liabilities for the six months ended March 31, 2024 and the fiscal years ended September 30, 2023 and 2022. There was no reconciliation performed between the financial position, cash flows and results of operations of Work Hangzhou and us.
69
Table of Contents
MANAGEMENT’S DISCUSSION AND ANALYSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with our consolidated financial statements and related notes that appear in this prospectus. In addition to historical consolidated financial information, the following discussion contains forward-looking statements that reflect our plans, estimates, and beliefs. Our actual results could differ materially from those discussed in the forward-looking statements. Factors that could cause or contribute to these differences include those discussed below and elsewhere in this prospectus, particularly in “Risk Factors” and elsewhere in this prospectus. See “Cautionary Note Regarding Forward Looking Statements.” All amounts in the fiscal years ended September 30, 2023 and 2022 are derived from our audited consolidated financial statements included elsewhere in this prospectus and the amounts in the six months ended March 31, 2024 and 2023 are derived from our unaudited consolidated financial statements included elsewhere in this prospectus. These financial statements have been prepared in accordance with U.S. GAAP.
Business Overview
We are a holding company with operating subsidiaries (collectively referred as the “Group”), and with all of our operations and assets in China. Our vision is to provide professional and reliable medical devices, both domestically and internationally. The PRC subsidiaries mainly manufacture and sell Class I and II medical devices, including endotracheal tubes, laryngeal mask airways, heat and moisture exchanging filters (HMEF), disposable breathing circuits, nebulizer kits, and yankauer suction sets. Besides Class I and II medical devices, the PRC subsidiaries also have retained the certificate of selling Class III disposable medical devices.
The PRC subsidiaries have three types of customers, i) direct end-user customers, which include hospitals, pharmacies, and medical institutions, ii) domestic distributor customers that distribute the PRC subsidiaries’ products to end-user customers in China, and iii) export distributor customers that distribute the PRC subsidiaries’ products to end-user customers in Asia, Africa, Europe, North America, South America, and Oceania.
Revenue decreased by $6,145,339, or approximately 31.18%, to $13,565,951 for the year ended September 30, 2023 from $19,711,290 for the year ended September 30, 2022. The decrease was mainly due to the decline in demand and unit price of masks and medical devices other than masks and was offset by the increase of commodity trading.
Net income decreased by $880,743, or approximately 93.3%, to $63,383 for the year ended September 30, 2023 from $944,126 for the year ended September 30, 2022.
For the six months ended March 31, 2024 and 2023, our revenues were $5,309,095 and $9,011,404, respectively, and our net income was $234,854 and $1,433,365, respectively. The decrease in sales was primarily due to the decrease in demand and unit price of masks, and was offset by the increase in sales of medical devices other than masks. We do not expect this trend to continue in future financial periods, and we estimate that the demand and unit price of masks have stabilized, as evidenced by the steady sales volume and unit price of masks from February 2024 to mid-July 2024. To mitigate these adverse effects, we have been strengthening our marketing efforts, focusing on medical devices other than masks, achieving strong sales performance for the six months ended March 31, 2024.
Key Factors Affecting Our Results of Operations
We believe the following key factors may affect our financial condition and results of operations:
• the PRC subsidiaries’ ability to effectively and efficiently conduct sales and marketing;
• the PRC subsidiaries’ ability to acquire sufficient raw materials and key components and obtain equipment and services from their suppliers in suitable quantity and quality;
• the PRC subsidiaries’ ability to effectively manage inventories; and
• the PRC subsidiaries’ ability to enhance our operational efficiency.
70
Table of Contents
Impact of COVID-19
Beginning in 2020, the COVID-19 pandemic resulted in quarantines, travel restrictions, and temporary closure of stores and facilities in China and elsewhere.
China lifted its travel restrictions and quarantine requirements in December 2022. We will continue to actively monitor the development of COVID-19, including outbreaks driven by its variants, and may take further actions to alter our business operations as may be required by governmental authorities or which we determine are in the best interests of our employees, customers, suppliers, and shareholders.
However, the extent of the impact of COVID-19 on our future financial results will be dependent on future developments, such as the length and severity of any resurgence, future government actions in response to COVID-19 on the global economy and capital markets, among many other factors, all of which remain highly uncertain and unpredictable. Given this uncertainty, it is impracticable for us to quantify the expected impact of COVID-19 on our future operations, financial condition, liquidity, and results of operations.
Recent Developments
On August 26, 2024, the Company closed its IPO of 2,000,000 Ordinary Shares, par value $0.0005 per share. The Ordinary Shares were priced at $4.00 per share, and the offering was conducted on a firm commitment basis.
On August 28, 2024, Kingswood Capital Partners, LLC, as the representative of the underwriters of the initial public offering of the Company, exercised its over-allotment option, in part, to purchase an additional 91,942 Ordinary Shares of the Company at the public offering price of US$4.00 per share. The closing for the sale of the over-allotment shares took place on August 29, 2024. As a result, the net proceeds of the Company’s IPO, including the proceeds from the sale of the over-allotment shares, totaled $5,654,525, after deducting underwriting discounts and other related expenses.
Results of Operations
The following table sets forth a summary of our consolidated statements of income for the fiscal years ended September 30, 2023 and 2022, and for the six months ended March 31, 2024 and 2023, respectively. This information should be read together with our consolidated financial statements and related notes included elsewhere in this prospectus. The results of operations in any period are not necessarily indicative of our future trends.
| | For the Six Months Ended
March 31, | | Change in
amount | | % of
change |
2024 | | 2023 | |
Net revenue from third parties | | $ | 5,075,986 | | | $ | 8,523,938 | | | $ | (3,447,952 | ) | | (40.5 | )% |
Net revenue from related party | | | 233,109 | | | | 487,466 | | | | (254,357 | ) | | (52.2 | )% |
Cost of revenue | | | (3,766,116 | ) | | | (5,111,338 | ) | | | 1,345,222 | | | (26.3 | )% |
Gross profit | | | 1,542,979 | | | | 3,900,066 | | | | (2,357,087 | ) | | (60.4 | )% |
Selling expenses | | | (1,094,833 | ) | | | (619,763 | ) | | | (475,070 | ) | | 76.7 | % |
General and administrative expenses | | | (777,056 | ) | | | (1,116,964 | ) | | | 339,908 | | | (30.4 | )% |
Research and development expenses | | | (178,783 | ) | | | (372,276 | ) | | | 193,493 | | | (52.0 | )% |
Total operating expenses | | | (2,050,672 | ) | | | (2,109,003 | ) | | | 58,331 | | | (2.8 | )% |
(Loss)/income from operations | | | (507,693 | ) | | | 1,791,063 | | | | (2,298,756 | ) | | (128.3 | )% |
Total other income (loss), net | | | 762,597 | | | | (137,027 | ) | | | 899,624 | | | (656.5 | )% |
Income before income tax expense | | | 254,904 | | | | 1,654,036 | | | | (1,399,132 | ) | | (84.6 | )% |
Income tax expense | | | (20,050 | ) | | | (220,671 | ) | | | 200,621 | | | (90.9 | )% |
Net income | | | 234,854 | | | | 1,433,365 | | | | (1,198,511 | ) | | (83.6 | )% |
71
Table of Contents
| | For the Years Ended
September 30, | | Change in
amount | | % of
change |
2023 | | 2022 | |
Net revenue | | $ | 13,565,951 | | | $ | 19,711,290 | | | $ | (6,145,339 | ) | | (31.2 | )% |
Cost of revenue | | | (9,422,967 | ) | | | (15,292,498 | ) | | | 5,869,531 | | | (38.4 | )% |
Gross profit | | | 4,142,984 | | | | 4,418,792 | | | | (275,808 | ) | | (6.2 | )% |
Selling expenses | | | (1,567,386 | ) | | | (1,032,685 | ) | | | (534,700 | ) | | 51.8 | % |
General and administrative expenses | | | (1,861,728 | ) | | | (2,563,879 | ) | | | 702,151 | | | (27.4 | )% |
Research and development expenses | | | (301,644 | ) | | | (183,900 | ) | | | (117,744 | ) | | 64.0 | % |
Total operating expenses | | | (3,730,757 | ) | | | (3,780,464 | ) | | | 49,707 | | | (1.3 | )% |
Income from operations | | | 412,227 | | | | 638,328 | | | | (226,101 | ) | | (35.4 | )% |
Total other income, net | | | (301,838 | ) | | | 475,233 | | | | (777,071 | ) | | (163.5 | )% |
Income before income tax expense | | | 110,389 | | | | 1,113,561 | | | | (1,003,172 | ) | | (90.1 | )% |
Income tax expense | | | (47,006 | ) | | | (169,435 | ) | | | 122,429 | | | (72.3 | )% |
Net income | | | 63,383 | | | | 944,126 | | | | (880,743 | ) | | (93.3 | )% |
Six Months Ended March 31, 2024 and 2023
Net revenue
| | For the Six Months Ended
March 31, |
| | | 2024 | | 2023 |
Masks | | $ | 566,549 | | $ | 4,752,892 |
Medical devices other than masks | | | 4,564,468 | | | 3,940,257 |
Commodity trading | | | 178,078 | | | 257,530 |
Others | | | — | | | 60,725 |
Net revenue | | $ | 5,309,095 | | $ | 9,011,404 |
Sales decreased by $3,702,309 or 41.1%, to $5,309,095 for the six months ended March 31, 2024 from $9,011,404 for the six months ended March 31, 2023. The decrease in sales was primarily due to the decrease in demand and unit price of masks, and was offset by the increase of sales of medical devices other than masks.
Sales of masks decreased by $4,186,343, or 88.1%, to $566,549 for the six months ended March 31, 2024 from $4,752,892 for the six months ended March 31, 2023. The decrease was mainly because (i) China lifted travel restrictions and quarantine requirements in December 2022, and the demand for masks and the sales volume of masks decreased for the six months ended March 31, 2024; and (ii) the Company shifted its marketing focus from masks to medical devices other than masks.
Medical devices other than masks sold are mainly anesthesia and respiratory consumables. The sales of medical devices increased by $624,211, or 15.8%, to $4,564,468 for the six months ended March 31, 2024 from $3,940,257 for the six months ended March 31, 2023. The increase was mainly attributable to the Group’s decision to intensify marketing efforts for medical devices other than masks, which accordingly led to higher sales volumes.
Cost of revenue
Cost of revenue primarily includes the cost of materials, direct labor, overhead, and other related incidental expenses that are directly attributable to the principal operations of the PRC subsidiaries. Cost of revenue decreased by $1,345,222, or approximately 26.3%, to $3,766,116 for the six months ended March 31, 2024 from $5,111,338 for the six months ended March 31, 2023. The decrease in the cost of revenue was smaller than the decrease in net revenue, mainly because the unit cost of medical devices other than masks was relatively higher than the unit cost of masks; therefore, the increased sales of medical devices other than masks partially offset the decreased of cost of revenue related to masks.
72
Table of Contents
Gross profit
Gross profit decreased by $2,357,087, or approximately 60.4%, to $1,542,979 for the six months ended March 31, 2024 from $3,900,066 for the six months ended March 31, 2023. Gross profit margin decreased to 29.1% for the six months ended March 31, 2024, as compared to 43.3% for the six months ended March 31, 2023. The decrease of gross profit margin was primarily due to the increased sales of medical devices other than masks with relative lower profit margin and the decreased sales of masks with relative higher profit margin for the six months ended March 31, 2024.
Selling expenses
Our selling expenses primarily consist of salaries, welfare expenses, marketing and promotion expenses, and freight expenses. Our selling and marketing expenses increased by $475,070, or approximately 76.7%, to $1,094,833 for the six months ended March 31, 2024 from $619,763 for the six months ended March 31, 2023, which was primarily due to more marketing and promotion expenses incurred for sales of medical devices other than masks.
General and administrative expenses
Our general and administrative expenses primarily consist of salaries and welfare expenses, allowance for doubtful accounts, depreciation and amortization, and professional fees. Our general and administrative expenses decreased by $339,908, or approximately 30.4%, to $777,056 for the six months ended March 31, 2024 from $1,116,964 for the six months ended March 31, 2023, primarily due to a decrease of allowance for doubtful accounts, which were mainly attributable to the Group’s improved collection of receivables for the six months ended March 31, 2024 compared to the six months ended March 31, 2023.
Research and development expenses
Our research and development expenses were incurred for the development of medical devices and technologies used for the manufacturing of medical devices. Our research and development expenses decreased by $193,493, or approximately 52.0%, to $178,783 for the six months ended March 31, 2024 from $372,276 for the six months ended March 31, 2023, which was primarily due to the cut down of related expenditures.
Other income (loss)
The total net other income was $762,597 for the six months ended March 31, 2024, and the net other expenses was $137,027 for the six months ended March 31, 2023.
The net other income for the six months ended March 31, 2024 primarily consisted of (i) $669,837 from the reassessment of a previously accrued disputed payable, now recognized as non-operating income due to recent resolution of the dispute; (ii) subsidy income of $345,245; and (iii) interest expenses of $252,485.
The net other expenses for the six months ended March 31, 2023 primarily consisted of interest expenses of $139,481.
Income tax expense
The income tax expense was $20,050 and $220,671 for the six months ended March 31, 2024 and 2023, respectively. The change was related to taxable income, primarily due to the decrease of profit before income tax.
Net income
As a result of the foregoing, our net income was $234,854 and $1,433,365 for the six months ended March 31, 2024 and 2023, respectively.
73
Table of Contents
Years Ended September 30, 2023 and 2022
Net revenue
The following table disaggregates our revenue by product categories for the years ended September 30, 2023 and 2022, respectively.
| | For the Year Ended
September 30, |
| | | 2023 | | 2022 |
Masks | | $ | 5,091,331 | | $ | 10,619,035 |
Medical devices other than masks | | | 7,997,540 | | | 8,747,886 |
Commodity trading | | | 325,429 | | | 106,643 |
Others | | | 151,651 | | | 237,726 |
Net revenue | | $ | 13,565,951 | | $ | 19,711,290 |
Sales decreased by $6,145,339, or 31.2%, to $13,565,951 for the year ended September 30, 2023 from $19,711,290 for the year ended September 30, 2022. The decrease in sales was primarily attributable to the decline in demand for medical devices other than masks and was offset by the new business of commodity trading.
Sales of masks decreased by $5,527,704, or 52.1%, to 5,091,331 for the year ended September 30, 2023 from $10,619,035 for the year ended September 30, 2022. The decrease was mainly because (a) the demand for masks decreased following China’s lifting of travel restrictions and quarantine requirements in December 2022, which lead to an 87.2% reduction in the sales volume of masks for the year ended September 30, 2023; and was partially offset by (b) the average unit sales price of masks for the year ended September 30, 2023 increased 28.3% compared with last year, which was attributable to the fact that the Company sold more high priced masks, such as the KN95 masks and customized patterned masks, other than regular medical face masks.
Medical devices other than masks were mainly anesthesia and respiratory related consumables. The sales of medical devices decreased by $750,346, or 8.6%, to $7,997,540 for the year ended September 30, 2023 from $8,747,886 for the year ended September 30, 2022. The decrease was mainly due to a slight decrease in market demand.
Commodity trading mainly involves trading in raw materials of masks such as melt-blown nonwovens. The increase was mainly due to the increased of market demand and our business expansion.
Cost of revenue
Cost of revenue primarily included cost of materials, direct labor, overhead, other related incidental expenses that are directly attributable to the principal operations, and inventory write-down. Cost of revenue decreased by $5,869,531, or approximately 38.4%, to $9,422,967 for the year ended September 30, 2023 from $15,292,498 for the year ended September 30, 2022. The decrease was mainly attributable to (a) the decreased sales for the year ended September 30, 2023; and (b) the sufficient supply of raw materials for mask production in the market, resulting in a low unit price of raw materials.
Gross profit
Gross profit decreased by $275,808, or approximately 6.2%, to $4,142,984 for the year ended September 30, 2023 from $4,418,792 for the year ended September 30, 2022. Gross profit margin increased to 30.5% for the fiscal year ended September 30, 2023, as compared to 22.4% for the fiscal year ended September 30, 2022. The increase of gross profit margin was primarily attributable to (a) relatively lower unit cost of masks, caused by an overall downward trend of deprecation cost by using a double declining balance method for deprecation of mask production machines; and (b) the increase of commodity trading with relative high profit margin for the fiscal year ended September 30, 2023.
Selling expenses
The selling expenses primarily consisted of salaries, welfare expenses, marketing and promotion expenses, and freight expenses. The selling and marketing expenses increased by $534,701, or approximately 51.8%, to $1,567,386 for the year ended September 30, 2023 from $1,032,685 for the year ended September 30, 2022, which was primarily due to the fact that the Company paid more bonuses to sales personnel at the end of December 2023 for their constant hard work during the pandemic.
74
Table of Contents
General and administrative expenses
The general and administrative expenses primarily consisted of salaries and welfare expenses, allowance for doubtful accounts, depreciation and amortization, and professional fees. The general and administrative expenses decreased by $702,151, or approximately 27.4%, to $1,861,728 for the year ended September 30, 2023 from $2,563,879 for the year ended September 30, 2022, which was primarily due to the fact that our PRC subsidiaries could not collect timely payments from some of their customers for the year ended September 30, 2022, and a significant amount of bad debt provision was made, but with the recovering economy since late 2022 and management’s increased efforts in payment collections, we believe that the liquidity risk from our customers has been alleviated, and a reduced bad debt provision was made for the year ended September 30, 2023. The decrease in general and administrative expenses was offset by the increase in salaries and welfare expenses.
Research and development expenses
The research and development expenses were incurred for the development of medical devices and technologies used for the manufacturing of medical devices. The research and development expenses increased by $117,744, or approximately 64.0%, to $301,644 for the year ended September 30, 2023 from $183,900 for the year ended September 30, 2022. The increase was primarily because we enhanced our collaboration with medical institutions and invested additional capital in R&D on technology and new product.
Other income
The total net other expense was $301,838 for the year ended September 30, 2023 and the total net other income was $475,233 for the year ended September 30, 2022.
The net other expenses for the year ended September 30, 2023 primarily consisted of interest expenses of $367,323.
The net other income for the year ended September 30, 2022 primarily consisted of received government subsidies of $1,186,641, which were partially offset by interest expense of $350,999 and loss on disposal of equipment of $360,409 due to equipment upgrading.
Income tax expense
Our PRC subsidiaries incurred income tax expenses related to taxable income of $47,006 and $169,435 for the years ended September 30, 2023 and 2022, respectively, primarily due to the decrease of profit before income tax.
Net income
As a result of the foregoing, the net income was $63,383 and $944,126 for the years ended September 30, 2023 and 2022, respectively.
Liquidity and Capital Resources
To date, the primary sources of liquidity consist of cash flows from the PRC subsidiaries’ operating activities, capital contributions from shareholders and loans from banks. The Group has financed its operations primarily through capital contributions and revenue from operations. As of March 31, 2024, we had cash, cash equivalents, and restricted cash of $1,080,639 and a total working capital deficit of $1,709,959. As of September 30, 2023, we had cash, cash equivalents and restricted cash of $1,637,283 and a total working capital surplus of $3,229,822. For the next 12 months from the issuance date of this prospectus, we plan to implement measures to meet the cash requirements, including: 1) strictly controlling and reducing expenses; 2) seeking additional credit facilities; and 3) seeking equity financing from shareholders. We believe the above measures will be adequate to meet the operation requirements in the next 12 months.
As of March 31, 2024, the gross balance of accounts receivable was $2,185,406, from which $937,766 was recorded as allowance for doubtful accounts. As of July 3, 2024, approximately $0.95 million of accounts receivable outstanding as of March 31, 2024 have been subsequently collected in cash. The Company believes the remaining outstanding balance, aside from the amount recorded as bad debt, can be collected before December 31, 2024.
75
Table of Contents
If we experience an adverse operating environment or incur unanticipated capital expenditure requirements, or if we accelerate our growth, then additional financing may be required. No assurance can be given, however, that additional financing, if required, would be available on favorable terms or at all. Such financing may include the use of additional debt or the sale of additional equity securities. Any financing which involves the sale of equity securities or instruments that are convertible into equity securities could result in immediate and possibly significant dilutions to the existing shareholders.
All of our operations are conducted in China and a majority portion of our revenue, expense, and cash are denominated in Renminbi (RMB). Current foreign exchange and other regulations in the PRC may restrict the PRC entities in their ability to transfer their net assets to us and our subsidiaries. However, we have no present plans to declare any dividends and we plan to retain our retained earnings to continue to grow our business. In addition, these restrictions have no impact on our ability to meet our cash obligations, as all of our current cash obligations are due within the PRC as of the date of this prospectus.
We have limited financial obligations denominated in U.S. dollars, thus, the foreign currency restrictions and regulations in the PRC on dividend distribution will not have a material impact on the liquidity, financial condition and results of operations of the Company.
Summary of Cash Flows
Six Months Ended March 31, 2024 and 2023
The following table summarizes our cash flows for the periods indicated:
| | For the six months ended
March 31, |
| | | 2024 | | 2023 |
Net cash (used in) provided by operating activities | | $ | (1,264,684 | ) | | $ | 719,455 | |
Net cash used in investing activities | | | (5,856,936 | ) | | | (522,487 | ) |
Net cash (used in) provided by financing activities | | | 6,556,704 | | | | (87,108 | ) |
Effect of exchange rate changes | | | 8,272 | | | | (44,829 | ) |
Net increase in cash, cash equivalents, and restricted cash | | $ | (556,644 | ) | | $ | 65,031 | |
Cash, cash equivalents and restricted cash, beginning of period | | | 1,637,283 | | | | 773,422 | |
Cash, cash equivalents and restricted cash, end of period | | $ | 1,080,639 | | | $ | 838,453 | |
Operating Activities
Net cash used in operating activities was $1,264,684 for the six months ended March 31, 2024, primarily derived from (a) a net income of $234,854, adjusted by depreciation and amortization of $751,585, movements of accounts payable not impacting cash flows of $688,797, written-off of inventory impairment of $154,188, reversal of allowance for doubtful accounts of $38,770, and deferred tax benefit of $27,784; (b) an increase in advances to suppliers of $3,882,872; and (c) a decrease in accounts payable of $2,051,296, and offset by (i) a decrease in accounts receivable of $2,208,310, mainly in line with the decrease of revenue; and (ii) a decrease in amounts due from related parties of $1,687,141, mainly due to the Company’s enhanced collection efforts, resulting in a large amount of repayments from related parties.
Net cash provided by operating activities was $719,455 for the six months ended March 31, 2023, primarily derived from (a) net income of $1,433,365, adjusted by depreciation and amortization of $827,806, allowance for doubtful accounts of 285,319, and deferred tax benefit of $46,486; (b) a decrease of advances to suppliers of $2,918,001, mainly due to the Group’s request for suppliers to expedite the delivery of raw materials in response to increasing production capacity; (c) an increase in accounts payable of $901,261, due to the increase in the purchase of raw materials in response to market demand; (d) an increase in deferred revenue of $516,086, as a result of our customers preparing for an increase in future purchases; and (e) a decrease in prepaid expenses and other current assets of $537,030, which was primarily due to a decrease of interest-free loans to third parties, and offset by (i) an increase of inventories of $3,332,434, mainly due to the increase in sales for the six months ended March 31, 2023, and preparation for the increase in future sales; (ii) an increase of amounts due from related parties of $1,555,467, mainly
76
Table of Contents
due to the advance to a related party for the initial public offering costs; (iii) an increase in accounts receivable of $912,324, which was mainly due to the increase of sales for six months ended March 31, 2023; and (iv) a decrease of income tax payable of $329,201.
Investing Activities
For the six months ended March 31, 2024, net cash used in investing activities was $5,856,936, which was derived from prepayment to a third-party for a building of $5,828,153 and purchases of fixed assets of $28,783.
For the six months ended March 31, 2023, net cash used in investing activities was $522,487, which was primarily derived from purchases of fixed assets of $538,628 and purchases of intangible assets of $137,369, and was offset by proceeds from disposal of properties and equipment of $153,510.
Financing Activities
For the six months ended March 31, 2024, net cash provided by financing activities was $6,556,704, which mainly consisted of (a) proceeds from short-term bank borrowings of $6,244,449, primarily from (i) a working capital loan agreement between Hangzhou Woli and China CITIC Bank dated December 20, 2023, which loan was in the principal amount of RMB42 million, with an annual interest rate of 4.20%, was guaranteed by the Group’s CEO, Shuang Wu, and secured by the CEO’s real properties, with a one-year term of loan from December 20, 2023, to December 19, 2024, and which loan is expected to be fully repaid by December 19, 2024; and (ii) proceeds from a working capital loan agreement between Hangzhou Shanyou and Bank of Jiangsu dated March 4, 2024, in the principal amount of RMB3 million, with an interest rate determined by the one-year Loan Prime Rate (“LPR”), set the day immediately before the loan agreement date, plus 60 basis points, with a term commencing March 4, 2024, to September 3, 2024, and which loan is expected to be fully repaid by September 3, 2024; and (b) the collection of interest-free loan payments from Liwei Zhang, Baiming Yu, Hangzhou Qingniu Medical Instrument Co., Ltd. (“Hangzhou Qingniu”), and Shuang Wu, who are all related parties, in the aggregate amount of $5,773,020, and from third parties of $2,686,425, which were offset by (i) loans made to Hangzhou Qingniu and Liwei Zhang in the aggregate amount of $2,985,963 and to certain third parties in the aggregate principal amount of $2,636,545; (ii) payment of initial public offering costs totaling $1,256,961; and (iii) repayment of short-term bank borrowings in the aggregate amount of $1,110,124, primarily consisted of (x) repayment of $693,828 under a working capital loan agreement between Hangzhou Shanyou and Zhejiang Xiaoshan Rural Commercial Bank dated September 7, 2022, which loan was in the principal amount of RMB19.5 million, with an interest rate determined by the one-year LPR, set the day immediately before the loan agreement date, plus 105 basis points, with a term commencing from September 7, 2022, to August 29, 2024, and which loan is expected to be fully repaid by August 29, 2024; and (y) repayment of $416,296 under the working capital loan agreement between Hangzhou Shanyou and Bank of Jiangsu dated March 4, 2024.
For the six months ended March 31, 2023, net cash used in financing activities was $87,108, which mainly consisted of (a) loans made to Hangzhou Shuige Technology Co., Ltd. (“Hangzhou Shuige”), Liwei Zhang, and Hangzhou Qingniu, who are all related parties, in the aggregate principal amount of $2,961,141; and (b) interests-free loans made to third parties in the aggregate principal amount of $392,800, which were offset by (i) the collection of loans from Hangzhou Shuige, Liwei Zhang, and Hangzhou Qingniu in the aggregate amount of $2,616,075; and (ii) proceeds from short-term bank borrowings in the aggregate principal amount of $650,758, primarily from a working capital loan agreement between Hangzhou Shanyou and Bank of Jiangsu dated March 31, 2023, the original loan principal of which was RMB3 million, with an interest rate determined by the one-year LPR, set the day immediately before the loan agreement date, plus 40 basis points, and which loan had a one-year term from March 31, 2023, to March 30, 2024, and has been fully repaid on March 4, 2024.
77
Table of Contents
Years Ended September 30, 2023 and 2022
The following table summarizes our cash flows for the periods indicated:
| | For the Years Ended
September 30, |
| | | 2023 | | 2022 |
Net cash provided by (used in) operating activities | | $ | 2,209,736 | | | $ | (2,258,948 | ) |
Net cash used in investing activities | | | (583,304 | ) | | | (1,347,165 | ) |
Net cash (used in) provided by financing activities | | | (728,719 | ) | | | 3,566,223 | |
Effect of exchange rate changes | | | (33,852 | ) | | | (59,819 | ) |
Net increase (decrease) in cash, cash equivalents, and restricted cash | | $ | 863,861 | | | $ | (99,709 | ) |
Cash, cash equivalents and restricted cash, beginning of period | | | 773,422 | | | | 873,131 | |
Cash, cash equivalents and restricted cash, end of period | | $ | 1,637,283 | | | $ | 773,422 | |
Operating Activities
Net cash provided by operating activities was $2,209,736 for the year ended September 30, 2023, primarily derived from (a) a net income of $63,383, adjusted by depreciation and amortization of $1,603,129, and addition of allowance for doubtful accounts of $149,503; (b) a decrease of advances to suppliers of $977,318, mainly due to the Group’s request that suppliers speed up delivery of raw materials in response to increasing production capacity; (c) an increase in accounts payable of $864,585, due to the increase in the purchase of raw materials in response to market demand; (d) a decrease in prepaid expenses and other current assets of $1,291,145, which was primarily due to a decrease in interest-free loans to third parties; and (e) an increase in accrued expenses and other liabilities of $1,688,370, due to increase in VAT tax payable and accrued payroll; and offset by (i) an increase in amounts due from related parties of $3,964,077, mainly due to the advance to a related party for the initial public offering costs and receivable of selling to related parties; (ii) an increase of inventories of $400,544, mainly due to the preparation for the increase in future sales; and (iii) a decrease of income tax payable of $136,508.
Net cash used in operating activities was $2,258,948 for the year ended September 30, 2022, primarily derived from (a) a decrease in accounts payable of $5,822,547, due to an increase of settlements for the year ended September 30, 2022; (b) an increase in accounts receivable of $4,234,997, due to the liquidation difficulties of our customers under the impact of pandemic; and (c) a decrease in deferred revenue of $751,375, due to a decrease in market demand for masks; which was offset by (a) a net income of $944,126, adjusted by depreciation and amortization of $3,258,267; (b) an increase in accrued expenses and other current liabilities of $917,303, which was mainly due to a decrease in loans to third-parties; and (c) a decrease in inventories of $2,053,675, which was primarily due to the decrease in sales for the year ended September 30, 2022.
Investing Activities
For the year ended September 30, 2023, net cash used in investing activities was $583,304, which was primarily derived from purchases of fixed assets of $455,391 and purchases of intangible assets of $127,913.
For the year ended September 30, 2022, net cash used in investing activities was $1,347,165, which primarily consisted of purchases of fixed assets of $2,012,546, offset by (a) disposal of equipment of $601,760 due to equipment upgrading; and (b) cash acquired from Shanghai Saitumofei of $63,621.
Financing Activities
For the year ended September 30, 2023, net cash used in financing activities was $728,719, which mainly consisted of (a) loans to related parties of $5,669,834; (b) repayment of short-term bank borrowings of $6,000,791; (c) loans to third parties of $5,669,834; and (e) payment of offering cost of $1,356,176; offset by (i) the collection of loans from related parties of $5,891,373; (ii) proceeds from short-term bank borrowings of $9,107,082; and (iii) repayment of loans to third parties of $2,682,706.
For the year ended September 30, 2022, net cash provided by financing activities was $3,566,223, primarily consisted of (a) proceeds from short-term bank borrowings of $7,248,367; (b) the collection of loans from related parties of $13,260,930; and (c) the collection of loans from third parties of $829,042, offset by (a) loans to related parties of $9,493,121; (b) repayment of short-term bank borrowings of $7,248,367; and (c) loans to third parties of $1,030,628.
78
Table of Contents
Capital Expenditures
Our capital expenditures are primarily incurred for the purpose of purchasing face mask production equipment. Our capital expenditures were $5,856,936 and $522,487 for the six months ended March 31, 2024 and 2023, respectively. Our capital expenditures were $583,304 and $2,012,546 for the years ended September 30, 2023 and 2022, respectively. We intend to fund our future capital expenditures with our existing cash balance and proceeds from this offering and expect to continue to make well-planned capital expenditures to meet the expected growth of our business.
Off-Balance Sheet Arrangements
We did not have during the periods presented, and we do not currently have, any off-balance sheet financing arrangements or any relationships with unconsolidated entities or financial partnerships, including entities sometimes referred to as structured finance or special purpose entities, that were established for the purpose of facilitating off-balance sheet arrangements or other contractually narrow or limited purposes.
Holding Company Structure
Our company, WORK Medical Technology Group LTD, is a holding company with no material operations of its own. We conduct our operations through the PRC subsidiaries in China. As a result, our ability to pay dividends depends upon dividends paid by the PRC subsidiaries. If our existing PRC subsidiaries or any newly formed ones incur debt on their own behalf in the future, the instruments governing their debt may restrict their ability to pay dividends to us. In addition, our subsidiaries in mainland China are permitted to pay dividends to us only out of its retained earnings, if any, as determined in accordance with PRC accounting standards and regulations. Under PRC law, each of our subsidiaries in mainland China are required to set aside at least 10% of its after-tax profits each year, if any, to fund certain statutory reserve funds until such reserve funds reach 50% of their registered capital. In addition, our subsidiaries in mainland China may allocate a portion of their after-tax profits based on PRC accounting standards to enterprise expansion funds and staff bonus and welfare funds at their discretion. The statutory reserve funds and the discretionary funds are not distributable as cash dividends. Remittance of dividends by a wholly foreign-owned company out of mainland China is subject to examination by the banks designated by SAFE. The PRC subsidiaries distributed dividends in the fiscal year ended September 30, 2021, and have made no further plans to pay dividends since such date and do not expect to do so unless and until they have generated sufficient accumulated profits and have met the requirements for statutory reserve funds.
Contractual Obligations
Commitments and Contingencies
From time to time, the PRC subsidiaries may be subject to certain legal proceedings, claims and disputes that arise in the ordinary course of business. Although the outcomes of these legal proceedings cannot be predicted, we do not believe these actions, in the aggregate, will have a material adverse impact on the PRC subsidiaries’ financial position, results of operations or liquidity.
Operating Lease
The Group did not have any operating lease contractual obligations, significant capital and other commitments, long-term obligations, or guarantees as of March 31, 2024 and September 30, 2023.
Seasonality
The nature of our business does not appear to be affected by seasonal variations.
Critical Accounting Policies and, Judgments and Estimates
We prepare our consolidated financial statements in accordance with U.S. GAAP. The preparation of financial statements in conformity with U.S. GAAP requires management to make judgments, estimates and assumptions that affect the reported amounts of assets and liabilities at the date of the financial statements and reported amounts of revenue and expenses during the reporting period. We continually evaluate these judgments and estimates based on our own experience, knowledge and assessment of current business and other conditions, and our expectations regarding
79
Table of Contents
the future based on available information and assumptions that we believe to be reasonable. Since the use of estimates is an integral component of the financial reporting process, our actual results could differ from those estimates. Some of our accounting policies require a higher degree of judgment than others in their application.
When reading our consolidated financial statements, you should consider our selection of critical accounting policies, the judgment and other uncertainties affecting the application of such policies and the sensitivity of reported results to changes in conditions and assumptions. Our critical accounting policies and practices include the following: (i) allowance for receivable; (ii) useful lives of property, plant and equipment and intangible assets; and (iii) inventories write-downs and the recoverability of long-lived assets. See “Note 2 — Summary of Significant Accounting Policies” to our consolidated financial statements for the disclosure of these accounting policies. We believe the following accounting estimates involve the most significant judgments used in the preparation of our financial statements.
Income taxes
Deferred tax assets and liabilities are measured using enacted tax rates expected to be applied to taxable income in the years when those temporary differences are expected to be recovered or settled. Deferred tax assets are reduced by a valuation allowance when, based upon the weight of available evidence, it is more likely than not that some portion or all of the deferred tax assets will not be realized. Current income taxes are provided for in accordance with the laws of the relevant taxing authorities. The amounts of allowance over deferred tax assets were $4,937 and $59,879 as of March 31, 2024 and September 30, 2023, respectively.
Quantitative and Qualitative Disclosures about Market Risks
The Group is also exposed to liquidity risk, which is the risk that the Group is unable to provide sufficient capital resources and liquidity to meet its commitments and business needs. Liquidity risk is controlled by the application of financial position analysis and monitoring procedures. When necessary, the Group will turn to other financial institutions and the shareholders to obtain short-term funding to address the liquidity shortage.
Inflation risk
To date, inflation in China has not materially impacted the Group’s results of operations. According to the National Bureau of Statistics of China, the year-over-year percentage changes in the consumer price index for 2021, 2022 and 2023 were increases of 0.9%, 2.0% and 0.2%, respectively. Although the Group has not been materially affected by inflation in the past, we can provide no assurance that the Group will not be affected in the future by higher rates of inflation in the PRC. For example, certain operating costs and expenses, such as employee compensation and office operating expenses, may increase as a result of higher inflation. Additionally, because a substantial portion of our assets consists of cash and cash equivalents, high inflation could significantly reduce the value and purchasing power of these assets. The Group is not able to hedge its exposure to higher inflation in China.
Interest rate risk
The Group’s exposure to interest rate risk primarily relates to the interest rate that its deposited cash can earn. Interest-earning instruments carry a degree of interest rate risk. The Group has not been exposed to material risks due to changes in interest rates. An increase, however, may raise the cost of any debt the Group may incur in the future.
Foreign currency translation and transaction
A substantial majority of our operating activities are denominated in RMB and all of our assets and liabilities are denominated in RMB, which is not freely convertible into foreign currencies. All foreign exchange transactions take place either through the PBOC or other authorized financial institutions at exchange rates quoted by PBOC. Approval of foreign currency payments by the PBOC or other regulatory institutions requires submitting a payment application form together with suppliers’ invoices and signed contracts. The value of RMB is subject to changes in central government policies and to international economic and political development affecting supply and demand in the China Foreign Exchange Trading System market.
80
Table of Contents
BUSINESS
Our Mission
Our mission is to become a world-leading manufacturer of medical devices, and we are committed to providing professional and reliable medical devices to the world.
Overview
In furtherance of our commitment to providing professional and reliable medical devices, both domestically and internationally, the PRC subsidiaries specialize in the research, development, manufacturing, and sales of medical devices as follow:
Hangzhou Shanyou
Among the PRC subsidiaries, only Hangzhou Shanyou is responsible for production, and only Hangzhou Shanyou has production lines. Hangzhou Shanyou mainly manufactures and sells Class I and II medical devices, including endotracheal tubes, laryngeal mask airways, heat and moisture exchanging filters (HMEF), disposable breathing circuits, nebulizer kits, and yankauer suction sets, etc. In addition to Class I and II medical devices, Hangzhou Shanyou focuses on manufacturing and sales of hygiene products and disposable medical supplies as well, including a filtering half mask and a non-powered air-purifying particle respirator.
Additionally, Hangzhou Shanyou has retained the certificate for selling Class III disposable medical devices. However, as of the date of this prospectus, Hangzhou Shanyou has no sale activities for Class III disposable medical devices.
Hangzhou Hanshi
Hangzhou Hanshi mainly focuses on the sales of Class II medical devices.
Shanghai Chuqiang
Shanghai Chuqiang focuses on the sales of Class II medical devices.
Shanghai Saitumofei
Shanghai Saitumofei focuses on the research and development of Class II and III medical devices, and biological and medical technology.
Hunan Saitumofei
Hunan Saitumofei focuses on the research and development of Class I, II and III medical devices.
Hangzhou Woli
Hangzhou Woli focuses on the sales of chemical products.
Competitive Strengths
We believe that the following strengths contribute to our success and are the differentiating factors that set us apart from our peers:
• A Deep Understanding of the Industry. Our COO, Baiming Yu, worked in a hospital as a doctor for nearly a decade, and he has been in the medical device industry for over two decades after he ended his medical practice in hospital. The three-decade experience furnish him with wide connections with people working in this industry, especially in Zhejiang Province, and a deep understanding of their specific needs for medical devices when providing medical services. Our subsidiary, which was founded by Mr. Yu in 2002, Hangzhou Shanyou has been manufacturing medical devices for over 20 years. Given the long-time operating history and interaction with the PRC subsidiaries’ clients, including hospitals, pharmacies and other medical institutions, the PRC subsidiaries have a deep understanding of the medical device industry, which enables them to develop and manufacture suitable and accommodative products tailored to the market. Meanwhile, the PRC subsidiaries’ R&D department has recruited a number of experienced
81
Table of Contents
medical professionals with extensive research experience, knowledge of the latest market and clinical needs of medical devices and the ability to develop suitable products to serve the demands efficiently. For more information about the R&D professionals, please refer to “— Research and Development (R&D).”
• Cost-effective Masks. Hangzhou Shanyou has recently upgraded its production equipment and it is able to manufacture more cost-effective products as a result. After transforming the production lines into fully automated ones, and automatic headband mask machines are put into use, the mask production of each production line has increased from 5,000 per day to 20,000 per day, with consistent product quality, and has decreased the production cost. Due to the boost of production efficiency, Hangzhou Shanyou expanded its production scale and increased the profit margin.
• Customized and Multifunctional Masks. Masks have been a necessity since the outbreak of COVID-19, and the market expects more from them than their basic function. Hence, customized and multifunctional masks have become increasingly popular over the past couple of years. In Contrast with the competitors that primarily produce masks with the basic function of reducing virus spreading, since the second half of 2021, Hangzhou Shanyou has upgraded its equipment, developing new production lines to produce customized masks with new features, which also can be used in various scenarios. Examples are tight fit masks and foldable masks. For those who work in industries which have stricter hygienic requirements, the PRC subsidiaries provide tight fit masks that reduce the gap between users’ faces and the masks, designed to decrease the possibilities of virus spreading. The PRC subsidiaries also provide foldable masks which reduce condensation on users’ glasses.
• Wide Distribution Network. The PRC subsidiaries maintain a wide distribution network which allows them to sell their products both domestically and internationally. For the six months ended March 31, 2024, they have approximately 849 domestic distributors and 22 exporting distributors. With the help of the domestic distributors, the PRC subsidiaries are able to sell their products in 34 provincial level administrative regions of China. The exporting distributors span across six continents through which the PRC subsidiaries can sell their products in over 30 countries, including Australia, Brazil, France, Germany, India, Italy, Japan, Netherlands, Saudi Arabia, South Africa, South Korea, Spain, Switzerland, U.K. and U.S. For more information about the distribution network, please refer to “— Distribution Network.”
Our Growth Strategies
We intend to develop our business and strengthen brand loyalty by implementing the following strategies:
• Continue to invest in the PRC subsidiaries’ research and department team. We believe that our success to date has in part resulted from the strong research and development capabilities. The R&D team consists of experienced medical professional background with extensive research experience, which allows the PRC subsidiaries to introduce new and more advanced products at competitive prices. We believe the R&D team has strong professional capabilities and medical expertise. Please refer to “— Research and Development (R&D).” We plan to deepen the PRC subsidiaries’ collaboration with medical institutions and invest additional capital in R&D on technology and new products to add new items to the PRC subsidiaries’ product portfolio.
• Expand the PRC subsidiaries’ sales and distribution network. We intend to expand the PRC subsidiaries’ sales and distribution network to cover new geographic markets, especially those of emerging countries, and cultivate new market demands, to gain bigger market shares in medical consumables and medical equipment markets and access a broader range of customers. We expect to continue to expand the PRC subsidiaries’ sales and distribution channels. In addition, the PRC subsidiaries actively attend expos, professional forums and seminars to keep informed about market trends, which affords them an opportunity to interact with customers and distributors to understand their needs and accommodate their orders.
• Strengthen the PRC subsidiaries’ quality control system and uphold the commitment to product quality. We intend to uphold the commitment to product quality items to ensure consistently high standards throughout the operations. To reach this goal, we plan to continue to maintain and enhance the PRC subsidiaries’ quality control systems across the entirety of the operations by replacing machines having a low degree of accuracy, organizing monthly training for employees in quality control departments, replacing disqualified suppliers, keeping track of developments of international, national, and local product quality standards, laws, and regulations, and increasing the frequency of quality inspection.
82
Table of Contents
Our Revenue Model
We generate revenue through the PRC subsidiaries’ manufacturing and sales of medical devices under their own brands. For the six months ended March 31, 2024 and the fiscal years ended September 30, 2023 and 2022, we recognized $5,309,095, $13,565,951, and $19,711,290 in revenue, respectively.
The PRC subsidiaries sell medical devices, both domestically and internationally. For the six months ended March 31, 2024 and the fiscal years ended September 30, 2023 and 2022, the revenue of domestic sales was $4,630,186, $12,598,261, and $18,291,527, accounting for 87%, 93%, and 93%, respectively, of our revenue, and the revenue of international sales was $678,909, $967,690, and $1,419,763, accounting for 13%, 7%, and 7%, respectively, of our revenue.
Products
In the Chinese market, the PRC subsidiaries’ products are sold in 34 provincial-level administrative regions. Internationally, the products are exported to more than 30 countries in Asia, Africa, Europe, North America, South America, and Oceania.
The PRC subsidiaries current product portfolio sold internationally includes 1) Class I disposable medical devices, such as, medical face masks, artery compression tourniquets, endotracheal tube holders, intubating stylets, guedel airways, etc.; 2) Class II disposable medical devices, such as, disposable breathing circuits, laryngeal mask airways, endotracheal tubes, anesthetic kit, oxygen face masks, HMEF, anesthesia masks, laryngoscope blades, yankauer suction sets, and nasal oxygen cannulas; 3) other medical devices, such as KN95 masks and filtering half mask; and 4) medical innovative devices and equipment, such as visualized prostatic dilatation catheter.
As of the date of this prospectus, the PRC subsidiaries have a total of 21 medical devices in their product portfolio. All of them are sold domestically and 15 of them are sold internationally.
The 21 products for the six months ended March 31, 2024 and the fiscal years ended September 30, 2023 and 2022 were as follows:
Class
Category | | Product Name | | Image | | Own
Brand/OEM/
Resale | | Use | | % of
Sales for the
six months
ended
March 31,
2024 | | % of
Sales in
the fiscal
year ended
September 30,
2023 | | % of
Sales in
the fiscal
year ended
September 30,
2022 |
Class I | | Medical Face Masks | | 
| | Own Brand/OEM | | Protection, commonly used by healthcare staff. | | 4.67% | | 16.9% | | 36.45% |
Class I | | FFP2 Masks | | 
| | Own Brand | | Protection, used by the general public. | | 0.27% | | 3.76% | | 4.19% |
Class I | | Artery Compression Tourniquets | | 
| | Own Brand | | Stop bleeding. | | 32.53% | | 20.27% | | 16.03% |
Class II | | Disposable Breathing Circuits | | 
| | Own Brand/OEM | | Conduct oxygen/anesthetic gases from machine to patient. | | 20.07% | | 12.9% | | 9.77% |
83
Table of Contents
Class
Category | | Product Name | | Image | | Own
Brand/OEM/
Resale | | Use | | % of
Sales for the
six months
ended
March 31,
2024 | | % of
Sales in
the fiscal
year ended
September 30,
2023 | | % of
Sales in
the fiscal
year ended
September 30,
2022 |
Class II | | Laryngeal Mask Airways | | 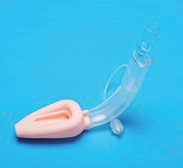
| | Own Brand/OEM | | Used as a temporary method to maintain an open airway. | | 7.78% | | 6.18% | | 5.32% |
Class II | | Endotracheal Tubes | | 
| | Own Brand/OEM | | Maintain air passage during intubation. | | 12.69% | | 7.41% | | 5.70% |
Class I | | Endotracheal Tube Holders | | 
| | Own Brand/OEM | | Fix an endotracheal tube and protect teeth. | | 5.48% | | 4.01% | | 2.85% |
Class II | | Anesthetic Kit | | 
| | Own Brand | | Used during general anesthesia. | | 4.06% | | 2.7% | | 2.16% |
Class II | | Face Masks | | 
| | Own Brand/OEM | | Transfer breathing oxygen gas from a storage tank to the patients’ lungs. | | 0.76% | | 1.32% | | 0.88% |
Class II | | Filters/HMEF | | 
| | Own Brand | | Filter viruses and bacteria in the breathing circuits. | | 1.62% | | 1.26% | | 0.84% |
N/A(1) | | KN95 Masks | | 
| | Own Brand | | Protection, used by the general public according to Chinese standard. | | 6.05% | | 20.8% | | 14.26% |
Class II | | Anesthesia Masks | | 
| | Own Brand/OEM | | Are used to deliver anesthetic gases of oxygen gases. | | 1.30% | | 0.6% | | 0.59% |
Class I | | Intubating Stylets | | 
| | Own Brand/OEM | | Assist to intubate an endotracheal tube. | | 0.78% | | 0.53% | | 0.27% |
84
Table of Contents
Class
Category | | Product Name | | Image | | Own
Brand/OEM/
Resale | | Use | | % of
Sales for the
six months
ended
March 31,
2024 | | % of
Sales in
the fiscal
year ended
September 30,
2023 | | % of
Sales in
the fiscal
year ended
September 30,
2022 |
Class II | | Laryngoscope Blades | | 
| | Own Brand | | Used to examine the larynx during intubation. | | 0.03% | | 0.06% | | 0.09% |
Class I | | Guedel Airways | | 
| | Own Brand/OEM | | Maintain a patient airway. | | 0.20% | | 0.13% | | 0.11% |
Class II | | Yankauer Suction Sets | | 
| | OEM | | Used for the removal of blood and liquids during surgery. | | 0.02% | | 0.08% | | 0.06% |
Class II | | Nasal Oxygen Cannulas | | 
| | OEM | | Provide patients with supplemental oxygen. | | 0.97% | | 0.26% | | 0.12% |
Class II | | SPO2 Sensor | | 
| | Own Brand | | Used for blood saturation monitoring. | | 0.11% | | 0.05% | | 0.08% |
Class II | | Nasogastric Tubes | | 
| | Own Brand | | Feed and provide certain medications to people through the nose. | | 0.06% | | — | | — |
Class II | | Personal protective equipment | | 
| | Resale | | Protection, commonly used by healthcare staff. | | 0.55% | | 0.77% | | 0.21% |
Class II | | Prostatic dilatation catheter | | 
| | Own Brand/OEM | | Therapy for symptoms caused by prostate obstruction | | — | | — | | — |
In addition to the above products, the PRC subsidiaries are also dedicated to developing new products and technologies, to better accommodate the clinical needs and thus improve medical efficiency. The following product is the example of the latest development.
85
Table of Contents
Visualized Prostatic Dilatation Catheter
Visualized prostatic dilatation catheter is a treatment for patients with benign prostatic hyperplasia. It dilates the hyperplastic prostatic tissue physically while observing the dilatation effect through endoscope. This technology is painless, operation-free, and minimally invasive, without any sequela, which can reduce the operation risk and improves the operation efficiency. The technology has following comparative advantages:
• It replaces the traditional spherical balloon with a columnar balloon which permits the prostate to be compressed more evenly. In addition, the product only dilates the hyperplastic prostate tissue, causing less damage to the urethral mucosa, thus the urethral mucosa will be repaired quickly after operation. In combination, the foregoing features may enhance therapeutic effects with limited complications;
• The product maintains the position firmly after the dilatation; therefore, it can ensure the dilating effect and stop bleeding by compression. In this way, it releases the external sphincter, and prevents urinary incontinence and massive hemorrhage as well; and
• The operation process only takes about 15 minutes. During the operation, the water balloon is compressed reducing the likelihood of hemorrhages.
Hangzhou Shanyou received the Medical Device Registration Certificate for the visualized prostatic dilatation catheter on October 11, 2023, which enables Hangzhou Shanyou to manufacture the product. However, as of the date of this prospectus, no visualized prostatic dilatation catheters have been sold, and therefore, no revenue has been generated from the sales of this product.
Licenses and Permissions
The following chart sets forth a summary of the licenses and permissions obtained by the PRC subsidiaries as of the date of this prospectus:
Company | | License/Permission | | Issuing Authority | | Validity(1) |
Work Age | | Business License | | Xiaoshan District Administration for Market Supervision | | Until April 27, 2052 |
Work Hangzhou | | Business License | | Xiaoshan District Administration for Market Supervision | | Long-term |
Hangzhou Shanyou | | Business License | | Xiaoshan District Administration for Market Supervision | | Long-term |
| | | Medical Device Production Certificate | | Zhejiang Province Medical Products Administration | | Until November 22, 2027 |
| | | Medical Device Operation Certificate | | Hangzhou City Administration for Market Supervision | | Until May 11, 2027 |
| | | Cosmetics production Certificate | | Zhejiang Province Medical Products Administration | | Until August 20, 2029 |
| | | Certificate of Class II Medical Device Operation Recordation | | Hangzhou City Administration for Market Supervision | | Long-term |
| | | Record Registration Form for Foreign Trade Business Operators | | Foreign Trade Operator Filing and Registration Authority | | Long-term |
| | | Certificate of the Customs of the People’s Republic of China on Registration of Customs Declaration Entity | | Hangzhou Customs District of PRC | | Long-term |
86
Table of Contents
Company | | License/Permission | | Issuing Authority | | Validity(1) |
| | | Certificate of Class I Medical Device Production Recordation | | Hangzhou City Administration for Market Supervision | | Long-term |
| | | Medical Device Online Sales Information Filing Form | | Hangzhou City Administration for Market Supervision | | Long-term |
| | | Internet Medicine Information Service Qualification Certificate | | Zhejiang Province Medical Products Administration | | Until February 26, 2029 |
| | | High Technology Enterprise Certificate | | Zhejiang Province Department of Science and Technology, Zhejiang Province Department of Finance, Zhejiang Province Taxation Bureau of the State Administration of Taxation | | Until December 8, 2026 |
| | | Medical Device Registration Certificate-Artery Compression Tourniquets | | Zhejiang Province Medical Products Administration | | Until July 21, 2029 |
| | | Medical Device Registration Certificate-Laryngeal Mask Airways | | Zhejiang Province Medical Products Administration | | Until December 25, 2024 |
| | | Medical Device Registration Certificate-Filters/HMEF | | Zhejiang Province Medical Products Administration | | Until April 17, 2027 |
| | | Medical Device Registration Certificate-Endotracheal Tubes | | Zhejiang Province Medical Products Administration | | Until August 8, 2029 |
| | | Medical Device Registration Certificate-General Anesthesia Tracheal Intubation Kit | | Zhejiang Province Medical Products Administration | | Until August 26, 2029 |
| | | Medical Device Registration Certificate-Nasogastric Tubes | | Zhejiang Province Medical Products Administration | | Until July 30, 2029 |
| | | Medical Device Registration Certificate-Disposable Breathing Circuits | | Zhejiang Province Medical Products Administration | | Until May 15, 2028 |
| | | Medical Device Registration Certificate-Guedel Airways | | Zhejiang Province Medical Products Administration | | Until December 14, 2027 |
| | | Medical Device Registration Certificate-Anesthesia Masks | | Zhejiang Province Medical Products Administration | | Until December 14, 2027 |
| | | Medical Device Registration Certificate-Disposable Pulse Oximetry Sensor | | Zhejiang Province Medical Products Administration | | Until June 22, 2027 |
| | | Medical Device Registration Certificate-Intubating Stylets | | Zhejiang Province Medical Products Administration | | Until June 16, 2029 |
| | | Medical Device Registration Certificate-Endotracheal Tube Holders | | Zhejiang Province Medical Products Administration | | Until December 28, 2025 |
87
Table of Contents
Company | | License/Permission | | Issuing Authority | | Validity(1) |
| | | Medical Device Registration Certificate-Laryngoscope Blades | | Zhejiang Province Medical Products Administration | | Until May 17, 2026 |
| | | Medical Device Registration Certificate-Medical Face Masks | | Zhejiang Province Medical Products Administration | | Until June 8, 2025 |
| | | Medical Device Registration Certificate-Medical Protective Mask | | Zhejiang Province Medical Products Administration | | Until January 4, 2026 |
| | | Medical Device Registration Certificate-Medical Surgical Mask | | Zhejiang Province Medical Products Administration | | Until June 8, 2025 |
| | | Medical Device Registration Certificate-Face Masks (Oxygen Mask, Non-rebreathing Mask, Nebulizer Mask) | | Zhejiang Province Medical Products Administration | | Until April 22, 2026 |
| | | Medical Device Registration Certificate-Arterial Compression Hemostasis Device | | Zhejiang Province Medical Products Administration | | Until July 9, 2029 |
| | | Medical Device Registration Certificate-Disposable Laryngoscopes Blade | | Zhejiang Province Medical Products Administration | | Until June 17, 2029 |
| | | Medical Device Registration Certificate-Disposable Prostate Expansion Catheter | | Zhejiang Province Medical Products Administration | | Until October 10, 2028 |
| | | Class I Medical Device Filing — Disposable Nasal Catheter Carbon Dioxide Detection Connector Tube Kit | | Hangzhou City Administration for Market Supervision | | Long-term |
| | | Class I Medical Device Filing — Nasal Oxygen Tube | | Hangzhou City Administration for Market Supervision | | Long-term |
Shanghai Chuqiang | | Business License | | Shanghai Jinshan District Administration for Market Supervision | | Until March 11, 2028 |
| | | Certificate of Class II Medical Device Selling Recordation | | Shanghai Jinshan District Administration for Market Supervision | | Long-term |
Hangzhou Hanshi | | Business License | | Hangzhou Xiaoshan District Administration for Market Supervision | | Long-term |
| | | Certificate of Class II Medical Device Selling Recordation | | Hangzhou City Administration for Market Supervision | | Long-term |
Shanghai Saitumofei | | Business License | | Shanghai Jiading District Administration for Market Supervision | | Until May 14, 2049 |
88
Table of Contents
Company | | License/Permission | | Issuing Authority | | Validity(1) |
Hunan Saitumofei | | Business License | | Changsha Wangcheng District Administration for Market Supervision | | Long-term |
| | | Medical Device Registration Certificate-Automatic Blood Cell Morphology Analyzer | | Hunan Province Medical Products Administration | | Until February 26, 2028 |
Hangzhou Woli | | Business License | | Xiaoshan District Administration for Market Supervision | | Long-term |
| | | Certificate of Class II Medical Device Selling Recordation | | Hangzhou City Administration for Market Supervision | | Long-term |
Hangzhou Youshunhe | | Business License | | Xiaoshan District Administration for Market Supervision | | Long-term |
Huangshan Saitumofei | | Business License | | Tunxi District Administration for Market Supervision | | Long-term |
89
Table of Contents
Manufacturing
Hangzhou Shanyou’s production lines are all located in Hangzhou, Zhejiang Province, the PRC. The PRC subsidiaries produce products and stock inventory of raw materials, components and finished goods at the facilities pursuant to the market demand, orders they receive or plan to receive, their production plan and capacity, and procurement information from their distributors. The production model is as follows:

This production process is subject to continuous review and monitoring by the quality control team to ensure that finished products are of the highest quality and meet customer requirements and ISO 13485 medical device quality management systems standard.
ISO 13485 is a widely used international standard for quality management of the medical device industry. Issued by the International Organization for Standardization (ISO), the ISO 13485 standard is an effective solution to meet the comprehensive requirements for a quality management system in the medical device industry.
In order to the maintain product safety and a high standard of product quality, the PRC subsidiaries implement a strict set of quality control policies and inspection protocols. These policies and protocols are enforced by the quality control team, senior management and officers along every step of the production to post-production process.
Certification of Production and Products
As a medical device manufacturing and sales company, the PRC subsidiaries are subject to extensive government regulation and supervision in China. Pursuant to PRC laws, the PRC subsidiaries must obtain production license or filing for Class I and II disposable medical devices, operation license or filing for Class II and III disposable medical devices, and filing or registration certificates for Class I and Class II disposable medical devices. As of the date of this prospectus, the PRC subsidiaries own all such licenses and certificates in China. Specifically, the PRC subsidiaries own a License of Production of Medical Devices, Registration Certificates for 21 Class II medical devices and the Filing of 2 Class I medical devices.
The National Medical Products Administration of the State Council is responsible for developing the classification rules for medical devices and maintaining catalogues of classified devices. Based on information on the production, distribution and use of medical devices, the National Medical Products Administration of the State Council analyzes and evaluates the risk levels of medical devices and adjusts the classification rules and classification catalogues.
“Class I” medical devices in China refer to medical devices with low risk, whose safety and effectiveness can be guaranteed through routine administration. Record-filing parties of domestic Class I medical devices shall submit record-filing materials to the drug regulatory authorities at the level of city divided into districts.
“Class II” medical devices in China refer to medical devices with medium risk, whose safety and effectiveness can be ensured by strict control, examination, and administration. Domestic Class II medical device shall be examined by the drug regulatory authorities of provinces, autonomous regions and centrally-administered municipalities which shall issue the Medical Device Registration Certificate upon approval after examination.
90
Table of Contents
“Class III” in China refer to medical devices with high risk, whose safety and effectiveness can be ensured by strict control, examination, and administration through special measures. Domestic Class III medical devices shall be examined by the National Medical Products Administration (“NMPA”), which shall issue the Medical Device Registration Certificate upon approval after examination. The registration certificate for a medical device is valid for five years and the registrant shall apply to the original registration departments for renewal at least six months prior to its expiration date.
Customers
The PRC subsidiaries have three types of customers, i) direct end-user customers, which include hospitals, pharmacies, and medical institutions, ii) domestic distributor customers that distribute the PRC subsidiaries’ products to end-user customers in China, and iii) export distributor customers that distribute the PRC subsidiaries’ products to end-user customers in Asia, Africa, Europe, North America, South America, and Oceania. The top ten countries and regions outside of mainland China where the products are sold are Saudi Arabia, Germany, Switzerland, Hong Kong, France, Poland, Netherlands, Mexico, Romania, and Russia.
As of March 31, 2024, the PRC subsidiaries had a total of 913 customers, of which 42 were direct end-user customers, 849 were domestic distributor customers, and 22 were export distributor customers. Since direct-end customers generally place smaller and less frequent orders, the PRC subsidiaries have gradually shifted their focus towards developing and maintaining relationships with distributor customers who are perceived to offer long-term and stable revenue contributions. As a result, even though the number of direct-end customers decreased from 64 for the six months ended March 31, 2023, to 42 for the six months ended March 31, 2024, there was an increase in domestic distributor customers from 800 to 849, export distributor customers from 10 to 22, and total customers from 874 to 913 over the same period.
Distributors usually purchase products from the PRC subsidiaries at a lower price and then resell the products to end customers, both domestically and internationally, at a comparatively higher price and earn the price difference. The PRC subsidiaries source their distributors through multiple channels: (i) industry exhibitions/expos, (ii) online platform, and (iii) distributors’ direct contact. For the six months ended March 31, 2024 and the fiscal years ended September 30, 2023 and 2022, the PRC subsidiaries had a total of 849, 892, and 867 domestic distributor customers, and 22, 22, and 37 are export distributor customers, respectively.
For the six months ended March 31, 2024, the PRC subsidiaries’ top three customers accounted for 5%, 5%, and 4%, respectively, of the revenue. For the fiscal year ended September 30, 2023, the PRC subsidiaries’ top three customers accounted for 9%, 8%, and 6%, respectively, of the revenue. For the fiscal year ended September 30, 2022, the top three customers accounted for 6%, 4%, and 4%, respectively, of the revenue. For the fiscal year ended September 30, 2021, the top three customers accounted for 49%, 15%, and 2%, respectively, of the revenue.
The PRC subsidiaries source their customers through multiple channels: (i) referrals from their present customers, (ii) industry exhibitions/expos such as China International Medical Equipment Fair, and (iii) provincial medical conferences.
Through the PRC subsidiaries’ distribution network, their products are sold to customers at all levels in China, and other countries and regions in Asia, Africa, Europe, North America, South America, and Oceania. The PRC subsidiaries target overseas customers mainly via exporting distributors and exhibitions. In order to market their products, gain more market share and secure more quality customers, the PRC subsidiaries frequently participate in, and promote their products at, specific medical device exhibitions/expos.
The PRC subsidiaries do not have long-term written sales agreements with their direct-end user customers. Each direct-end user customer sale is typically governed by a brief purchase-order based sales agreement. The key terms of the sales agreements with direct-end user customers (including those agreements with their top customers) include:
• the product’s name, type, quantity, and price;
• quality standard — medical device qualifications, including business license, medical device production and operation licenses, medical device registration certificates, inspection report, etc.;
• delivery time, method and payment terms;
91
Table of Contents
• breach of contract terms, including remedies, such as refunds and return of products (e.g., direct-end user customers are entitled to refunds and may return the product if the wrong product is delivered or the product does not meet agreed upon quality standards);
• shipping costs, which are typically borne by the seller; and
• dispute solutions, including bringing a lawsuit at the local court where the direct-end user customers are located, if negotiations are unsuccessful.
The PRC subsidiaries have no offices or subsidiaries in foreign countries and currently have no intention to establish any offices in foreign countries in the future. In the course of dealing with overseas customers, the PRC subsidiaries have maintained stable business relationships, however, such business relationships are not memorialized in any long-term agreements, but are rather provided for in short-form order sheets. In respect of their international sales, the risk of loss is borne by the customers upon delivery of the products.
In addition, in the course of dealing with the local distributors overseas, such distributers undertake all obligations with respect to the local rules and regulations and taxes regarding the importation of medical products.
The PRC subsidiaries face an inherent risk of liability claims or complaints from customers. When the products are found to be defective, they are required to recall the products according to usage of trade. When customers file product liability claims against the PRC subsidiaries, conflict of laws and product liability laws of the countries or regions where they are located are applicable. Jurisdiction is determined by the sales agreements, and laws of the countries or regions where the customers are located will determine the issue if the sales agreements are absent of jurisdiction selection clauses.
As of the date of this prospectus, the PRC subsidiaries are not aware of any warnings, investigations, prosecutions, disputes, claims or other proceedings in respect of medical devices they manufacture or distribute overseas, nor have they been penalized or can foresee any penalties to be made by any overseas jurisdiction with respect to medical device safety.
Please refer to “— Marketing and Sales — Distribution Network” for more details about the business and sales agreements with the PRC subsidiaries’ distributor customers.
For the six months ended March 31, 2024 and the fiscal years ended September 30, 2023 and 2022, revenue generated from domestic distributor customers, domestic direct end-user customers and overseas distributor customers amounted to (i) $4,580,267, $49,919, and $678,909, (ii) $11,990,599, $607,662, and $967,690, and (iii) $17,150,575, $1,140,952, and $1,419,763, respectively, constituting (i) 86%, 1%, and 13%, (ii) 90%, 4%, 6%, and (iii) 87%, 6%, 7%, respectively, of our total revenue.
Suppliers
The PRC subsidiaries source their suppliers through multiple channels: (i) industry exhibitions/expos, (ii) online platform, and (iii) suppliers’ direct contact.
The PRC subsidiaries’ suppliers are those providing raw materials for the manufacturing of the products.
The raw and auxiliary materials include meltblown, non-woven fabrics, steel spring, OPP membrane, methyl silicone oil, etc. All of which are purchased from certified and qualified suppliers in China. The PRC subsidiaries’ raw materials supply has been very stable for many years and are easily sourced due to the PRC subsidiaries’ geographical location. The prices of raw materials are not volatile and they, along with the PRC subsidiaries’ supply chain, have not been impacted by the recent conflict between Russia and Ukraine, as of the date of this prospectus. At the beginning of 2020, the price of mask raw materials surged and the logistics were affected by the COVID-19 pandemic.
However, since Jiangsu province and Zhejiang province, where the PRC subsidiaries are located and conduct their business, are the core supply areas of mask raw materials and the pandemic was effectively controlled in these areas, so the PRC subsidiaries had sufficient raw materials and the prices were not materially impacted during the pandemic. Recently, China has substantially lifted COVID-19 restrictions by removing mandatory centralized quarantine, compulsory testing, and sweeping lockdowns. The raw materials our PRC subsidiaries source from their suppliers are sufficient, and the prices of these raw materials remain stable.
92
Table of Contents
According to the Company, it is a common practice in the industry of medical devices for companies to purchase raw materials from a considerable number of suppliers. As of the date of this prospectus, the PRC subsidiaries have a total number of 130 suppliers. Although there are lots of suppliers available in the market, our PRC subsidiaries believe that they have established healthy and stable relationships with their significant suppliers through years of cooperation. These significant suppliers, in the aggregate, accounted for over 34%, 34%, and 27% of their raw material purchases for the six months ended March 31, 2024 and the fiscal year ended September 30, 2023 and 2022. For the six months ended March 31, 2024, the top three significant suppliers represented approximately 15%, 11%, and 8% of the total supplies purchased, respectively. For the year ended September 30, 2021, the top three significant suppliers represented approximately 18%, 16%, and 5% of the total supplies purchased, respectively. For the fiscal year ended September 30, 2023, the top three significant suppliers represented approximately 13%, 11%, and 9% of the total supplies purchased, respectively. For the year ended September 30, 2022, the top three significant suppliers represented approximately 16%, 11%, and 7% of the total supplies purchased, respectively. See “Risk Factors — Risks Related to the PRC Subsidiaries’ Business and Industry — Our success will be dependent upon our PRC subsidiaries’ ability to maintain relationships with existing suppliers who are critical and necessary to the production of our PRC subsidiaries’ products and to create relationships with new suppliers.” There are no minimum purchase requirements with any of the suppliers, including these significant ones. Each supplier order is typically governed by a brief purchase-order based purchase agreement. The key terms of the procurement agreements (including those agreements with our significant suppliers) include:
• the product’s name, type, quantity, and price;
• quality terms which are typically expressed with reference to national or industry standards;
• delivery time, method and payment terms. Shipping costs are the responsibility of the supplier; and
• breach of contract terms, including refund and return of products, compensatory damages. If the supplier cannot deliver the product within the time agreed, or if the products do not meet the stated quality standard, the supplier must compensate the PRC subsidiaries for losses caused, including treble damages if the products are defective or counterfeit. In the event the PRC subsidiaries cannot timely pay, liquidated damages are due to the supplier.
Marketing and Sales
The PRC subsidiaries market and sell their products through multiple channels: (i) through their sales team, and (ii) through their distribution network, including their domestic and export distributors.
Sales Team
As of the date of this prospectus, the PRC subsidiaries have a sales team of 38 employees. Their sales team provides them with direct access to their customers and is capable of promptly addressing customers’ needs. There are 4 team leaders leading their respective teams to market the PRC subsidiaries’ products, both domestically and internationally.
The PRC subsidiaries’ sales team promotes the products in the following ways: (i) advertising on search engine websites such as Baidu and 360; (ii) participating in online bidding on the provincial drug procurement platforms; (iii) attending exhibitions and expos; (iv) attending medical conferences and introducing the products; (v) developing business relation with local distributors and promoting the products; (vi) conducting online searches to obtain public information of potential customers from enterprise yellow pages or enterprise official websites, establishing contact with them by ascertaining their product demand and purchasing intention via telephone calling or social media applications, such as WeChat and QQ.
The compensation package for the sales team includes vacation, social insurance, fixed base salaries and commissions based on the revenue or collection they achieve. The PRC subsidiaries provide their sales team with regular training and internally developed systems to assist them in quickly becoming proficient and productive sales personnel. The employment agreements with the sales team members include, contract period (fixed time or indefinite duration), job description, occupational hazard protection, termination provisions (termination at will is prohibited), and compliance with the Labor Law of the People’s Republic of China and the Labor Contract Law of the People’s Republic of China in all material aspects.
93
Table of Contents
Distribution Network
According to the Company, it is a common practice in the medical device industry for companies to rely on a considerable number of distributors to sell their products. Distributors usually purchase products from the PRC subsidiaries at a discounted price and then resell the products to end customers, both domestically and internationally.
The PRC subsidiaries’ domestic distributors cover 34 provincial-level administrative regions of PRC for the resale of the products in the Chinese market. Domestic distributors market and distribute the products in the regions where they are located and secured approximately 865, 1,032, and 1,021 domestic customers for the PRC subsidiaries for the six months ended March 31, 2024 and the fiscal years ended September 30, 2023 and 2022, respectively, including hospitals and medical institutions.
The PRC subsidiaries’ exporting distributors can be classified into two categories: those located outside of China that only distribute the products internationally, and those located in China that distribute the products, both domestically and internationally. The total number of direct and indirect customer relationships established overseas through the exporting distributors was approximately 48 as of March 31, 2024.
The PRC subsidiaries do not have long-term written agreements with their distributors. Each distributor order is typically governed by a brief purchase-order based sales agreement. The key terms of the distributor purchase agreements include:
• the product’s name, type, quantity, and price;
• qualifications, including business license, medical device production and operation licenses, medical device registration certificates, and inspection report. (The absence of any of these qualifications will result in termination of the agreements);
• delivery method and payment terms: shipping costs are typically borne by the PRC subsidiaries and payment shall be made before delivery;
• risk of loss, which is typically borne by the PRC subsidiaries until delivery;
• breach of contract terms, including refunds and return of products (e.g., distributors are entitled to refunds and may return a product if the wrong product is delivered, or the product does not meet agreed upon quality standards); and
• dispute solutions, including bringing a lawsuit at the local court where the distributors are located.
See “Risk Factors — Risks Related to the PRC Subsidiaries’ Business and Industry — Our PRC subsidiaries rely in part on third-party distributors to place their products into the market and they may not be able to control their distributors.”
Research and Development (“R&D”)
The PRC subsidiaries invest in R&D, aiming to develop new products and improve their existing products to accommodate the market needs. The R&D expenses totaled approximately $178,783, $356,160, and $183,900 for the six months ended March 31, 2024 and the fiscal years ended September 30, 2023 and 2022, respectively. R&D expenses mainly consist of applicable personnel, design, sample manufacturing and materials expenses. As of March 31, 2024, the PRC subsidiaries have a total of 16 employees in the R&D department.
The PRC subsidiaries expect that the R&D expenses will increase significantly in the future, as they continue to develop new products, enhance their existing products and technologies, and perform activities related to obtaining additional regulatory approval.
The PRC subsidiaries adhere to a market-oriented R&D approach and actively cooperate with universities, hospitals, medical institutions, and distributors in sorting out their R&D orientation based on the real market demand. The market-oriented R&D approach includes the following:
• Independent R&D. The PRC subsidiaries place great emphasis on independent R&D. For the six months ended March 31, 2024 and the fiscal years ended September 30, 2023 and 2022, the PRC subsidiaries invested $178,783, $356,160, and $183,900 in purchasing tooling, equipment, raw materials, and paying
94
Table of Contents
researchers’ salaries, accounting for 3.37%, 2.20%, and 0.93% of our total revenue, respectively. The PRC subsidiaries have expanded their R&D department since 2022 and searched for new research fields, and hence, they expect to increase their ratio of R&D investment to revenue to 6% to 10% in the following three years. Visualized prostatic dilatation catheter is the latest products of the research team.
• Universities and Hospitals to Factories. Many universities and hospitals have research centers where they develop and patent certain R&D results. The PRC subsidiaries from time to time communicate with research personnel from research centers and keep themselves informed of the latest R&D and patents, and if needed by their customers, purchase patents from the research centers and research, develop and manufacture products with such patents. For instance, Hangzhou Shanyou entered into a patent transfer agreement (“Jiaxing Hospital Agreement”) with The Second Hospital of Jiaxing City (the “Hospital”) on April 1, 2021 (the “Agreement Date”). Set forth below are the material terms of the agreement:
• Nature of the patent transferred: a cuff device for endotracheal intubation, which can automatically inflate and deflate and monitor the pressure of columnar balloon;
• Patent expiration date: May 6, 2029;
• Payment terms: one-time payment of RMB60,000 (US$8,737) shall be paid by Hangzhou Shanyou to the Hospital within 30 days of the Agreement Date. As of the date of this prospectus, Hangzhou Shanyou has paid RMB60,000 under the Jiaxing Hospital Agreement;
• Hangzhou Shanyou’s rights: (i) Hangzhou Shanyou is entitled to own the patent; and (ii) Hangzhou Shanyou is entitled to improve the inventions and creations derived from the patent. The resulting new achievements of substantial or creative technological progress derived from Hangzhou Shanyou’s work shall belong to Hangzhou Shanyou;
• Hangzhou Shanyou’s obligations: (i) Hangzhou Shanyou is required to complete the patent transfer registration within 90 days of the Agreement Date; and (ii) Hangzhou Shanyou is required to pay liquidated damages of 30% of the total contract price, which is RMB18,000 (US$2,621), to the Hospital, if Hangzhou Shanyou fails to pay the contract price in full on time;
• The Hospital’s rights: (i) the Hospital is entitled to a one-time payment of RMB60,000; and (ii) after transferring the patent to Hangzhou Shanyou, the Hospital is also entitled to improve the inventions and creations derived from the patent. The resulting new achievements of substantial or creative technological progress derived from the Hospital’s work shall belong to the Hospital;
• The Hospital’s obligations: (i) the Hospital will furnish Hangzhou Shanyou with all documents submitted to or received from China National Intellectual Property Administration, including patent specification, patent certificate, and the latest payment receipt of patent annual renewal fee, within 60 days since the date the Hospital received the payment of RMB60,000 from Hangzhou Shanyou; and (ii) the Hospital will ensure that the patent transfer will not infringe on any legitimate right of any third party, and the Hospital is required to assume all responsibility if a third party accuses Hangzhou Shanyou of patent infringement; failure to do so will result in a refund to be issued by the Hospital to Hangzhou Shanyou within 30 days;
• Mutual rights and obligations: (i) for the duration of the Jiaxing Hospital Agreement, any party that makes improvements to the patent should promptly notify the other party; (ii) if both parties collaborate on technical improvements to the patent and if neither party applies for a patent for the improved technology, the other party has a duty of confidentiality regarding the improved technology, and it shall not disclose, license, or transfer the improved technology to others without permission; and (iii) for substantial technological progress jointly made by both parties, the right to apply for a patent is jointly owned by both parties;
• Termination: the Jiaxing Hospital Agreement can be terminated because of the occurrence of an event of force majeure; and
• Dispute resolution: any dispute arising from the Jiaxing Hospital Agreement will be resolved by negotiation and mediation. If negotiation and mediation fail, legal action shall be taken in the people’s court where the Hospital is situated.
95
Table of Contents
In addition, Hangzhou Hanshi entered into a patent transfer agreement with Zhejiang University (Zhejiang University Agreement) on December 26, 2019. Set forth below are the material terms of the agreement:
• Nature of the patent transferred: a new type of expanded visualized prostatic dilatation catheter;
• Patent expiration date: November 8, 2028;
• Payment terms: one-time payment of RMB315,000 (US$45,868) shall be paid by Hangzhou Hanshi to Zhejiang University within five days of the date of the Zhejiang University Agreement. As of the date of this prospectus, Hangzhou Hanshi paid RMB319,725 (US$46,556) under the Zhejiang University Agreement, of which RMB315,000 is the contract price and the remaining RMB4,725 (US$688) is the patent transfer service fee charged by China Intellectual Property Trading Center for the patent transfer from Zhejiang University to Hangzhou Hanshi;
• Hangzhou Hanshi’s rights: Hangzhou Hanshi is entitled to own the patent;
• Hangzhou Hanshi’s obligations: (i) Hangzhou Hanshi will complete the patent transfer registration; and (ii) Hangzhou Hanshi is required to pay Zhejiang University liquidated damages of 1% of the total contract price per day (RMB3,125 (US$455)), if Hangzhou Shanyou’s designated contact person fails to (a) complete the relevant work assigned according to the agreed upon time, method and place; (b) ensure that it will not be difficult or impossible for Hangzhou Hanshi to perform the Zhejiang University Agreement due to personnel changes; and (c) guarantee Hangzhou Hanshi’s performance of the Zhejiang University Agreement in an appropriate time, manner and standard;
• Zhejiang University’s rights: (i) Zhejiang University is entitled to a one-time payment of RMB315,000;
• Zhejiang University’s obligations: (i) Zhejiang University will furnish Hangzhou Hanshi with patent application acceptance notice, patent applications documents, and related technical documents before January 10, 2020. Failure to do so will result in a payment of RMB50,000 (US$7,281) in liquidated damages to be paid by Zhejiang University to Hangzhou Hanshi; and (ii) Zhejiang University will ensure that the patent transfer will not infringe on any legitimate right of any third party. Failure to do so will result in a payment of RMB50,000 in liquidated damages to be paid by Zhejiang University to Hangzhou Hanshi;
• Mutual rights and obligations: (i) neither party shall restrict the other’s technological competition and development; and (ii) both parties are entitled to improve the inventions and creations derived from the patent;
• Termination: the Zhejiang University Agreement can be terminated, if: (i) an event of force majeure occurs; (ii) changes occur in national industry policy or there is a change in the technical project manager, within six months since the date of the agreement; or (iii) Hangzhou Hanshi fails to pay the contract price for over three months; and
• Dispute resolution: any dispute arising from the Zhejiang University Agreement should be resolved by negotiation and mediation. If negotiation and mediation fail, legal action shall be taken in the people’s court where Zhejiang University is situated.
• Customers’ Feedback from Sales Representatives and Distributors. Sales representatives and distributors receive feedback and proposals from end-users from time to time. The PRC subsidiaries review the feedback and proposals on a regular basis. Prior to developing a product improvement or new product, the PRC subsidiaries assess end-users’ feedback from their sales representatives and distributors in order to better identify the changing needs and demands of end-users.
As of the date of the prospectus, the PRC subsidiaries have 29 registered patents in mainland China. Faced with the ever-changing market demands, they continue to invest in acquiring new patents and technologies that are tailored to the market’s fast-changing requirements.
96
Table of Contents
Competition
The medical device industry is intensely competitive, subject to rapid change and significantly affected by new product introductions and other market activities of industry participants. The PRC subsidiaries compete or plan to compete with manufacturers of disposable medical devices. Some of these competitors are large, well-capitalized companies with greater market share, resources and experience than the PRC subsidiaries have. As a consequence, they are able to spend more on product development, marketing, sales and other product initiatives than the PRC subsidiaries can. The PRC subsidiaries also compete with smaller medical device companies that have only one product or a limited range of products. The PRC subsidiaries’ current competitors include Henan Tuoren Medical Device Co., Ltd., Guangzhou Weili Medical Device Co., Ltd., and Zhejiang Sujia Medical Device Co., Ltd. The PRC subsidiaries compete based on factors such as price, value, customer support, brand recognition, reputation, and product functionality, reliability, and compatibility.
Although there can be no assurance that the PRC subsidiaries will be able to continue to compete successfully in the future, we believe that the PRC subsidiaries can compete successfully with these companies by offering products of better quality at comparable prices.
Quality Control
Medical device are medical products directly applied to the human body, which is closely related to the life and health of users. Quality and safety are always the PRC subsidiaries’ core value. Reliable, safe and stable product quality is an important driving factor for maintaining market competitiveness. Through long-term business dealings with major hospitals and medical institutions across China, the PRC subsidiaries believe that they have developed a sophisticated quality control management system in accordance with the requirements of Chinese laws and regulations.
The quality control management system fully covers the whole process of manufacturing, which consists of three parts: raw materials inspection, production check, and product examination.
Raw materials are inspected when they arrive at Hangzhou Shanyou’s manufacturing facilities. Hangzhou Shanyou’s inspectors check their names, sizes, models, quantities, packaging, and supplier qualifications, to make sure they are in accordance with the purchasing order or receipt. After the materials pass the inspection, the inspectors put them on record and issue a notice of inbound, suggesting them to be put in storage. The quality control managers check the raw materials again, and review the record and notice, granting approval for storage if the materials are compatible with the description of the record and notice.
Hangzhou Shanyou has made process flow diagrams for all the products, specifying technological parameters and critical parts for quality control. Quality examiners will inspect the production process, to confirm if the production strictly follows the diagrams.
Hangzhou Shanyou inspects all its products before shipping them to customers. The following will be checked and examined after production and before Hangzhou Shanyou ships its products to customers: 1) whether the production strictly follows the procedures listed on the process flow diagram; 2) whether key and/or special processes of production are verified; 3) whether modifications of production assignments, raw materials, and production techniques, if applicable, are made according to instructions; and 4) whether all raw materials are checked, including their names, sizes, models, quantities, packaging, and supplier qualifications.
Hangzhou Shanyou prioritizes product quality management. It is committed to strengthening the professional ethics and cultivating quality consciousness of its employees and forming a strict quality management system, which it believes is in line with international standards.
The quality control management programs have earned Hangzhou Shanyou a number of quality-related manufacturing designations. In 2020, Hangzhou Shanyou received an EC certification, which certifies the compliance of its quality management system and technical documentation with European regulations for medical devices and the requirements of Annex V of directive 93/42/EEC have been met for its products (such as endotracheal tubes, laryngeal mask airways, laryngoscope blades, face masks and suction catheters).
In the same year, Hangzhou Shanyou also received ISO 13485 system certification for over 20 products, including nasogastric tubes, catheter mounts, intubating stylets, artery compression tourniquets, and endotracheal tube holders, demonstrating their ability to provide such safe and high-quality medical devices that meet ISO requirement.
97
Table of Contents
In 2023, Hangzhou Shanyou also registered 17 products with the FDA (registration number: 3007127332), including a surgical respirator, nasal oxygen cannula, reservoir bag and nasopharyngeal airway, allowing their products to enter the U.S. market. It has 18 products that have passed the inspections administered by the local authorities in Zhejiang province and obtained the registration certificates.
However, despite the quality control management system, Hangzhou Shanyou cannot eliminate the risks of errors, defects, or failures. Hangzhou Shanyou may fail to detect or cure defects as a result of a number of factors, many of which are outside our control, including:
• technical or mechanical malfunctions in the production process;
• human error or malfeasance by quality control personnel;
• tampering by third parties; and
• defective raw materials or equipment.
Failure to detect quality defects in the products could result in patient injury, customer dissatisfaction, or other problems that could harm Hangzhou Shanyou’s reputation and business, expose it to liability, and adversely affect its revenue and profitability. Relevant PRC laws and regulations were formulated to strengthen the administration of rules pertaining to product quality, as well as to clarify the rules on product liability, protect consumers and maintain social and economic order. Products offered for sale in China must meet the relevant quality and safety standards. Violations of state or industrial standards for health, safety and any other related violations may result in civil liabilities and penalties, such as compensation for damages, fines, suspension, or shutdown of business, as well as confiscation of products illegally produced for sale and the sales proceeds of such products.
On December 9, 2019, Hangzhou Shanyou was required to recall a batch of disposable breathing circuits and arrange internal rectification, since a connection part was insufficiently welded at the machine end and a circuit did not pass the leakage test. Hangzhou Shanyou recalled all of the breathing circuits involved and arranged the internal rectification. After investigation and evaluation, Hangzhou Shanyou confirmed the involved products would not cause harm to human health and all the affected goods were disposed of.
On November 16, 2020, Hangzhou Shanyou was required to recall 20,000 disposable medical masks by Heilongjiang Provincial Drug Administration, because the mask strings failed to comply with an elastic requirement. Hangzhou Shanyou recalled the affected batch of masks.
The PRC subsidiaries’ products are subject to various laws and regulations relating to medical devices with various requirements in details, including but not limited to the registration and filings of medical devices, the production and operation license, the production and quality management. Although the PRC subsidiaries endeavor to stay in compliance with such laws and regulations, we cannot assure you that the PRC subsidiaries comply with relevant laws and regulations at all times. Any such failure may have a material and adverse effect on the PRC subsidiaries’ and our business, financial condition, and results of operations.
As of the date of the prospectus, we or the PRC subsidiaries are not aware of any other investigations, prosecutions, disputes, claims or other proceedings in respect of quality issues, nor have we or the PRC subsidiaries been penalized additionally or can foresee any penalty to be made by any related PRC government authorities.
Financing
The PRC subsidiaries enter into financing agreements with banks to finance their operations. Set forth below is a detailed description of the PRC subsidiaries’ financing agreements which were filed as exhibits to our registration statement on Form F-1, initially filed with the SEC on April 24, 2023:
1. A comprehensive credit contract between Hangzhou Shanyou and Bank of Beijing dated September 29, 2020. It is a revolving credit contract with an aggregate loan principal of RMB8 million. The term of each loan drawn under the revolving credit contract is 12 months, and the period of loan withdrawal is 12 months starting on the date of this contract. Hangzhou Shanyou fully repaid the loan on September 5, 2022.*
98
Table of Contents
2. A loan contract affiliated with the comprehensive credit contract between Hangzhou Shanyou and Bank of Beijing dated September 29, 2020. The loan principal was RMB8 million, with an interest rate determined by the one-year LPR, set the day immediately before the withdrawal date, plus 65 basis points. The withdrawal period was from September 29, 2020 to September 28, 2021, and the loan period was one year from the date of withdrawal, with the final maturity date of September 28, 2022. Hangzhou Shanyou fully repaid the loan on September 6, 2021.
3. A working capital loan contract between Hangzhou Shanyou and Bank of Jiangsu dated January 26, 2021. The loan principal was RMB5 million, with an interest rate determined by the one-year LPR set the day immediately before the contract date, plus 75 basis points, which was 4.6%. The date of loan withdrawal was January 26, 2021, and Hangzhou Shanyou fully repaid the loan on January 25, 2022.
4. A loan contract between Hangzhou Shanyou and Zhejiang Xiaoshan Rural Commercial Bank dated December 22, 2016. The loan principal was RMB15 million, with an interest rate of 6.06% per month. The term of the loan was from December 22, 2016 to November 21, 2021. Hangzhou Shanyou fully repaid the loan on November 21, 2021.
5. A working capital loan contract between Hangzhou Shanyou and Zhejiang Xiaoshan Rural Commercial Bank dated January 6, 2020. The loan principal was RMB4 million, with an interest rate determined by the one-year LPR, set the day immediately before the contract date, plus 216 basis points. The term of the loan was from January 6, 2020 to January 5, 2021. Hangzhou Shanyou fully repaid the loan on January 5, 2021.
6. A working capital loan contract between Hangzhou Shanyou and Zhejiang Xiaoshan Rural Commercial Bank dated January 15, 2020. The loan principal was RMB3 million, with an interest rate determined by the one-year LPR, set the day immediately before the contract date, plus 216 basis points. The term of loan was from January 15, 2020 to January 14, 2021. Hangzhou Shanyou fully repaid the loan on January 14, 2021.
7. A working capital loan contract between Hangzhou Shanyou and Zhejiang Xiaoshan Rural Commercial Bank dated March 2, 2020. The loan principal was RMB3.5 million, with an interest rate determined by the one-year LPR, set the day immediately before the contract date, plus 117 basis points. The term of loan was from March 2, 2020 to February 27, 2021. Hangzhou Shanyou fully repaid the loan on February 27, 2021.
8. A working capital loan contract between Hangzhou Shanyou and Zhejiang Xiaoshan Rural Commercial Bank dated June 28, 2020. The loan principal was RMB5 million, with an interest rate determined by the one-year LPR, set the day immediately before the contract date, plus 137 basis points. The term of loan was from June 28, 2020 to June 27, 2021. Hangzhou Shanyou fully repaid the loan on June 27, 2021.
9. A working capital loan contract between Hangzhou Shanyou and Zhejiang Xiaoshan Rural Commercial Bank dated July 2, 2020. The loan principal was RMB5 million, with an interest rate determined by the one-year LPR, set the day immediately before the contract, plus 137 basis points. The term of loan was from July 2, 2020 to July 1, 2021. Hangzhou Shanyou fully repaid the loan on July 1, 2021.
10. A working capital loan contract between Hangzhou Shanyou and Zhejiang Xiaoshan Rural Commercial Bank dated September 29, 2021. The loan principal was RMB10 million, with an interest rate determined by the one-year LPR, set the day immediately before the contract date, plus 100 basis points. The term of loan was from September 29, 2021 to September 23, 2022. Hangzhou Shanyou fully repaid the loan on September 1, 2022.
11. A working capital loan contract between Hangzhou Shanyou and Zhejiang Xiaoshan Rural Commercial Bank dated September 29, 2021. The loan principal was RMB19.5 million, with an interest rate determined by the one-year LPR, set the day immediately before the contract date, plus 137 basis points. The term of loan was from September 29, 2021 to September 23, 2022. Hangzhou Shanyou fully repaid the loan on September 7, 2022.
12. A working capital loan contract between Hangzhou Shanyou and Zhejiang Xiaoshan Rural Commercial Bank dated September 1, 2022. The loan principal was RMB10 million, with an interest rate determined by the one-year LPR, set the day immediately before the contract date, plus 70 basis points. The term of loan was from September 1, 2022, to August 31, 2023. Hangzhou Shanyou and Zhejiang Xiaoshan
99
Table of Contents
Rural Commercial Bank agreed on August 30, 2023, to extend the maturity date to August 29, 2024, and the interest rate was adjusted to be determined by the one-year LPR, set the day immediately before the contract date, plus 105 basis points. Hangzhou Shanyou and Zhejiang Xiaoshan Rural Commerical Bank agreed on August 26, 2024, to extend the maturity date to August 25, 2025, and the interest rate was adjusted to be determined by the one-year LPR, set the date immediately before the contract date, plus 115 basis points. Hangzhou Shanyou intends to fully repay the loan by August 25, 2025.
13. A working capital loan contract between Hangzhou Shanyou and Zhejiang Xiaoshan Rural Commercial Bank dated September 2, 2022. The loan principal was RMB8 million, with an interest rate determined by the one-year LPR, set the day immediately before the contract date, plus 105 basis points. The term of loan was from September 2, 2022, to August 30, 2023. Hangzhou Shanyou and Zhejiang Xiaoshan Rural Commercial Bank agreed on August 29, 2023, to extend the maturity date to August 28, 2024, and the interest rate was adjusted to be determined by the one-year LPR, set the day immediately before the contract date, plus 95 basis points. Hangzhou Shanyou and Zhejiang Xiaoshan Rural Commercial Bank agreed on August 26, 2024, to extend the maturity date to August 25, 2025, and the interest rate was adjusted to be determined by the one-year LPR, set the date immediately before the contract date, plus 105 basis points. Hangzhou Shanyou intends to fully repay the loan by August 25, 2025.
14. A working capital loan contract between Hangzhou Shanyou and Zhejiang Xiaoshan Rural Commercial Bank dated September 7, 2022. The loan principal was RMB5 million, with an interest rate determined by the one-year LPR, set the day immediately before the contract date, plus 105 basis points. The term of loan was from September 7, 2022, to August 30, 2023. Hangzhou Shanyou and Zhejiang Xiaoshan Rural Commercial Bank agreed on August 29, 2023, to extend the maturity date to August 28, 2024, and the interest rate was adjusted to be determined by the one-year LPR, set the day immediately before the contract date, plus 95 basis points. On August 26, 2024, Hangzhou Shanyou and Zhejiang Xiaoshan Rural Commercial Bank agreed to extend the maturity date to August 25, 2025, and the interest rate was adjusted to be determined by the one-year LPR, set the date immediately before the contract date, plus 85 basis points. Hangzhou Shanyou intends to fully repay the loan by August 25, 2025.
15. A working capital loan contract between Hangzhou Shanyou and Zhejiang Xiaoshan Rural Commercial Bank dated September 7, 2022. The loan principal was RMB19.5 million, with an interest rate determined by the one-year LPR, set on the day immediately before the contract date, plus 105 basis points. The term of loan was from September 7, 2022, to August 30, 2023. Hangzhou Shanyou and Zhejiang Xiaoshan Rural Commercial Bank agreed on August 30, 2023, to extend the maturity date to August 29, 2024. The interest rate remains the same. On August 29, 2024, Hangzhou Shanyou repaid RMB5 million of the outstanding RMB19.5 million loan to Zhejiang Xiaoshan Rural Commercial Bank. On August 26, 2024, Hangzhou Shanyou and Zhejiang Xiaoshan Rural Commercial Bank agreed to extend the maturity date of the remaining outstanding RMB14.5 million loan to August 25, 2025, and the interest rate was adjusted to be determined by the one-year LPR, set the date immediately before the contract date, plus 115 basis points. Hangzhou Shanyou intends to fully repay the remaining outstanding RMB14.5 million loan by August 25, 2025.
16. A working capital loan contract between Hangzhou Shanyou and Bank of Jiangsu dated March 31, 2023. The loan principal was RMB3 million, with an interest rate determined by the one-year LPR, set the day immediately before the contract date, plus 40 basis points. The term of loan was from March 31, 2023, to March 30, 2024. Hangzhou Shanyou fully repaid the loan on March 4, 2024.
17. A working capital loan contract between Hangzhou Shanyou and China CITIC Bank dated June 28, 2023. The loan principal was RMB9 million, with an interest rate determined by the one-year LPR, set on the contract date, plus 35 basis points. The term of loan was from June 28, 2023, to June 27, 2024. Hangzhou Shanyou fully repaid the loan on June 27, 2024.
18. A working capital loan contract between Hangzhou Woli and China CITIC Bank dated December 20, 2023. The loan principal was RMB42 million, with an interest rate determined by the one-year LPR, set on the contract date, plus 75 basis points. The term of loan was from December 20, 2023, to December 19, 2024. Hangzhou Shanyou intends to fully repay the loan by December 19, 2024.
100
Table of Contents
19. A working capital loan contract between Hangzhou Shanyou and Bank of Beijing dated June 29, 2023. The loan principal was RMB10 million, with an interest rate determined by the one-year LPR, set the day immediately before the date of loan drawdown. The term of loan was from June 29, 2023, to June 29, 2024. Hangzhou Shanyou fully repaid the loan on June 28, 2024.
20. A working capital loan contract between Hangzhou Shanyou and Bank of Jiangsu dated March 4, 2024. The loan principal was RMB3 million, with an interest rate determined by the one-year LPR, set the day immediately before the contract date, plus 60 basis points. The term of loan was from March 4, 2024, to September 3, 2024. Hangzhou Shanyou and Bank of Jiangsu agreed on September 3, 2024 to extend the maturity date to September 2, 2025, and the interest rate was adjusted to be determined by the one-year LPR, set the date immediately before the contract date, plus 70 basis points. Hangzhou Shanyou intends to fully repay the loan by September 2, 2025.
21. A working capital loan contract between Hangzhou Shanyou and China CITIC Bank (Hangzhou) dated July 26, 2024. The loan principal was RMB6 million, with an interest rate determined by the one-year LPR, set on the contract date, plus 35 basis points. The term of loan was from July 26, 2024 to July 19, 2025. Hangzhou Shanyou intends to fully repay the loan by July 19, 2025.
22. A working capital loan contract between Hangzhou Shanyou and Zhejiang Xiaoshan Rural Commercial Bank dated July 11, 2024. The loan principal was RMB5 million, with an interest rate determined by the one-year LPR, set on the contract date, plus 75 basis points. Hangzhou Shanyou intends to fully repay the loan by July 10, 2025.
Environmental Matters
Hangzhou Shanyou’s production activities are governed by PRC laws and regulations. The wastewater the Hangzhou Shanyou generates is sanitary wastewater, which can be disposed directly into municipal pipelines. The corner wastes generated are cleaned and collected by the cleaning personnel on time, and transported to the municipal garbage disposal site for treatment by the local sanitation department. Solid wastes generated during operation are collected and sent to relevant manufacturers for recycling. If new products are developed in the future, Hangzhou Shanyou will take corresponding environmental protection measures according to relevant laws and regulations.
As of the date of this prospectus, except as disclosed in this prospectus, neither we nor Hangzhou Shanyou are aware of any warning, investigations, prosecutions, disputes, claims or other proceedings in respect of environmental protection, nor has Hangzhou Shanyou been punished or can it foresee any punishment to be made by any government authorities of the PRC with regard thereto.
Employees
As of March 31, 2024, September 30, 2023, 2022 and 2021, the PRC subsidiaries had a total of 226, 216, 278 and 214 full time employees, respectively. The following table sets forth the number of the full-time employees categorized by areas of operations as of December 19, 2024:
Function/Department | | Number |
Manufacturing | | 124 |
Research and Development | | 14 |
General | | 28 |
Sales and Marketing | | 38 |
Management | | 17 |
Total | | 221 |
101
Table of Contents
Our success depends on the PRC subsidiaries’ ability to attract, motivate, train and retain qualified personnel. We believe the PRC subsidiaries offer their employees competitive compensation packages and an environment that encourages self-development and, as a result, have generally been able to attract and retain qualified personnel and maintain a stable core management team.
As required by PRC laws and regulations, the PRC subsidiaries participate in various employee benefit plans that are organized by municipal and provincial governments, including, among other things, pension, medical insurance, unemployment insurance, maternity insurance, on-the-job injury insurance, and housing fund plans through a PRC government-mandated benefit contribution plan. The PRC subsidiaries are required under PRC laws to make contributions to employee benefit plans at specified percentages of the salaries, bonuses and certain allowances of their employees. As of the date of this prospectus, (i) some of the PRC subsidiaries have not completed the social insurance registration and the housing fund registration; (ii) the PRC subsidiaries did not make contributions in the full amount for the social insurance fund and housing provident fund for their employees, as required under the relevant PRC laws and regulations; and (iii) the PRC subsidiaries did not make contributions in the housing fund for some employees. For the six months ended March 31, 2024 and the fiscal years of 2023 and 2022, $141,340, $287,629, and $295,009 of contributions for social insurance fund, respectively, should have been made but were not. For the six months ended March 31, 2024 and the fiscal years of 2023 and 2022, $44,530, $90,620, and $92,945 of contributions for the housing provident fund, respectively, should have been made but were not. Please see “Risk Factors — Risks Related to Doing Business in China — Failure to make adequate contributions to various employee benefit plans and withhold individual income tax on employees’ salaries as required by PRC regulations may subject the PRC subsidiaries to penalties” on page 29.
We believe the PRC subsidiaries maintain a good working relationship with their employees, and the PRC subsidiaries have not experienced any material labor disputes. None of their employees are represented by a labor union.
Properties
The PRC subsidiaries’ headquarters, manufacturing facilities and office spaces are located in Hangzhou, Zhejiang Province, Changsha, Hunan Province, and Shanghai, in the PRC.
Properties the PRC Subsidiaries Own
The PRC subsidiaries own the premises of their headquarters, manufacturing facilities, office spaces, and a warehouse, which cover an aggregate building area of approximately 356,176 square feet, with the breakdown set for in the below table:
Description/Use | | Owner | | Location | | Area
(square
feet) |
Office space & manufacturing facility | | Hangzhou Shanyou | | No.138, Building 1 – 3, Louta Town, Xiaoshan District, Hangzhou | | 97,003 |
Manufacturing facility | | Hangzhou Shanyou | | No.138, Building 5, Louta Town, Xiaoshan District, Hangzhou | | 103,780 |
Manufacturing facility | | Hangzhou Shanyou | | No.138, Building 6, Louta Town, Xiaoshan District, Hangzhou | | 147,266 |
Warehouse(1) | | Hangzhou Shanyou | | No.138, Building 5, Louta Town, Xiaoshan District, Hangzhou | | 8,127 |
Total | | | | | | 356,176 |
Leased Properties
In addition to the above-mentioned properties that the PRC subsidiaries own, they currently lease several properties in Hangzhou, Shanghai and Hunan for an aggregate area of approximately 80,263 square feet for warehouses and office space.
102
Table of Contents
The breakdown of the leased properties is as follows:
Lessor | | Lessee | | Location | | Area
(Square
Feet) | |
Rent
| | Term | | Use |
Hangzhou Tianxia Weaving Co., Ltd. | | Hangzhou Shanyou | | Building No. 113, Louta Town, Xiaoshan District | | 21,528 | | $9,840 (RMB70,000.00) | | May 30, 2024 to November 29, 20241 | | Sterilization Warehouse |
Hangzhou Xiaoshan Louta Guancun Stock Economic Union | | Hangzhou Shanyou | | Development District | | 2,828 | | $345.29 (RMB2,456.25) | | January 1, 2023 to December 31, 2025 | | Driveway |
Hangzhou Xiaoshan Louta Guancun Stock Economic Union | | Hangzhou Shanyou | | Development District | | 9,831 | | $962.96 (RMB6,850.00) | | January 1, 2023 to December 31, 2025 | | Driveway |
Louta Town Village Committee | | Hangzhou Shanyou | | Development District | | 45,208 | | $1,955 (RMB12,600.00) from January 1, 2011 to December 31, 2013 $3,910 (RMB25,200.00) from January 1, 2014 to December 31, 2027 | | January 1, 2011 to December 31, 2027 | | Warehouse and Parking Area |
Ailiu Real Estate Co., Ltd. | | Shanghai Chuqiang | | Caojing Town, Jinshan District, Shanghai | | 108 | | $372 (RMB2,400.00) | | February 27, 2018 to February 26, 2028 | | Office |
Changsha Yixianglong Property Management Co., Ltd. | | Hunan
Saitumofei | | Room 204, 201, Building A42, Lian Dong Jin Yu Industrial Centre, Wang Cheng Economic and Technological Development Zone, Changsha | | 646 | | $337.39 (RMB2,400.00) | | January 2, 2023 to January 1, 2026 | | Office |
Hunan Hua Mao Doctor Medical Technology Co., Ltd. | | Hunan
Saitumofei | | Room 201, 204, Building A42, Lian Dong Jin Yu Industrial Center, Wang Cheng Economic and Technological Development Zone, Changsha | | 646 | | $553.99 (RMB4,000) | | November 1, 2024 to October 31, 2029 | | Office and Manufacturing Facility |
Jufu (Shanghai) Enterprise Management Consulting Co., Ltd. | | Shanghai Saitumofei | | Room 145, Zone A, 1/F, Building 6, No. 4997, Bao’an Highway, Anting Town, Shanghai | | 114 | | $689.28 (RMB5,029.7) | | June 1, 2024 to May 31, 2025 | | Office |
Huangshan Tunxi District State-owned Assets Investment and Operation Co., Ltd. | | Huangshan Saitumofei | | No. 6-2 – 6-3, Xingyu Road, Tunxi District, Huangshan | | 427 | | $1,715.03 (RMB12,383) | | August 1, 2024 to July 31, 2025 | | Storefront |
103
Table of Contents
Hangzhou Shanyou’s manufacturing facilities, located in No.138, Louta Town, Xiaoshan District, Hangzhou, have capacity for 200 workers, and their annual production capacity is demonstrated in the following table:
Medical Devices | | Number
(thousand) |
Anesthetic Kits | | 200 |
Endotracheal Tube Holders | | 1,500 |
Disposable Breathing Circuits | | 1,000 |
Nasogastric Tubes | | 200 |
Foreskin Circumcision | | 500 |
Artery Compression Tourniquets | | 1,000 |
HMEF | | 500 |
Intubating Stylets | | 500 |
SPO2 Sensor | | 500 |
Anesthesia Masks | | 200 |
Guedel Airways | | 2,000 |
Nasal Oxygen Cannulas | | 1,500 |
Yankauer Suction Sets | | 500 |
Oxygen Transferring Masks | | 5,000 |
Nebulizer Masks | | 15,000 |
Wireless Analgesic Pumps | | 50 |
Masks | | 474,500 |
Intellectual Property
The PRC subsidiaries’ business is dependent on a combination of trademarks, patents, domain names, and other proprietary rights in order to protect the PRC subsidiaries’ intellectual property rights. As of the date of this prospectus, the PRC subsidiaries have 16 registered trademarks, 29 registered patents, one domain name and one copyright in mainland China.
Trademarks
Set forth below is a detailed description of the PRC subsidiaries’ registered trademarks:
Country | | Trademark | | Trademark
Registration
No. | | Trademark
Name | | Trademark
Registration
Date | | Trademark
Classes | | Trademark
Owner | | Trademark
Term |
China | | 
| | 3496763 | | Work 沃克 | | 09/21/2004 | | 10 | | Hangzhou Shanyou | | 09/21/2004 – 09/20/2034 |
China | | 
| | 45207397 | | Work 沃克 | | 04/07/2021 | | 10 | | Hangzhou Shanyou | | 04/07/2021 – 04/06/2031 |
China | | 
| | 36105130 | | Botailong 博泰龙 | | 11/28/2019 | | 10 | | Hangzhou Shanyou | | 11/28/2019 – 11/27/2029 |
China | | 
| | 70105831 | | WORK, sanyou | | 10/28/2023 | | 3 | | Hanagzhou Shanyou | | 10/28/2023 – 10/27/2033 |
China | | 
| | 70112405 | | WORK, sanyou | | 11/07/2023 | | 5 | | Hanagzhou Shanyou | | 11/07/2023 – 11/06/2033 |
China | | 
| | 70113945 | | WORK, sanyou | | 11/07/2023 | | 10 | | Hanagzhou Shanyou | | 11/07/2023 – 11/06/2033 |
China | | 
| | 70117006 | | WORK, sanyou | | 10/28/2023 | | 24 | | Hanagzhou Shanyou | | 10/28/2023 – 10/27/2033 |
China | | 
| | 70107445 | | WORK, sanyou | | 08/28/2023 | | 36 | | Hanagzhou Shanyou | | 08/28/2023 – 08/27/2033 |
China | | 
| | 70107427 | | WORK, sanyou | | 08/28/2023 | | 35 | | Hanagzhou Shanyou | | 08/28/2023 – 08/27/2033 |
China | | 
| | 70106256 | | WORK, sanyou | | 08/28/2023 | | 44 | | Hanagzhou Shanyou | | 08/28/2023 – 08/27/2033 |
104
Table of Contents
Country | | Trademark | | Trademark
Registration
No. | | Trademark
Name | | Trademark
Registration
Date | | Trademark
Classes | | Trademark
Owner | | Trademark
Term |
China | | 
| | 70104692 | | WORK, sanyou | | 08/28/2023 | | 42 | | Hanagzhou Shanyou | | 08/28/2023 – 08/27/2033 |
China | | 
| | 70104659 | | WORK, sanyou | | 10/28/2023 | | 41 | | Hanagzhou Shanyou | | 10/28/2023 – 10/27/2033 |
China | | 
| | 70104259 | | WORK, sanyou | | 08/28/2023 | | 9 | | Hanagzhou Shanyou | | 08/28/2023 – 08/27/2033 |
China | | 
| | 70102953 | | WORK, sanyou | | 08/28/2023 | | 16 | | Hanagzhou Shanyou | | 08/28/2023 – 08/27/2033 |
China | | 
| | 70093576 | | WORK, sanyou | | 10/28/2023 | | 21 | | Hanagzhou Shanyou | | 10/28/2023 – 10/27/2033 |
China | | 
| | 70091370 | | WORK, sanyou | | 11/07/2023 | | 11 | | Hanagzhou Shanyou | | 11/07/2023 – 11/06/2033 |
Patents
According to the PRC Patent Law promulgated by the SCNPC on March 12, 1984, and last amended on October 17, 2020 with effect from June 1, 2021, and as advised by our PRC counsel, AllBright Law Offices (Fuzhou), during the patent protection period, a patent can be renewed every year to preserve the exclusive proprietary rights in the patent by paying an annual fee commencing from the year in which the patent right is granted; otherwise, the patent right will be terminated in mainland China. Once a patent is expired, it cannot be renewed, however, the patent can still be used. The PRC subsidiaries closely monitor the payment date of each patent and have, as of the date of this prospectus, paid patent fees in a timely manner to maintain the patent’s validity, except for the few patents that the PRC subsidiaries do not intend to continue to own. The Company does not expect that the loss of any particular patent would have a materially adverse effect on the PRC subsidiaries’ business and the Group’s financial condition and results of operations, for the following reasons: i) through the firm relationship with the suppliers and customers, professional brand loyalty and stable sales channels established during the protection period, we believe that the PRC subsidiaries’ products can maintain stable sales performances after the patent protection period; ii) the core customers of the PRC subsidiaries are hospitals at all levels in China, and these hospitals strictly choose their suppliers based on suppliers’ product quality and quantity, timeliness of delivery, reputation, etc.; and iii) the PRC subsidiaries plan to continuously keep investing in R&D and developing new patents. Therefore, after the protection period, even though other manufactures can produce medical devices with the PRC subsidiaries’ patents, it is expected that the PRC subsidiaries will still be able to sell their products to their core customers. In addition, the PRC subsidiaries expect to constantly improve and upgrade existing technologies, which can generate new technical patents, which may preserve their prominence in this field despite any patent losses. Set forth below is a detailed description of registered patents which are all owned by the PRC subsidiaries:
Country | | Patent No. | | Patent Name | | Patent
Publication
Date | | Patent Type | | Patent
Validity
Period | | Patent Owner |
China | | ZL201822130552.0 | | A carbon dioxide absorption tank 1 | | 07/31/2020 | | Utility model | | 12/18/2028 | | Hangzhou Shanyou |
China | | ZL201520791869.2 | | An arterial compression hemostat | | 03/02/2016 | | Utility model | | 10/14/2025 | | Hangzhou Shanyou |
China | | ZL202021052943.6 | | A new-type anti-allergy mask | | 04/09/2021 | | Utility model | | 06/10/2030 | | Hangzhou Shanyou |
China | | ZL202021230920.X | | A new-type vaginal dilator | | 05/25/2021 | | Utility model | | 06/29/2030 | | Hangzhou Shanyou |
China | | ZL201530411996.0 | | Compression hemostat | | 04/13/2016 | | Appearance design | | 10/23/2025 | | Hangzhou Shanyou |
China | | ZL201721459172.0 | | Hemostat and hemostat module | | 06/28/2019 | | Utility model | | 11/03/2027 | | Hangzhou Shanyou |
China | | ZL201930731098.1 | | Prostate dilatation catheter (one balloon) | | 10/30/2020 | | Appearance design | | 12/26/2029 | | Hangzhou Hanshi |
China | | ZL201922387773.0 | | A prostate dilatation catheter | | 02/02/2021 | | Utility model | | 12/26/2029 | | Hangzhou Hanshi |
105
Table of Contents
Country | | Patent No. | | Patent Name | | Patent
Publication
Date | | Patent Type | | Patent
Validity
Period | | Patent Owner |
China | | ZL202022331716.3 | | An umbilical cord ligation ring for newborn | | 09/28/2021 | | Utility model | | 10/19/2030 | | Hangzhou Shanyou |
China | | ZL201922420338.3 | | A new-type prostate dilatation catheter | | 02/02/2021 | | Utility model | | 12/26/2029 | | Hangzhou Hanshi |
China | | ZL202022231860.X | | A new type of protective mask | | 12/3/2021 | | Utility model | | 10/09/2030 | | Hangzhou Shanyou |
China | | ZL202022374054.8 | | An umbilical cord ligature | | 11/30/2021 | | Utility model | | 10/22/2030 | | Hangzhou Shanyou |
China | | ZL202022374829.1 | | A new type of umbilical cord ligation ring for newborns | | 11/30/2021 | | Utility model | | 10/22/2030 | | Hangzhou Shanyou |
China | | ZL201920630410.2 | | A tracheal intubation can automatically inflate and deflate monitoring pressure cuff device | | 04/04/2020 | | Utility model | | 05/06/2029 | | Hangzhou Shanyou |
China | | ZL201821836490.9 | | A visual prostate expander | | 05/22/2020 | | Utility model | | 11/08/2028 | | Hangzhou Hanshi |
China | | ZL202010604739.9 | | A umbilical cord clamping device | | 10/21/2022 | | Invention | | 06/28/2040 | | Hangzhou Shanyou and Hangzhou Obstetrics and Gynecology Hospital |
China | | ZL202221864363.6 | | Filtration mechanism and a new type of mask containing the said filtration mechanism | | 02/03/2023 | | Utility model | | 07/17/2032 | | Hangzhou Shanyou |
China | | ZL202221867065.2 | | Filter and novel mask containing the filter | | 02/03/2023 | | Utility model | | 07/17/2032 | | Hangzhou Shanyou |
China | | ZL202221867105.3 | | A new type of filter mask | | 03/21/2023 | | Utility model | | 07/17/2032 | | Hangzhou Shanyou |
China | | ZL201910748225.8 | | An umbilical cord cutting device | | 01/23/2024 | | Invention | | 08/13/2039 | | Hangzhou Shanyou and Hangzhou Obstetrics and Gynecology Hospital |
China | | ZL201910748013.X | | A kind of umbilical cord clamp and its processing process | | 09/29/2023 | | Invention | | 08/13/2039 | | Hangzhou Shanyou |
China | | ZL201811548926.9 | | A kind of carbon dioxide absorption tank | | 03/19/2024 | | Invention | | 12/17/2038 | | Hangzhou Shanyou |
China | | ZL202010605813.9 | | A novel umbilical cord clamping device | | 01/23/2024 | | Invention | | 06/28/2040 | | Hangzhou Shanyou and Hangzhou Obstetrics and Gynecology Hospital |
China | | ZL202011602759.9 | | Data labeling processing method, device, and system | | 07/18/2023 | | Invention | | 12/28/2040 | | Shanghai Saitumofei |
China | | ZL202330142507.0 | | Umbilical cord clamp | | 09/26/2023 | | Appearance design | | 03/22/2038 | | Hangzhou Shanyou and Hangzhou Obstetrics and Gynecology Hospital |
China | | ZL202320646770.8 | | A clamping device for the umbilical cord | | 10/27/2023 | | Utility model | | 03/22/2033 | | Hangzhou Shanyou and Hangzhou Obstetrics and Gynecology Hospital |
China | | ZL202221818091.6 | | A clamping and connecting device for umbilical cords | | 07/21/2023 | | Utility model | | 07/11/2032 | | Hangzhou Shanyou and Hangzhou Obstetrics and Gynecology Hospital |
106
Table of Contents
Country | | Patent No. | | Patent Name | | Patent
Publication
Date | | Patent Type | | Patent
Validity
Period | | Patent Owner |
China | | ZL202111621435.4 | | A clamping device for umbilical cord clamps | | 07/16/2024 | | Invention | | 12/27/2041 | | Hangzhou Shanyou and Hangzhou Obstetrics and Gynecology Hospital |
China | | ZL202330861318.9 | | Breathing circuit tube | | 07/16/2024 | | Appearance design | | 12/27/2038 | | Hangzhou Shanyou |
Domain Name
The PRC subsidiaries own a Certification of Global Top Level Domain Name of “hzsy120.com”, with a registration date of August 15, 2003 and an expiration date of August 15, 2031.
Copyrights
Set forth below is a detailed description of our registered copyright, which is owned by Shanghai Saitumofei:
Country | | Copyright No. | | Copyright Name | | Copyright
Publication
Date | | Copyright Type | | Copyright
Application Date |
China | | 2020SR1798799 | | White blood cell classification system based on convolutional neural network V1.0 | | Unpublished | | software copyright | | 12/11/2020 |
The term of protection of this computer software copyright shall be 50 years, expiring on December 31 of the fiftieth year after the first publication of such software. However, if such software has not been published within fifty years from the date on which its development has been completed, it shall be no longer protected under relevant regulations in the mainland China, and the term of protection could not be extended. The owner of software copyright has the right to decide whether to make the software available to the public. Even though the above computer software is unpublished, the rights of software copyright owner are still protected by relevant regulations.
Insurance
The PRC subsidiaries provide social security insurance including pension, medical insurance, unemployment insurance, maternity insurance, on-the-job injury insurance and housing fund plans through a PRC government-mandated benefit contribution plan for their employees. They do not carry any key-man life insurance or product liability insurance and have not purchased any property insurance or business interruption insurance. The PRC subsidiaries have determined that the costs of insuring for related risks and the difficulties associated with acquiring such insurance on commercially reasonable terms make it impractical. Please refer to “Risk Factors — Risks Related to the PRC Subsidiaries’ Business and Industry — The PRC subsidiaries do not have any product liability, business interruption, or property insurance and they may incur liabilities that are not covered by insurance, which could expose them to significant costs and business disruption” on page 39.
Legal Proceedings
As of the date of this prospectus, neither we nor the PRC subsidiaries are a party to any material legal or administrative proceedings. From time to time, the PRC subsidiaries may be subject to various claims and legal actions arising in the ordinary course of business. Litigation or any other legal or administrative proceeding, regardless of the outcome, is likely to result in substantial cost and diversion of the PRC subsidiaries’ resources, including management’s time and attention. Furthermore, as of the date of this prospectus, the PRC subsidiaries are not a party to any international claims or litigation with respect to defective products or other matters.
107
Table of Contents
REGULATIONS
This section summarizes the principal PRC laws, rules and regulations relevant to the PRC subsidiaries’ business and operations and the Hong Kong laws and regulations which are relevant to the business. This summary does not purport to be a complete description of all laws and regulations, which apply to the PRC subsidiaries’ business and operations. Investors should note that the following summary is based on relevant laws and regulations in force as of the date of this prospectus, which may be subject to change.
The Classification, Registration and Filing of Medical Devices
According to the Regulations on the Supervision and Administration of Medical Devices (as amended in 2021) (the “2021 Medical Device Regulation”) which was promulgated by the State Council on February 9, 2021 and took effect on June 1, 2021, the CFDA, now known as the NMPA, is in charge of the national supervision and administration of medical devices. The relevant departments under the State Council shall be responsible for the supervision of medical devices within their respective scope of authorities. The food and drug supervision and administration departments of the local governments at the county level and above are responsible for the supervision and administration of medical devices within their own administrative districts.
In China, medical devices have been classified into three categories based on the degree of risk. Class I medical devices shall refer to those devices with low degree of risk and whose safety and effectiveness can be ensured through routine administration. Class II medical devices shall refer to those devices with medium degree of risk and whose safety and effectiveness shall be strictly controlled and administered. Class III medical devices shall refer to those devices with high degree of risk and whose safety and effectiveness must be strictly controlled and administered with special measures.
The products the PRC subsidiaries currently produce and sell in China are Class I and Class II medical devices. As of the date of this prospectus, the PRC subsidiaries have complied with the relevant laws and regulations regarding the production and sale of medical devices in all material aspects, and each of the PRC subsidiaries has obtained the licenses required for their respective business operations in accordance with relevant regulations.
Furthermore, according to the 2021 Medical Device Regulation, enterprises that export medical devices should ensure that the exported medical devices meet the legal requirements of the importing country or region.
Pursuant to the Measures for the Administration of Registration and Record-filing of Medical Devices promulgated by the State Administration for Market Regulation (“SAMR”) on August 26, 2021 and came into effect on October 1, 2021, Class II and Class III medical devices are subject to product registration management. Class I medical devices are subject to product filing management. Record-filing parties of domestic Class I medical devices shall submit record-filing materials to the drug regulatory authorities at the cities with subordinate districts. Domestic Class II medical device shall be examined by the drug regulatory authorities of provinces, autonomous regions and centrally-administered municipalities which shall issue the Medical Device Registration Certificate upon approval after examination. Domestic Class III medical devices shall be examined by the NMPA which shall issue the Medical Device Registration Certificate upon approval after examination. The registration certificate for a medical device is valid for five years and the registrant shall apply to the original registration departments for renewal at least six months prior to its expiration date.
According to the Measures for the Administration of Registration and Record-filing of Medical Devices, the clinical evaluation shall be conducted for the registration or record-filing of medical devices, except for circumstances specified under any of the following circumstances: (i) the medical device has a clear working mechanism, finalized design and mature production process, and the marketed medical devices of the same kind have been used in clinical application for years (number unspecified) with no record of serious adverse events, and does not change the general purpose of the medical device; or (ii) any other circumstance where the safety and effectiveness of the medical device can be proved through non-clinical evaluation. On September 16, 2021, the NMPA issued its Announcement on Issuing Catalog of Medical Devices Exempted from Clinical Evaluation, which came into effect on October 1, 2021 and was amended on July 20, 2023.
108
Table of Contents
For certain Class III medical devices that are subject to clinical trials with high risk to the human body, approval from the NMPA is required before clinical trials. On September 14, 2020, the NMPA issued the Notice on the List of Class III Medical Devices Subject to Clinical Trial Approval (2020 Revision), which revised the original List of Class III Medical Devices Subject to Clinical Trial Approval and came into effect on September 14, 2020.
According to the Measures for the Administration of Registration and Filing of Medical Devices, the registrants of medical devices shall take the initiative to carry out post-marketing research, further confirm the safety, effectiveness and quality controllability of the medical devices and strengthen the continuous management of the medical devices on the market. In case of any substantial change of the designs, raw materials, production technologies, scopes of application and application methods, etc., of the registered medical device products of Class II or Class III, which may affect the safety or effectiveness of such medical devices, the registrants shall apply to the original registration departments for changing registration.
In addition, Measures for the Administration of Registration and Record-filing of Medical Devices provide details on other aspects such as product development and manufacturing, clinical evaluation, registration system verification, product registration, change of registration, renewal of registration, product filing, etc. It also states special registration procedures, such as the innovative product registration procedure, the priority registration procedure, the contingency registration procedure, etc.
Production and Quality Management of Medical Devices
Pursuant to the 2021 Medical Devices Regulation and the Administrative Measures of Production of Medical Devices (2017), promulgated by the CFDA, amended and effective from November 17, 2017, a producer of medical devices shall satisfy the following conditions: (i) possess production sites, environmental conditions, production equipment and professional technicians that are suitable for such medical device produced; (ii) possess organizations or professional examination staff and examination equipment that carry out quality examination for such medical device produced; (iii) formulate a management system which ensures the quality of such medical device; (iv) have capability of after-sale services suitable for such medical device produced; and (v) satisfy the requirements as prescribed in production R&D and production technique documents.
On March 10, 2022, the SAMR promulgated the Administrative Measures of Production of Medical Devices (2022) (SAMR Notice No.53 [2022]), which came into effective on May 1, 2022 and replace the Administrative Measures of Production of Medical Devices (2017). From May 1, 2022, new applications for medical device production and business activities shall go through licensing or filing according to the relevant provisions of the 2021 Medical Devices Regulation and the Administrative Measures of Production of Medical Devices (2022).
The enterprises engaging in the production of medical devices of Class I shall complete record-filing with NMPA at city level where such entity is located; and an entity engaging in the production of medical devices of Class II or III shall obtain a production permit of medical devices from NMPA at provincial level. The Permit for the Medical Devices Production is valid for 5 years and the registrant shall apply to the original departments that issued such permit for renewal at least 6 months prior to its expiration date.
The Production Measures and the Standards on Production and Quality Management of Medical Devices (the “Standards on Production and Quality Management”) which was promulgated on December 29, 2014 and came into effect on March 1, 2015, stipulates that an enterprise engaging in the production of medical devices shall establish and effectively maintain a quality control system in accordance with the requirements of the Standards on Production and Quality Management. The enterprise engaging in the production of medical devices shall regularly conduct comprehensive self-inspection on the operation of quality management system in accordance with the requirements of the Standards on Production and Quality Management and submit a self-inspection report to the provincial branches of the NMPA or the local branches at the prefectural city level before the end of every year. The enterprise shall establish its procurement control procedures and assess its suppliers by establishing an examination system to ensure the purchased products are in compliance with the statutory requirements. The enterprise shall record the procurement, production and inspection of raw materials. Such records shall be true, accurate, complete and traceable. The enterprise shall apply risk management to the whole process of design and development, production, sales and after-sale services. The measures being adopted shall be applicable to risks associated with the related products.
109
Table of Contents
Pursuant to The Notice of Four Guidelines including On-site Inspection Guidelines for the Standards on Production and Quality Management of Medical Devices promulgated on September 25, 2015 and came into effect on the same date, during the course of on-site verification of the registration of medical devices and on-site inspection of production permit (including changing production permit), the inspection team shall, in accordance with the guidelines, issue recommended conclusions for on-site inspections, which shall be divided into “Passed,” “Failed” or “Reassessment after rectification.” During the supervision and inspection, if it is found that the requirements of the key items or ordinary items that may have direct impact on product quality are not satisfied, the enterprise shall suspend production and go through rectification.
If found that the requirements of the ordinary items are not satisfied, and it does not directly affect product quality, the enterprise shall rectify in a prescribed time. The regulatory authorities shall examine and verify the recommended conclusions and on-site inspection materials submitted by the inspection group and issue the final inspection results.
Clinical trials on medical devices shall be conducted by organizations that possess relevant qualifications as required by the Good Clinical Practice for Medical Devices Trial. According to the Good Clinical Practice for Medical Devices, which became effective on June 1, 2016, the Good Clinical Practice includes full procedures of clinical trial of medical devices, including, among others, the protocol design, conduct, monitoring, verification, inspection, and data collection, recording, analysis and conclusion and reporting procedure of a clinical trial.
Prior to a clinical trial, the applicant shall complete the preclinical study of the investigational medical devices, including product design (structural composition, working principle and mechanism of action, intended use and scope of application, applicable technical requirements) and quality inspection, animal trial and risk analysis, and the result shall support the clinical trial, and the applicant also shall enter into an agreement in writing with the clinical trial organization and researchers regarding matters such as the design of the trial, quality control of the trial, division of responsibility in the trial.
Quality inspection results shall include a self-inspection report and a registration inspection report valid within one year and issued by an eligible inspection institution. Clinical trial of medical devices shall be conducted in two or more clinical trial institutions of medical devices. Before subjects participate in a clinical trial, investigators shall provide the subjects or guardians of persons without or with limited capacity for civil conduct with extensive details about the clinical trial, including known and foreseeable risks and possible adverse events. After full and detailed explanation, the subjects or their guardians and investigators shall sign the informed consent forms with their names and dates. The clinical trial shall be subject to the approval of the ethics committee of clinical trial institution of medical devices. The medical devices included in the List of Class III Medical Devices Requiring Clinical Trial Approval shall also be approved by the NMPA. Before a clinical trial, the applicant shall file with the supervision and administration departments of the provinces, autonomous regions or municipalities where it is located.
On March 30, 2022, the NMPA promulgated the Notice of Releasing Good Clinical Practice for Medical Devices (NMPA Notice No.21 [2022]), which came into effective on May 1, 2022 and replace the Good Clinical Practice for Medical Devices (2016). According to the NMPA Notice No.21 [2022], within the territory of mainland China, the relevant activities of clinical trials of medical devices for the purpose of applying for registration (including in vitro diagnostic reagents) shall comply with the Good Clinical Practice for Medical Devices. The Good Clinical Practice for Medical Devices covers the whole process of medical device clinical trials, including the design, implementation, supervision, inspection, data collection, data recording, data preservation, data analysis, data summary and data reporting of medical device clinical trials.
Laws and Regulations Relating to Medical Devices Operation
Pursuant to the 2021 Medical Device Regulation and the Measures for the Supervision and Administration of Medical Devices Operation, promulgated on July 30, 2014, effective on October 1, 2014 and amended on November 17, 2017, an enterprise engaging in the operation of medical devices shall have business premises and storage conditions suitable for the operation scale and scope, and shall have the quality management system and quality management institution or quality management personnel suitable for the medical devices it operates. An enterprise engaged in the operation of Class II medical devices shall make filing with the local food and drug supervision and administration department at the level of city with districts and provide proofing materials for its satisfying the relevant conditions of engaging in the operation of medical devices, while an enterprise engaged in the operation of Class III medical devices shall
110
Table of Contents
apply for an operation permit to the local food and drug supervision and administration department at the level of city with districts and provide proofing materials for its satisfying the relevant conditions of engaging in the operation of such medical devices.
The food and drug supervision and administration department which accepts operation permit application shall grant the Permit for Medical Devices Operation if the enterprise meets the prescribed requirements. The Permit for Medical Devices Operation is valid for five years and may be renewed pursuant to the relevant regulations. An enterprise engaging in the operation of medical devices shall not operate or use any medical device that has not been legally registered, has no qualification certificate, or is outdated, invalid or eliminated.
The Measures for the Supervision and Administration of Medical Devices Operation was amended on March 10, 2022 by the SAMR and took effective on May 1, 2022, according to which there’s no need for a registered holder or record holder of medical devices selling its registered or filed medical devices in its domicile or production site to have the operation permit or filing for its operation of medical devices but shall meet the specified operating conditions, while it still need to have the operation permit or filing if it store and sell medical devices in other places.
Tender Processes for Medical Devices
According to the Notice on Further Strengthening the Administration of Centralized Procurement of Medical Devices issued on June 21, 2007, all non-profit medical institutions under all levels of government and state-owned enterprises from different industries shall participate in the centralized procurement of medical devices.
The centralized procurement of medical devices must follow the basic principles of openness, fairness, impartiality and good faith, and the procurement method is mainly based on public bid invitation. All localities should formulate and improve centralized procurement procedures and implementation measures for medical devices in accordance with relevant laws and regulations and in light of local actual conditions, to ensure fair and open procedures. Local authorities shall strictly implement the advance announcement of bidding and procurement information, the announcement of bid evaluation methods and the announcement of bidding results. After the results of the bidding are confirmed, the medical institution should be organized to sign the contract in time and supervise the implementation.
According to the Government Procurement Law of the People’s Republic of China (the “Government Procurement Law”) promulgated by SCNPC on June 29, 2002, and last amended on August 31, 2014, and the Regulations for Implementation of the Government Procurement Law of the People’s Republic of China (the “Implementation Regulations”) promulgated by State Council on January 30, 2015 and came into effect on March 1, 2015, the term “government procurement” means the use of fiscal funds by all levels of State authorities, institutions and social organizations to procure goods, projects and services that fall within the catalogue for centralized procurement formulated in accordance with the law or that are above the procurement limits. Government procurement shall combine centralized and decentralized procurement. The scope of centralized procurement shall be defined by the catalogue for centralized procurement promulgated by the people’s governments at provincial level and above. The catalogue for centralized procurement for government procurement items that fall within the central government budget shall be determined and promulgated by the State Council. The catalogue for centralized procurement for government procurement items that fall within the local government budget shall be determined and promulgated by the people’s government or its authorized institution of provinces, autonomous regions and municipalities directly under the central government. Government procurement items that are included in the catalogue for centralized procurement shall be subject to centralized procurement. The limits for government procurement for government procurement items that fall within the central government budget shall be determined and promulgated by the State Council. The limits for government procurement for government procurement items that fall within local government budgets shall be determined and promulgated by the people’s government or its authorized institution of provinces, autonomous regions and municipalities directly under the central government. Government procurement contracts shall be in writing. A supplier shall be prosecuted for legal liability if it sub-contracts the government procurement contract.
In the event that buyers procure goods or services whose budget amount is more than the standard of public bidding amount, the public bidding must be adopted.
As of the date of this prospectus, we believe the PRC subsidiaries have been in full compliance with such law in all material respects when the PRC subsidiaries have participated in bidding procedures and, as of the date of this prospectus, none have received any notifications with any non-incompliance of the related rules.
111
Table of Contents
Regulations Relating to Advertisements of Medical Devices
The SAMR promulgated the Interim Measures for the Administration of the Examination and Administration of Drugs, Medical Devices, Health Foods, and Formula Foods for Special Medical Purposes (the “Examination Interim Measures”) on December 24, 2019, which came into effect on March 1, 2020. The Examination Interim Measures stipulates that the advertisements for medical devices shall not be released without being reviewed and the contents of a medical device advertisement shall be based on the contents of the registration certificate or filing certificate approved by the drug administrations, or the registered or filed product instructions. Where the medical device advertisement involves the name, scope of application, functional mechanism or structure or composition, etc. of the medical device, the scopes of the registration certificate or filing certificate, or registered or filed product instruction shall not be exceeded. The validity period of the advertisement approval number for drugs, medical devices, health food and formula food for special medical purposes shall be consistent with the shortest validity period of the product registration certificate, filing certificate or production license. If no valid period is prescribed in the product registration certificate, filing certificate or production license, the valid period of the advertisement approval number shall be two years.
Medical Device Product Export
According to the Regulations on Application for Export Certificate of Medical Devices promulgated on January 6, 1996, the State Administration of Medicine (now known as the NMPA) shall examine the safety and legality of medical device products produced by domestic enterprises, and issue export certificates in accordance with international practice to prove that the products have obtained lawful production licenses within the territory of China.
Pursuant to the Regulations on the Administration of Export Sales Certificates of Medical Devices promulgated on June 1, 2015 and came into effect on September 1, 2015, if the registration certificate for a medical device and production permit for a medical device have been obtained in China, or the medical device registration and production filing have been completed, the food and drug supervision and administration department may issue a Medical Device Product Export Sales Certificate to the relevant manufacturing enterprise. The validity term of the Medical Device Product Export Sales Certificate should not exceed the earliest deadline for the various documents submitted by the enterprise in the application materials, and the maximum validity term shall not exceed two years.
According to the Customs Law of the PRC (Amended in 2021) (“Customs Law”) which was passed by the Standing Committee of the National People’s Congress (“SCNPC”) on April 29, 2021, where a consignee or consignor of importing or exporting goods or a customs clearing enterprise handles customs declaration procedures, they shall be subject to registration by customs in accordance with law. Customs clearing personnel shall obtain the occupational qualifications for customs clearances in accordance with law.
According to the Provisions of the Customs of the PRC on the Administration of Registration of Customs Declaration Entities (Revised in 2018), which was promulgated by the General Administration of Customs on May 29, 2018 and became effective as of July 1, 2018, registration of declaring entities shall be divided into the registration of declaring enterprises, for which approval from the relevant competent authority directly under the General Administration of Customs or the authorized customs affiliate shall be the pre-condition to undertake the declaration procedures at the customs and the registration of consignees or consignors of imported or exported goods, for which no additional approvals need to be obtained. On November 19, 2021, the Provisions of the PRC on the Administration of the Recordation of Customs Declaration Entities were promulgated and became effective on January 1, 2022. The Provisions of the Customs of the PRC on the Administration of Registration of Customs Declaration Entities (Revised in 2018) was repealed simultaneously. According to the Administration of the Recordation of Customs Declaration Entities, the registration of customs declaring entities has been completely changed to recordation, and the recordation is implemented for both customs declaration entities and the consignor or consignee of imported and exported goods. As of the date of the prospectus, the Certificate of the Customs of the People’s Republic of China on Registration of Customs Declaration Entity, certifying the PRC subsidiaries’ export activities, is valid.
112
Table of Contents
Medical Device Recalls
Pursuant to the Administrative Measures for Medical Device Recalls, which was promulgated on January 25, 2017 and came into effect on May 1, 2017, in accordance with the severity of harm, medical device recalls are divided into: (i) Class I recall, where the circumstances leading to the recall may cause or have caused serious health hazards; (ii) Class II recall, where the circumstances leading to the recall may cause or have caused temporary or reversible health hazards; or (iii) Class III recall where, the circumstances leading to the recall are not likely to cause harm.
Medical device manufacturers shall determine the recall class based on the specific situation and properly design and implement the recall plan based on the recall class and the sale and use of the medical devices. In terms of Class I recall, the recall notice shall be published on the NMPA website and major media. In terms of Class II and Class III recalls, the recall notice shall be published on the website of the food and drug administrative authority of the provinces, autonomous regions or municipalities.
Online Sales of Medical Device
According to the Administration and Supervision Measures of Online Sales of Medical Devices, which was promulgated on December 20, 2017 and came into effect on March 1, 2018, enterprises engaged in online sales of medical devices must be medical device manufacture and operation enterprises with medical devices production licenses or operation licenses or enterprises which have been filed for record in accordance with laws and regulations, and shall carry out online sales activities of medical devices through their own website or the third-party platform of online medical devices transaction services. The enterprise engaging in online sales of medical devices through its own website shall obtain an Internet Drug Information Services Qualification License according to the law and have the appropriate office space and technical conditions for data backup and failure recovery in line with the business scale. The third-party platform provider shall obtain an Internet Drug Information Services Qualification License in accordance with the law, have the appropriate office space and technical conditions for data backup and failure recovery in line with the business scale, and set up a special medical device network quality and safety management organization or equip medical device quality and safety management personnel.
Environmental Protection
Pursuant to the Environmental Protection Law of the PRC (Amended in 2014) promulgated on April 24, 2014 and became effective on January 1, 2015, the waste discharge licensing system has been implemented in the PRC and entities that discharge medical sewage to water bodies directly or indirectly shall obtain a waste discharge license. Furthermore, installations for the prevention and control of pollution at a construction project must be designed, built and commissioned together with the principal part of the project. Pursuant to the Environmental Impact Assessment Law of the PRC (Amended in 2018) promulgated on October 28, 2002, become effective on September 1, 2003 and last amended on December 29, 2018, the State implements administration by classification on the environmental impact of construction projects according to the level of impact on the environment. The project facility owner shall prepare an environmental impact report, or an environmental impact form or complete an environmental impact registration form (the “Environmental Impact Assessment Documents”) for reporting and filing purpose. If the Environmental Impact Assessment Documents of a construction project have not been reviewed by the approving authority in accordance with the law or have not been granted approval after the review, the project facility owner is prohibited from commencing construction works. For a construction project for which an Environmental Impact Statement is prepared, the project facility may go into production or be delivered for use only after it undergoes an inspection and acceptance has been made by the facility owner.
Pursuant to the Administrative Regulations on Environmental Protection in Construction Projects (Revised in 2017) promulgated on November 29, 1998, and last amended on July 16, 2017, upon the completion of construction, the project facility owner shall inspect the production lines to determine if they comply with relevant regulations of environmental protection and make Inspection and Acceptance Reports of Environmental Protection. Failure to inspect the production lines to determine if they comply with relevant regulations of environmental protection and make Inspection and Acceptance Reports of Environmental Protection will result in an order to complete relevant procedures, and a penalty between RMB0.2 million and RMB1 million, will be imposed by local authorities. If the project facility owner fails to complete relevant procedures within the stipulated period, the local authorities will impose a penalty between RMB1 million and RMB2 million. Furthermore, if material environmental pollution or ecological damage is caused by such failure, the project facility owner will be ordered to stop production or stop
113
Table of Contents
using the construction project by the PRC regulatory authorities. Pursuant to the Interim Measures for Environmental Protection Acceptance of Completion of Construction Projects promulgated on November 20, 2017, and become effective on the same day, if the project facility owner fails to inspect construction projects and make Inspection and Acceptance Reports of Environmental Protection, its non-compliance will be recorded in Credibility Archive and will be disclosed to the public.
Pursuant to the Law of the PRC on Prevention and Control of Environmental Pollution Caused by Solid Wastes (Revised in 2020), promulgated on April 29, 2020 and taken effect on September 1, 2020, all entities and individuals shall take measures to reduce the output of solid waste, promote the comprehensive utilization of solid waste, and reduce the harmfulness of solid waste. Each enterprise generating industrial solid waste shall obtain a discharge permit, establish a sound accountability system for the prevention and control of environmental pollution caused by industrial solid waste in the whole process of generation, collection, storage, transportation, utilization and treatment, set up a management ledger for industrial solid waste to faithfully keep records on the sorts, quantity, whereabouts, storage, utilization and treatment, etc., of industrial solid waste generated, so as to realize the traceability and querying of industrial solid waste, and take measures for the prevention and control of environmental pollution caused by industrial solid waste. Furthermore, the construction of projects which discharge solid waste and the construction of project for storage, use and treatment of solid waste shall be carried out upon the appraisal regarding their effects on environment and in compliance with the relevant state regulations concerning the management of environmental protection in respect of construction projects. The necessary supporting facilities for the prevention and control of environmental pollution caused by solid wastes as specified in the environmental impact assessment documents of the construction project shall be designed, constructed and put into operation simultaneously with the major construction works of the construction project. No construction projects shall be permitted to be put into operation or to use before its facilities for the prevention and control of environmental pollution caused by solid wastes have been inspected and accepted by the competent department of environmental protection that examined and accepted the environmental impact assessment documents. On December 20, 2019, the Catalogue for Classified Administration of Sewage Discharge Permits for Fixed Pollution Sources was promulgated, according to which the state shall implement pollutant discharge registration administration with respect to entities that discharge pollutants in small quantities or discharge pollutants that have little impact on the environment.
Pursuant to the Law of the PRC on Prevention and Treatment of Water Pollution (Amended in 2017) promulgated on June 27, 2017 and taken effect on January 1, 2018, the environmental protection department of the State Council formulates national waste discharge standards. Enterprises that discharge waste into water shall pay a treatment fee. Each of the local environmental protection bureaus is authorized to regulate water pollution within each of their respective jurisdictions by formulating more specific local standards, and may impose penalties for violation, including suspending operations. The water pollutants discharged into the facilities for central treatment of urban sewage shall conform to the national or local standards for the discharge of water pollutants. Furthermore, the environmental impact assessment shall be conducted on new construction, reconstruction and construction expansion projects or other installations on water which directly or indirectly discharge pollutants into the water according to law. The water pollution prevention and treatment facilities of a construction project must be designed, constructed and put into operation simultaneously with the major construction works of the said construction project. The water pollution prevention and treatment facilities shall comply with the requirements of approved or filed environmental impact assessment documents.
Fire Prevention Management
According to the Fire Prevention Law of the PRC promulgated on April 29, 1998, which was amended on October 28, 2008, April 23, 2019, and April 29, 2021, and the Interim Provisions on the Administration of Fire Protection Design Review and Acceptance of Construction Projects promulgated on April 1, 2020 and amended on October 30, 2023, the design and construction of fire prevention features for construction projects shall conform to state’s fire prevention technical standards. Project owners, design entities, construction entities, project supervision entities, etc., shall be responsible for the fire protection design and the quality of the fire protection for construction of the projects. With respect to construction projects, such projects are subject to inspection and acceptance for fire protection. Project facility owners of special construction projects shall file applications with competent authorities for inspection and acceptance for fire protection. These special construction projects include: (1) sports stadiums, halls, showrooms of public exhibition halls or museums, with a gross floor area of more than 20,000 square meters; (2) terminals of civil airports, waiting rooms of passenger transport stations, or waiting rooms of passenger transport docks, with a gross floor area of more than 15,000 square meters; (3) hotels, restaurants, shopping malls or farmers markets,
114
Table of Contents
with a gross floor area of more than 10,000 square meters; (4) movie theaters, reading rooms of public libraries, for-profit indoor fitness or leisure venues, outpatient buildings of hospitals, teaching buildings of universities, libraries, canteens, production and processing workshops of labor-intensive enterprises, temples or churches, with a gross floor area of more than 2,500 square meters; (5) children’s rooms, children’s game rooms or other indoor activity spaces of nurseries or a kindergartens, ward buildings of nursing home, welfare centers, hospitals or sanitariums, teaching buildings, libraries or cafeterias of primary or secondary schools, collective dormitories of schools, or staff collective dormitories of labor-intensive enterprises, with a gross floor area of more than 1,000 square meters; (6) song and dance halls, video halls, projection rooms, Karaoke halls, nightclubs, recreation halls, sauna bathrooms, Internet cafes, bars, restaurants, teahouses or cafes with entertainment functions, with a gross floor area of more than 500 square meters; (7) Class I high-rise residential buildings as set forth in the national technical standards of fire protection for construction projects; (8) urban rail transports, tunnels, large-scale power generation, transformation or distribution projects; (9) plants for production, warehouses for storage, or special stations or docks for loading and unloading of inflammable and explosive hazardous materials, or filling stations, supply stations or pressure regulation stations of flammable and explosive gases and liquid; (10) state authority office buildings, electric power dispatching buildings, telecommunication buildings, postal buildings, disaster prevention commanding and dispatching buildings, radio and television buildings or archives buildings; (11) construction projects under any of the circumstances set forth in examples (1) to (6) mentioned above; or (12) public buildings other than examples (10) and (11), and the gross floor area of a single building is more than 40,000 square meters or the height of the building is more than 50 meters.
Apart from these special construction projects mentioned above, for other construction projects, such as Hangzhou Shanyou’s production lines, a project facility owner shall prepare the Inspection and Acceptance Reports of Fire Protection for the records of the competent authorities, and such authorities shall conduct a random inspection thereof.
If a project facility owner places into service a construction project which was required to undergo a fire protection inspection and acceptance and prepare an Inspection and Acceptance Report of Fire Protection, but has not undergone such inspection and acceptance and has failed to prepare such Inspection and Acceptance Report of Fire Protection, or has failed the inspection and acceptance, or failed to suspend the use of construction project for which such project facility owner prepared the Inspection and Acceptance Report of Fire Protection for the records of the competent authorities but is later found to be unqualified in a random inspection, such project facility owner will be ordered to stop using the affected construction project, stop production or business operations and a fine of between RMB30,000 and RMB300,000 may be imposed by competent departments. If a project facility owner has undergone a fire protection inspection and acceptance and has already prepared an Inspection and Acceptance Report of Fire Protection but fails to submit the report to the competent authorities for the records, such project facility owner will be subject to a fine of RMB5,000.
As of the date of this prospectus, Hangzhou Shanyou, the PRC subsidiary that operates all production lines, has not prepared the Inspection and Acceptance Reports of Fire Protection for the records of the competent authorities, which may result in it being ordered to stop the use of its production lines and being subject to a fine of between RMB30,000 and RMB300,000. Since all of its products are manufactured by operation of such production lines, any such development could materially and adversely affect its and our business, financial condition and results of operations. If Hangzhou Shanyou conducts the inspection and acceptance and prepares the Inspection and Acceptance Reports of Fire Protection but does not submit them to the competent authorities for the records, it will be subject to a fine of RMB5,000. See “Risk Factors — Risks Related to the PRC subsidiaries’ Business and Industry — The PRC subsidiaries are subject to a variety of fire protection laws that could be costly for them to comply with, and they could incur liability if they fail to comply with such laws, which could adversely affect the Group as a whole” on page 42.
Construction Management
According to the Administrative Measures for Construction Permits of Building Projects promulgated by the Ministry of Housing and Urban-Rural Development on June 25, 2014, and last amended on March 30, 2021, for construction and decoration works in respect of various housing construction and auxiliary facilities thereof, installation of circuits, pipelines and equipment, as well as construction of infrastructural works for cities and towns, the construction enterprise shall apply for construction permits from the competent department in accordance with the regulations of the Administrative Measures for urban-rural development of housing of the local people’s government at or above county level where the construction is located prior to the commencement of works. It is not necessary to apply for construction permits for construction works with an investment amount less than RMB0.3 million or of which the gross floor area is less than 300 square meters. The administrative authority in charge of housing and urban-rural
115
Table of Contents
development of the people’s government of a province, autonomous region or municipality directly under the Central Government may, in accordance with the specific circumstances prevailing in their respective regions, readjust these limits and notify the department under the State Council responsible for construction for its records. Those who have not obtained the construction permits shall be ordered by the license-issuing authority which has jurisdiction to stop the construction, to correct any such lapse within a time limit, and pay a fine of between 1% to 2% of the contract price of the construction project.
According to the Regulations on the Quality Management of Construction Engineering promulgated by the State Council on April 23, 2019, a construction project owner shall, within 15 days from the date of the completion and acceptance inspection of the construction project, submit the inspection and acceptance report of the construction project and the approval documents issued by the planning, public security, fire control, environmental protection and other departments to the construction administrative department or other relevant departments for the record. If it fails to do so, it shall be ordered to make corrections and pay a fine of between RMB0.2 million to RMB0.5 million. Furthermore, where the project facility is placed into service without being subject to completion inspection and acceptance, the construction owner shall be ordered to make corrections and pay a fine of between 2% to 4% of the contract price of the project. Where there is any loss resulting therefrom, such construction owner shall be liable to compensate for any such loss.
As of the date of this prospectus, as to the production lines of Hangzhou Shanyou, which were placed into service without having been subject to completion inspection and acceptance, it did not obtain a construction license prior to the commencement of construction nor undergone the completion inspection and acceptance nor did it prepare the Inspection and Acceptance Reports of Construction for the records of the competent authorities, therefore, Hangzhou Shanyou could be fined (i) an amount equal to 1% to 2% of the contract price of the production lines construction (approximately $16,500 to $33,000),for its failure to obtain a construction license prior to the commencement of construction, (ii) plus a fine of between RMB0.2 million to RMB0.5 million (approximately $28,000 to $70,000) for its failure to prepare the Inspection and Acceptance Reports of Construction for the records of the competent authorities and make adequate rectification, and (iii) a fine of between 2% to 4% of the contract price of the production lines construction (approximately $33,000 to $66,100), for its failure to complete inspection and acceptance before placing the production lines into service. As a result, the aggregate amount of potential fine could be between $77,500 to $169,100.
Regulation on Foreign Investment Restrictions
Investment activities in mainland China by foreign investors are principally governed by the Catalog of Industries for Encouraging Foreign Investment (2022 Edition), or the Catalog, as promulgated by the Ministry of Commerce of the People’s Republic of China (“MOFCOM”), and the National Development and Reform Commission (“NDRC”) on October 26, 2022, and the Special Administrative Measures for Access of Foreign Investment (2021 Edition), or the Negative List (2021), as promulgated on December 27, 2021. According to the Negative List (2021), our businesses operated in the PRC do not fall into the restricted or prohibited categories.
In addition, a foreign-invested enterprise in the PRC is required to comply with other regulations on its incorporation, operation and changes. On March 15, 2019, the National People’s Congress (“NPC”) adopted the Foreign Investment Law (the “FIL”), which became effective on January 1, 2020. Pursuant to the FIL, PRC will grant national treatment to foreign-invested entities, except for those foreign-invested entities that operate in industries that fall within “restricted” or “prohibited” categories as prescribed in the Negative List (2021) to be released or approved by the State Council.
On December 26, 2019, the State Council promulgated the Implementation Rules to the Foreign Investment Law, which became effective on January 1, 2020. The implementation rules further clarify that the state encourages and promotes foreign investment, protects the lawful rights and interests of foreign investors, regulates foreign investment administration, continues to optimize a foreign investment environment, and advances a higher-level opening. On December 30, 2019, the MOFCOM and SAMR jointly promulgated the Measures for Information Reporting on Foreign Investment, which became effective on January 1, 2020. Pursuant to the Measures for Information Reporting on Foreign Investment, where a foreign investor carries out investment activities in mainland China, directly or indirectly, the foreign investor or the foreign-invested enterprise shall submit the investment information to the competent commerce department.
116
Table of Contents
On February 17, 2023, the CSRC issued the New Administrative Rules Regarding Overseas Listings, which came into force on March 31, 2023. The New Administrative Rules Regarding Overseas Listings refine the regulatory system for domestic company’s overseas offering and listing by subjecting both direct and indirect overseas offering and listing activities to the filing-based administration, and clearly defines the circumstances where provisions for direct and indirect overseas offering and listing apply and relevant regulatory requirements.
According to the New Administrative Rules Regarding Overseas Listings, among other things, a domestic company in the PRC that seeks to offer and list securities on overseas markets shall fulfill the filing procedures with the CSRC as per requirement of the Trial Administrative Measures. Where a domestic company seeks to directly offer and list securities on overseas markets, the issuer shall file with the CSRC. If an issuer offers securities on the same overseas market where it has previously offered and listed securities subsequently, filings shall be made with the CSRC within 3 working days after the offering is completed. Upon occurrence of any material event, such as change of control, investigations or sanctions imposed by overseas securities regulatory agencies or other relevant competent authorities, change of listing status or transfer of listing segment, or voluntary or mandatory delisting, after an issuer has offered and listed securities on an overseas market, the issuer shall submit a report thereof to CSRC within three working days after the occurrence and public disclosure of such event. Further, an overseas securities company that serves as a sponsor or lead underwriter for overseas securities offering and listing by domestic companies shall file with the CSRC within 10 working days after signing its first engagement agreement for such business, and submit to the CSRC, no later than January 31 each year, an annual report of its business activities in the previous year associated with overseas securities offering and listing by domestic companies. If an overseas securities company has entered into engagement agreements before the effectuation of the Trial Administrative Measures and is serving in practice as a sponsor or lead underwriter for overseas securities offering and listing by domestic companies, it shall file with the CSRC within 30 working days after the Trial Administrative Measures take effect.
Under the New Administrative Rules Regarding Overseas Listings, a domestic company is prohibited from overseas offering and listing if any of the following circumstances is involved: (1) where such securities offering and listing is explicitly prohibited by provisions in laws, administrative regulations and relevant state rules; (2) where the intended securities offering and listing may endanger national security as reviewed and determined by competent authorities under the State Council in accordance with laws; (3) where the domestic company intending to make the securities offering and listing, or its controlling shareholders and the actual controller, have committed crimes such as corruption, bribery, embezzlement, misappropriation of property or undermining the order of the socialist market economy during the latest three years; (4) where the domestic company intending to make the securities offering and listing is suspected of committing crimes or major violations of laws and regulations, and is under investigation according to law, and no conclusion has yet been made thereof; and (5) where there are material ownership disputes over equity held by the domestic company’s controlling shareholder or by other shareholders that are controlled by the controlling shareholder and/or actual controller. Moreover, a domestic company that seeks to offer and list securities on overseas markets shall abide by certain other regulatory requirements as set out in the New Administrative Rules Regarding Overseas Listings, including, without limitation to, compliance with laws of national secrecy, foreign investment, cybersecurity, data security, cross-border investment and financing, foreign exchange, and other laws and relevant provisions.
The Trial Administrative Measures also provides that if the issuer both meets the following criteria, the overseas securities offering and listing conducted by such issuer will be deemed as indirect overseas offering by PRC domestic companies: (i) 50% or more of any of the issuer’s operating revenue, total profit, total assets or net assets as documented in its audited consolidated financial statements for the most recent fiscal year is accounted for by domestic companies; and (ii) the main parts of the issuer’s business activities are conducted in mainland China, or its main place(s) of business are located in mainland China, or the majority of senior management staff in charge of its business operations and management are PRC citizens or have their usual place(s) of residence located in mainland China. Where an issuer submits an application for initial public offering to competent overseas regulators, such issuer must file with the CSRC within three working days after such application is submitted. The Trial Administrative Measures also require subsequent reports to be filed with the CSRC on material events, such as change of control or voluntary or forced delisting of the issuer(s) who have completed overseas offerings and listings.
Pursuant to the New Administrative Rules Regarding Overseas Listings, the CSRC will conclude the filing procedures and publish the filing results on the CSRC website within 20 working days after receiving the filing documents if the filing documents are complete and in compliance with stipulated requirements. However, during the filing process, the CSRC may request the Company to supply additional documents or may consult with competent authorities, the time
117
Table of Contents
for which will not be counted in the 20 working days. We are required to file with the CSRC within three working days after any subsequent securities offering is completed, pursuant to the New Administrative Rules Regarding Overseas Listings.
According to the Trial Administrative Measures, if a domestic company fails to fulfill filing procedures, or offers and lists securities on an overseas market in violation of the measures, the CSRC shall order rectification, issue warnings to such domestic company, and impose a fine of between RMB1 million and RMB10 million. Directly liable persons-in-charge and other directly liable persons shall be warned and each imposed a fine of between RMB0.5 million and RMB5 million. Controlling shareholders and actual controllers of the domestic company that organize or instruct the aforementioned violations shall be imposed a fine of between RMB1 million and RMB10 million.
On February 24, 2023, the CSRC promulgated the Provisions on Strengthening Confidentiality and Archives Administration of Overseas Securities Offering and Listing by Domestic Companies (the “Confidentiality and Archives Administration Provisions”), which also became effective on March 31, 2023. According to the Confidentiality and Archives Administration Provisions, domestic companies that seek overseas offering and listing (either in direct or indirect means) and the securities companies and securities service (either incorporated domestically or overseas) providers that undertake relevant businesses shall institute a sound confidentiality and archives administration system, and take necessary measures to fulfill confidentiality and archives administration obligations. They shall not leak any state secret or working secret of government agencies, or harm national security and public interests. Therefore, a domestic company that plans to, either directly or through its overseas listed entity, publicly disclose or provide to relevant individuals or entities including securities companies, securities service providers and overseas regulators, any documents and materials that contain state secrets or working secrets of government agencies, shall first obtain approval from competent authorities according to laws, and file with the secrecy administrative department at the same level. The above-mentioned documents and materials that, if leaked, will be detrimental to national security or public interest, therefore, the domestic company shall strictly fulfill relevant procedures stipulated by applicable regulations.
Furthermore, the Confidentiality and Archives Administration Provisions stipulates that a domestic company that provides accounting archives or copies of accounting archives to any entities, including securities companies, securities service providers and overseas regulators and individuals, shall fulfill due procedures in compliance with applicable regulations. Working papers produced in mainland China by securities companies and securities service providers in the process of undertaking businesses related to overseas offering and listing by domestic companies shall be retained in mainland China. Where such documents need to be transferred or transmitted to areas outside of mainland China, relevant approval procedures stipulated by regulations shall be followed.
Regulation on Foreign Exchange Control
In 1996, China published The Foreign Currency Administration Regulations, and late on amended on January 14, 1997 and August 5, 2008. This Regulation has been the major one governing the foreign exchange activities in China. Under this Regulation, the Renminbi is convertible for foreign currency account items, including the distribution of dividends, interest payments and trade and service-related foreign exchange transactions. Conversion of Renminbi into foreign currency for capital account items, such as, loans, investment in securities and repatriation of investments, however, is subject to the registration of the Sate Administration of Foreign Exchange (“SAFE”) or its local counterparts.
Under the Regulation and relevant rules, foreign-invested enterprises may buy, sell and remit foreign currencies at banks authorized to conduct foreign exchange transactions for settlement of currency account transactions after providing valid commercial documents and, in the case of capital account item transactions, only after registration with the SAFE and, as the case may be, other relevant PRC government authorities as required by law.
According to the Overseas Investment Regulation which was issued in 2014, capital investments directed outside of mainland China by domestic or foreign-invested enterprises are also subject to restrictions, which include registration filing with Ministry of Commerce, even though the Notice on Further Improving and Adjusting the Foreign Exchange Management Policy for Capital Account (“No. 2 Notice”) passed in February of 2014 by SAFE has made domestic enterprises much easier releasing foreign currency overseas to foreign companies, including connected companies.
The conversion of Renminbi into foreign currencies, including U.S. dollars, has been based on rates set by the People’s Bank of China. On July 21, 2005, the PRC government changed its policy of pegging the value of the Renminbi to the U.S. dollar. Under the new policy, the Renminbi will be permitted to fluctuate within a band against a basket of certain foreign currencies. The PRC subsidiaries receive a significant portion of their revenue in Renminbi, which is not a
118
Table of Contents
freely convertible currency. Under the current structure, our income will be primarily derived from dividend payments from our subsidiaries in China. Even though the PRC subsidiaries may remit the income from China to anywhere deemed desirable, the fluctuation of exchange rate may be a disadvantage to us if Renminbi depreciates.
On January 26, 2017, the SAFE promulgated the Notice on Improving the Check of Authenticity and Compliance to Further Promote Foreign Exchange Control (the “SAFE Circular 3”), which stipulates several capital control measures with respect to the outbound remittance of profit from domestic entities to offshore entities, including (i) under the principle of genuine transaction, banks shall check board resolutions regarding profit distribution, the original version of tax filing records and audited financial statements; and (ii) domestic entities shall hold income to account for previous years’ losses before remitting the profits. Moreover, pursuant to the SAFE Circular 3, domestic entities shall make detailed explanations of the sources of capital and utilization arrangements, and provide board resolutions, contracts and other proof when completing the registration procedures in connection with an outbound investment.
On October 23, 2019, the SAFE promulgated the Circular of the State Administration of Foreign Exchange on Further Promoting Cross-border Trade and Investment Facilitation (the “SAFE Circular 28”), which expressly allows foreign-invested enterprises that do not have equity investments in their approved business scope to use their capital obtained from foreign exchange settlement to make domestic equity investments as long as there is a truthful investment and such investment is in compliance with the foreign investment-related laws and regulations.
On December 4, 2023, SAFE issued the Circular of the State Administration of Foreign Exchange on Further Deepening Reforms to Facilitate Cross-Border Trade and Investment, which stipulated that domestic equity transferors (including institutions and individuals) can directly remit to the capital project settlement account the equity transfer consideration funds paid in foreign currency by domestic entities, as well as the foreign exchange funds raised by domestic enterprises listed overseas. The funds in the capital project settlement account can be independently settled and utilized.
Regulation on Foreign Exchange Registration of Offshore Investment by PRC Residents
In October of 2005, SAFE promulgated a Notification known as “Notification 75,” in which SAFE requires PRC residents to register their direct establishment or indirect control of an offshore entity (referred to in Notification as “special purpose vehicle.”), where such offshore entity was established for the purpose of overseas financing, provided that PRC residents contribute their legally owned assets or equity into such entity. In July of 2014, this Notification was replaced by Notification 37, “Notification on Relevant Issues Concerning Foreign Exchange Control on Domestic Residents’ Offshore Investment and Financing and Returning Investment through Special Purpose Vehicles,” which expanded SAFE oversight scope to include overseas investment registration as well. Meanwhile, Notification 37 also covers more areas such as PRC residents paying capital contribution with overseas assets or equity. Furthermore, Notification 37 requires amendment to the registration where any significant changes with respect to the special purpose vehicle capitalization or structure of the PRC resident itself (such as capital increase, capital reduction, share transfer or exchange, merger or spin off). On February 13, 2015, the SAFE promulgated a Notice on Further Simplifying and Improving Foreign Exchange Administration Policy on Direct Investment, or Notice 13, which became effective on June 1, 2015 and was amended on December 30, 2019. Under Notice 13, applications for foreign exchange registration of inbound foreign direct investments and outbound overseas direct investments, including those required under Notification 37, will be filed with qualified banks instead of SAFE. The qualified banks will directly examine the applications and accept registrations under the supervision of SAFE.
Regulation on Dividend Distributions
The PRC subsidiary, WFOE, is wholly foreign-owned enterprises under the PRC law. The principal regulations governing the distribution of dividends paid by WFOE include Corporate Law (1993) as lastly amended in December 2023, which will be effective on July 1, 2024, the Foreign Investment Law and its Implementing Regulations, and the Enterprise Income Tax Law (2007) as lastly amended in 2018 and its Implementation Regulations (2007) as lastly amended in 2019.
Under these requirements, foreign-invested enterprises may pay dividends only out of their accumulated profit, if any, as determined in accordance with PRC accounting standards and regulations. A PRC company is required to allocate at least 10% of their respective accumulated after-tax profits each year, if any, to fund certain capital reserve funds
119
Table of Contents
until the aggregate amount of these reserve funds have reached 50% of the registered capital of the enterprises. A PRC company is not permitted to distribute any profits until any losses from prior fiscal years have been offset. Profits retained from prior fiscal years may be distributed together with distributable profits from the current fiscal year.
On March 16, 2007, the NPC enacted the Enterprise Income Tax Law, and on December 6, 2007, the State Council issued the Implementation Regulations on the Enterprise Income Tax Law, both of which became effective on January 1, 2008. The Enterprise Income Tax Law was lately amended on December 29, 2018 and the Implementation Regulations on the Enterprise Income Tax Law was lately amended on April 23, 2019. Under this law and its implementation regulations, dividends payable by a foreign-invested enterprise in the PRC to its foreign investor who is a non-resident enterprise will be subject to a 10% (5% for Hong Kong residents) withholding tax, unless any such foreign investor’s jurisdiction of incorporation has a tax treaty with the PRC that provides for a lower withholding tax rate.
M&A Rules and Regulation on Overseas Listings
On August 8, 2006, six PRC regulatory agencies, Ministry of Commerce, the State Assets Supervision and Administration Commission, the State Administration for Taxation, the State Administration for Industry and Commerce, CSRC and SAFE, jointly adopted the Regulation on Mergers and Acquisitions of Domestic Enterprises by Foreign Investors, or so called the M&A Rules and amended it on June 22, 2009. The M&A Rules purport, among other things, to require that offshore SPVs that are controlled by PRC entities or individuals and that have been formed for overseas listing purposes through acquisitions of PRC domestic interests held by such PRC entities or individuals, obtain the approval of the CSRC prior to publicly listing their securities on an overseas stock exchange. After the FIL and its implementation regulations became effective on January 1, 2020, the provisions of the M&A Rules remain effective to the extent they are not inconsistent with the FIL and its implementation regulations.
Furthermore, on July 6, 2021, the General Office of the Central Committee of the Communist Party of China and the General Office of the State Council jointly promulgated the Opinions on Strictly Cracking Down on Illegal Securities Activities in Accordance with the Law, pursuant to which Chinese regulators are required to accelerate rulemaking related to the overseas issuance and listing of securities, and update the existing laws and regulations related to data security, cross-border data flow, and management of confidential information.
Following the promulgation of the Cybersecurity Law and Data Security Law, on February 17, 2023, the CSRC issued the New Administrative Rules Regarding Overseas Listings, which came into force on March 31, 2023. The New Administrative Rules Regarding Overseas Listings provided certain examination and filing mechanisms with respect to a Chinese domestic enterprise’s offering of securities overseas.
As there are still uncertainties regarding the interpretation and implementation of such regulatory guidance in the future, we cannot assure you that we or the PRC subsidiaries will be able to comply with new regulatory requirements relating to our future overseas capital-raising activities and we or the PRC subsidiaries may become subject to more stringent requirements with respect to matters including CSRC approval requirements, data privacy and cross-border investigation and enforcement of legal claims.
While the application of the M&A Rules and the New Administrative Rules Regarding Overseas Listings remains unclear, the CSRC currently has not issued any definitive rule or interpretation concerning whether offerings such as our offering are subject to the CSRC approval procedures; and despite the lack of any definitive rule or interpretation from CSRC, the main purpose of the M&A rule is for national security and national industrial policy and so far none of the Chinese companies that have completed their public listing in the U.S. have obtained such approval; and the PRC subsidiaries’ business operations in China do not belong to a prohibited industry by foreign investment.
However, there is still uncertainty as to how the M&A Rules and the New Administrative Rules Regarding Overseas Listings will be interpreted and implemented in the future. If the CSRC or other PRC regulatory agencies subsequently determine that CSRC approval is required for this offering, we or the PRC subsidiaries may need to apply for remedial approval from the CSRC and we or the PRC subsidiaries may be subject to penalties and administrative sanctions administered by these regulatory agencies. These regulatory agencies may impose fines and penalties on the PRC subsidiaries’ operations in the PRC, limit the PRC subsidiaries’ operating privileges in the PRC, delay or restrict the repatriation of the proceeds from this offering into the PRC, or take other actions that could materially adversely affect the PRC subsidiaries’ and our business, financial condition, results of operations, reputation and prospects, as well as the trading price of our Ordinary Shares. The CSRC or other PRC regulatory agencies may also take actions requiring us, or making it advisable for us, to halt this offering before settlement and delivery of our Ordinary Shares.
120
Table of Contents
Restriction on Foreign Ownership
The principal regulation governing foreign ownership of businesses in the PRC is Guidance Catalogue for Industrial Structure Adjustments (2024 edition), effective as of February 1, 2024 (the “Catalogue”). The Catalogue classifies the various industries into three categories: encouraged, restricted and prohibited. Risks and uncertainties relating to PRC regulation of medical device businesses include new laws, regulations or policies may be promulgated or announced that will regulate medical device. If these new laws, regulations or policies are promulgated, additional licenses may be required for the PRC subsidiaries’ operations. If the PRC subsidiaries’ operations do not comply with these new regulations after they become effective, or if the PRC subsidiaries fail to obtain any licenses required under these new laws and regulations, the PRC subsidiaries could be subject to penalties and their business operations could be disrupted.
Regulations on Offshore Parent Holding Companies’ Direct Investment in and Loans to their PRC Subsidiaries
China has been open to foreign direct and indirect investments. An offshore company may invest in a PRC company. Such investment is subject to the FIL. Under the FIL, foreign investments no longer need to be approved by Chinese government, but only need to register the investment with Chinese regulatory agency.
However, Chinese government still has foreign exchange control policy. The money transfer in or out of China is still under tight control. So, shareholder loans made by offshore parent holding companies to their PRC subsidiaries are regarded as foreign debts for regulatory purposes, which debts are subject to a number of PRC laws and regulations, including the PRC Foreign Exchange Administration Regulations, Administration Rules on the Settlement, Sale and Payment of Foreign Exchange, the Statistical Monitoring of Foreign Debt Tentative Provisions, and the Provisional Measures on Administration of Foreign Debt.
Pursuant to the Provisional Measures on Administration of Foreign Debt (the “Foreign Debt Measures”) issued by the State Development Planning Commission (revised), Ministry of Finance and SAFE in January 2003 and became effective on March 1, 2003, which was amended on July 26, 2022 and became effective on September 1, 2022, any loans provided by us to the subsidiary in mainland China in foreign currencies shall be classified as foreign debt under the Foreign Debt Measures. According to the Foreign Debt Measures, the sum of cumulative accrued amounts of medium-term to long-term foreign loans and balance amounts of short-term foreign loans taken by a foreign investment enterprise shall be limited to the difference between the total project investment amount approved by the government and the amount of registered capital. Foreign investment enterprises may take foreign loans freely within the scope of difference.
On January 12, 2017, the PBOC issued the Notice of People’s Bank of China on Matters Concerning Macro-prudential Management on All-round Cross-border Financing (the “No. 9 Notice”), which improved the policy framework of the cross-border financing. The No. 9 Notice clarifies the new calculation methods of the upper limit of the risk-weighted balance for all types of cross-border financing, in particular, the upper limit for risk-weighted balance for cross-border financing equals to the capital or the net assets multiplied by the leverage rate of cross-border financing and the macro-prudential adjustment parameters. In the case of the subsidiary in mainland China, the capital or the net assets is calculated at the net assets of each subsidiary, the leverage rate for cross-border financing for an enterprise is 2, and the macro-prudential adjustment parameter is 1 (the “All-Round Mode”). On March 11, 2020, the PBOC and SAFE promulgated the Circular of the People’s Bank of China and the State Administration of Foreign Exchange on Adjusting the Macro-prudential Regulation Parameter for Full-covered Cross-border Financing, which provides that based on the current macro economy and international balance of payments, the macro-prudential regulation parameter as set forth in the Notice 9 is updated from 1 to 1.25. On January 7, 2021, the PBOC and SAFE promulgated the Notice of PBC and SAFE on Adjusting the Macro-prudential Adjustment Parameter for Cross-border Financing of Companies, which provides that the macro-prudential regulation parameter of companies is updated from 1.25 to 1. Currently, the implementation of the foregoing methodologies in cross-border financing have not been formally determined by the PBOC and the SAFE. In the practice, according to the Q&A on Macro-prudential Regulation Parameter for Full-Covered Cross-border Financing (Phase I) (the “Q&A”) issued by the SAFE on May 27, 2017, FIEs shall submit a written filing report to the local foreign exchange bureau when they handle the foreign debt signing filing (registration) for the first time after the issuance of the Q&A, so as to clarify the cross-border financing management mode they choose during the transition period. If the All-Round Mode is selected, the latest audited net assets data shall be reported at the same time. Once the cross-border financing management mode is determined, it shall not be changed. Alternatively, if we choose to use the All-Round Mode, the amount of loans we can make to the subsidiary
121
Table of Contents
in mainland China as calculated according to the No. 9 Notice and the Notice of PBC and SAFE on Adjusting the Macro-prudential Adjustment Parameter for Cross-border Financing of Companies will not be more than 2 times of the net assets of such entities.
Moreover, as the debtors of cross-border financing, the subsidiary in mainland China is also required to comply with certain registration formalities for execution of foreign debt contracts with the foreign exchange bureau at the locality within fifteen working days after signing the contracts according to the Notice of State Administration of Foreign Exchange on Promulgation of the Administrative Measures on Registration of Foreign Debt which was promulgated by the SAFE in April 2013 and revised in May 2015.
Pursuant to the Circular of the National Development and Reform Commission on Promoting the Administrative Reform of the Record-filing and Registration System for the Issuance of Foreign Debt by Enterprises promulgated on September 14, 2015 (“Circular 2044”), before the issuance of foreign loans, enterprises shall first apply to the NDRC for record-filing and registration procedures and shall report the information on the issuance to NDRC within 10 business days after completion of each issuance. The term “foreign loan” shall mean RMB-denominated or foreign currency-denominated debt instruments with a maturity of one year or more which are issued overseas by domestic enterprises and their controlled overseas enterprises or branches and for which the principal and interest are repaid as agreed, including bonds issued overseas and long and medium-term international commercial loans, and so forth. On January 5, 2023, the NDRC issued the Administrative Measures for Review and Registration of Medium- and Long-term Foreign Debt of Enterprises, or Foreign Debt Registration Measures, which came into effect on February 10, 2023, and Circular 2044 is, therefore invalid. The Foreign Debt Registration Measures not only further clarifies the scope of debt instruments, including senior debt, perpetual debt, capital debt, medium-term note, convertible bond, exchangeable bond, financial leasing, and commercial loan, but also changes the record-filing and registration system to the review and registration system. According to the Foreign Debt Registration Measures, before incurring foreign debt, an enterprise shall obtain the Certificate of Review and Registration of Enterprise Incurrence of Foreign Debt, or the Certificate of Review and Registration, and complete the review and registration formalities. Without review and registration, no foreign debt may be incurred. An enterprise shall, within ten working days after incurring each foreign debt, report the information of incurring foreign debt to the review and registration authority via the network system, including the main operating indicators of the enterprise and the information about the foreign debt incurred, among others, and report the corresponding information about the foreign debt incurred within ten working days after the expiration of Certificate of Review and Registration. The above provisions shall apply to indirect overseas incurrence of foreign debt by domestic enterprises, which means that enterprises whose main business activities are in China, issuing bonds or borrowing commercial loans overseas in the name of enterprises registered overseas, based on the equity, assets, earnings or other similar rights and interests of the domestic enterprises, are subject to these provisions. However, the NDRC has not issued any other further explanation for the implementation of the Foreign Debt Registration Measures. In the practice, the NDRC’s attitude on whether foreign-invested enterprises with foreign loans with a term of more than one year need to register is still not completely unified, and it is generally determined on a case-by-case basis.
Regulations Relating to Employment and Social Welfare
Regulations on Employment
The major PRC laws and regulations that govern employment relationship are the PRC Labor Law, or the Labor Law (issued by the SCNPC on July 5, 1994, came into effect on January 1, 1995 and revised on August 27, 2009 and December 29, 2018, the PRC Labor Contract Law, or the Labor Contract Law, promulgated by the SCNPC on June 29, 2007 and became effective on January 1, 2008, and then amended on December 28, 2012 and became effective on July 1, 2013, and the Implementation Rules of the Labor Contract Law of the PRC, or the Implementation Rules of the Labor Contract Law, issued by the State Council on September 18, 2008 and came into effect on the same day. According to the aforementioned laws and regulations, labor relationships between employers and employees must be executed in written form. The laws and regulations above impose stringent requirements on the employers in relation to entering into fixed-term employment contracts, hiring of temporary employees and dismissal of employees. As prescribed under the laws and regulations, employers shall ensure its employees have the right to rest and the right to receive wages no lower than the local minimum wages. Employers must establish a system for labor safety and sanitation that strictly abide by state standards and provide relevant education to its employees. Violations of the Labor Contract Law and the Labor Law may result in the imposition of fines and other administrative liabilities and/or incur criminal liabilities in the case of serious violations.
122
Table of Contents
Regulations on Social Insurance and Housing Provident Fund
According to the Social Insurance Law of PRC, which issued by the SCNPC on October 28, 2010 and came into effect on July 1, 2011 and was latest revised on December 29, 2018, enterprises and institutions in mainland China shall provide their employees with welfare schemes covering pension insurance, unemployment insurance, maternity insurance, work-related injury insurance, medical insurance and other welfare plans. The employer shall apply to the local social insurance agency for social insurance registration within 30 days from the date of its formation. And it shall, within 30 days from the date of employment, apply to the social insurance agency for social insurance registration for the employee. Any employer who violates the regulations above shall be ordered to make correction within a prescribed time limit; if the employer fails to rectify within the time limit, the employer and its directly liable person will be fined. Meanwhile, the Interim Regulation on the Collection and Payment of Social Insurance Premiums, issued by the State Council on January 22, 1999 and came into effect on the same day and was recently revised on March 24, 2019, prescribes the details concerning the social securities.
Apart from the general provisions about social insurance, specific provisions on various types of insurance are set out in the Regulation on Work-Related Injury Insurance, issued by the State Council on April 27, 2003, came into effect on January 1, 2004 and revised on December 20, 2010, the Regulations on Unemployment Insurance, issued by the State Council on January 22, 1999 and came into effect on the same day, the Trial Measures on Employee Maternity Insurance of Enterprises, issued by the Ministry of Labor on December 14, 1994 and came into effect on January 1, 1995. Enterprises subject to these regulations shall provide their employees with the corresponding insurance.
According to the Regulation Concerning the Administration of Housing Provident Fund, implemented since April 3, 1999 and latest amended on March 24, 2019, any newly established entity shall make deposit registration at the housing accumulation fund management center within 30 days as of its establishment. After that, the entity shall open a housing accumulation fund account for its employees in an entrusted bank. Within 30 days as of the date an employee is recruited, the entity shall make deposit registration at the housing accumulation fund management center and seal up the employee’s housing accumulation fund account in the bank mentioned above within 30 days from termination of the employment relationship.
Any entity that fails to make deposit registration of the housing accumulation fund or fails to open a housing accumulation fund account for its employees shall be ordered to complete the relevant procedures within a prescribed time limit. Any entity failing to complete the relevant procedures within the time limit will be fined RMB10,000 to RMB50,000. Any entity that fails to make deposits to the housing provident fund within the time limit or has any shortfall in payment of housing provident fund will be ordered to make the payment or make up the shortfall within the prescribed time limit, otherwise, the housing provident management center is entitled to apply for compulsory enforcement with the People’s Court. As of the date of this prospectus, (i) some of the PRC subsidiaries have not completed the social insurance registration and the housing fund registration; (ii) the PRC subsidiaries did not make contributions in the full amount for the social insurance fund and housing provident fund for their employees, as required under the relevant PRC laws and regulations; and (iii) the PRC subsidiaries did not make contributions in the housing fund for some employees. For the six months ended March 31, 2024 and the fiscal years of 2023 and 2022, $141,340, $287,629, and $295,009 of contributions for social insurance fund, respectively, should have been made but were not. For the six months ended March 31, 2024 and the fiscal years of 2023 and 2022, $44,530, $90,620, and $92,945 of contributions for the housing provident fund should have been made but were not. See “Risk Factors — Risks Related to Doing Business in China — Failure to make adequate contributions to various employee benefit plans and withhold individual income tax on employees’ salaries as required by PRC regulations may subject the PRC subsidiaries to penalties” on page 29.
Regulations Relating to Tax
Enterprise income tax
According to the EIT Law, which was promulgated by the SCNPC on March 16, 2007 and last amended and effective on December 29, 2018, and the Enterprise Income Tax Implementation Regulations of the PRC (the “EITIR”), which was promulgated by the State Council on December 6, 2007 and last amended and effective on April 23, 2019, the enterprise income tax of both domestic and foreign-invested enterprises is unified at 25% with certain exceptions. According to the EIT Law, enterprises are classified as “resident enterprises” and “non-resident enterprises.” Pursuant to the EIT Law and the EITIR, PRC resident enterprises typically pay an enterprise income tax at the rate of 25%, while non-PRC resident enterprises without any branches in the PRC should pay an enterprise income tax in connection with
123
Table of Contents
their income from the PRC at the tax rate of 10%. Enterprises established under the laws of foreign countries or regions whose “de facto management bodies” (i.e., establishments that carry out substantial and overall management and control over production and operations, personnel, accounting and properties) are located in the PRC are considered as PRC tax resident enterprises, and will generally be subject to enterprise income tax at the rate of 25% of their global income.
Pursuant to the EIT Law, enterprises qualified as “High and New Technology Enterprises” are entitled to a 15% enterprise income tax rate rather than the 25% uniform statutory tax rate. The preferential tax treatment continues as long as an enterprise can retain its “High and New Technology Enterprise” status, which certificate is valid for a period of three years and renewable.
Value-added tax
According to Provisional Regulations on Value-added Tax of the PRC, which were promulgated by the State Council on December 13, 1993 and last amended on November 19, 2017, and the Implementing Rules for the Interim Regulations on Value-added Tax of the PRC promulgated by Ministry of Finance on December 25, 1993 and last amended on November 1, 2011, all enterprises and individuals that engage in the sale of goods, the provision of processing, repair and replacement services, the sale of services, intangible assets or immovable properties and the importation of goods within the territory of the PRC must pay value-added tax.
Dividend Withholding tax
According to the EIT Law and the EITIR, dividends paid by foreign-invested companies to their foreign investors that are non-resident enterprises as defined under the law are subject to withholding tax at a rate of 10%, unless otherwise provided in the relevant tax agreements entered into with the central government of the PRC. Pursuant to the Arrangement Between the Mainland of China and the Hong Kong Special Administrative Region for the Avoidance of Double Taxation on Income (the “Double Tax Avoidance Arrangement”) promulgated on August 21, 2006, if a Hong Kong resident enterprise is determined by the competent PRC tax authority to have satisfied the relevant conditions and requirements under such Double Tax Avoidance Arrangement, the withholding tax rate on the dividends the Hong Kong resident enterprise receives from a PRC resident enterprise may be reduced to 5% from 10% applicable under the EIT Law and the EITIR. However, based on the Notice of the State Taxation Administration on Certain Issues with Respect to the Enforcement of Dividend Provisions in Tax Treaties promulgated and took into effect on February 20, 2009 by the State Taxation Administration (the “STA”), if the relevant PRC tax authorities determine, in their discretion, that a company benefits from such reduced income tax rate due to a structure or arrangement that is primarily tax-driven, such PRC tax authorities may adjust the preferential tax treatment. Based on the Notice of the State Taxation Administration on the Recognition of Beneficial Owners in Tax Treaties, which was promulgated by STA on February 3, 2018 and came into effect on April 1, 2018, a comprehensive analysis will be used to determine beneficial ownership based on the actual situation of a specific case combined with certain principles, and if an applicant was obliged to pay more than 50% of its income to a third country (region) resident within 12 months of the receipt of the income, or the business activities undertaken by an applicant did not constitute substantive business activities including substantive manufacturing, distribution, management and other activities, the applicant was unlikely to be recognized as a beneficial owner to enjoy tax treaty benefits.
Regulations on Intellectual Property
Trademarks
Trademarks are protected by the PRC Trademark Law adopted in 1982 and lastly amended in 2019, as well as the Implementation Regulation of the PRC Trademark Law adopted by the State Council in 2002 and amended in 2014. The Trademark Office of China National Intellectual Property Administration handles trademark registrations. Trademarks can be registered for a term of ten years and can be repeatedly extended for another ten-year term at the time of expiry. The PRC Trademark Law has adopted a “first-to-file” principle with respect to trademark registration. As of the date of this prospectus, the PRC subsidiaries have registered 16 trademarks, all of which are fully owned and in use by us. According to Chinese Trademark Law, if anyone has a dispute the officially registered trademarks, he can file a petition to the review board of the Trademark Office, requesting a comprehensive review that may result in the revoking the registered trademarks. As of the date of this prospectus, the PRC subsidiaries have not received any such kind of petition.
124
Table of Contents
Patents
According to the PRC Patent Law promulgated by the SCNPC on March 12, 1984 and last amended on October 17, 2020 with effect from June 1, 2021, and its latest Implementation Rules promulgated by the State Council on December 11, 2023 and took into effect on January 20, 2024, the National Intellectual Property Administration is responsible for administering patents in the PRC. Inventions, utility models, and designs with the features of novelty, inventiveness and practical applicability, are three kinds of patent defined and protected under China’s Patent Law. The State Intellectual Property Office is responsible for examining and approving patent applications. Once the application is approved, the applicants can have their patent under Chinese legal protection for a long term since its application date, which is 20 years for invention and ten years for utility models and designs.
Domain Names
Internet domain name registration and related matters are primarily regulated by the Measures on Administration of Internet Domain Names, which were promulgated by the MIIT on August 24, 2017 and taken effect on November 1, 2017, and the Detailed Rules for the Implementation of National Top-level Domain Name Registration, which were promulgated by China Internet Network Information Center and took into effect on June 18, 2019. Domain name owners are required to register their domain names and the MIIT is in charge of the administration of PRC internet domain names. The domain name services follow a “first come, first file” principle. The applicants will become the holders of such domain names upon the completion of the registration procedures.
Copyrights
Pursuant to the PRC Copyright Law promulgated by the SCNPC on September 7, 1990 and last amended on November 11, 2020 (the latest revision became effective from June 1, 2021) and the Implementing Regulations of the PRC Copyright Law promulgated by the State Council on August 2, 2002, last amended on January 30, 2013 (the latest revision became effective from March 1, 2013), PRC nationals, legal persons, and other organizations may copyright their works, whether published or not, which works include, among others, works of literature, art, natural science, social science, engineering technology and computer software. Pursuant to the Regulations on the Protection of Computer Software promulgated by the State Council in December 2001, and most recently amended in January 2013, and the Rules for the Registration of Computer Software Copyright, which was promulgated by the China Copyright Office and came into effect in February 2002, anyone who publishes, revises or translates computer software without obtaining the prior approval of the computer software copyright holders shall bear civil liability to the copyright owner as a consequence of harming the copyright. The corporate computer software copyright is valid for a term of 50 years, i.e., until December 31st of the 50th year, starting from the date as of first publication. Computer software copyright owners shall register at the registration institution authorized by the PRC Copyright Office to obtain the computer software copyright registration certificates as primary evidence of the computer software copyright being registered.
Regulations Relating to Anti-Monopoly, Anti-Corruption and Anti-Bribery
On August 30, 2007, the Anti-Monopoly Law, which was promulgated by the SCNPC, and amended on June 24, 2022 which amendment became effective on August 1, 2022, stipulates the regulation of market monopoly. The Anti-Monopoly Law prohibits the conclusion of a monopoly agreement between business operators, the abuse of a dominant market position by a business operator and concentration of undertakings which has or may have an effect of excluding or limiting market competition. The newly revised Anti-Monopoly Law proposes to increase the fines on business operators liable for illegal concentration to “no more than ten percent of the preceding year’s sales revenue of the business operators if the concentration of business operators has or may have an effect of excluding or limiting competition; or a fine of up to RMB5 million if the concentration of business operators does not have an effect of excluding or limiting competition.” Furthermore, the relevant authority may require business operators to declare where there is evidence that the resulting concentration has or may have the effect of eliminating or restricting competition, even if the level of such concentration does not reach the filing threshold. If the business operators fail to make a declaration, the relevant authority will conduct an investigation according to law.
Pursuant to the Anti-Unfair Competition Law of the PRC promulgated by the SCNPC on September 1, 1993 and last amended on April 23, 2019, a business operator shall not resort to bribery to seek a transaction opportunity or competitive advantage by offering money or goods or by any other means, to (i) any employee of the counterparty in a transaction, (ii) any entity or individual entrusted by the counterparty in a transaction to handle relevant affairs, or
125
Table of Contents
(iii) any other entity or individual that takes advantage of powers or influence to influence a transaction. A business operator may expressly offer a discount to the counterparty or pay commissions to the intermediaries of a transaction in the course of transaction activities, which shall be properly recorded at both parties’ accounting books. Any commercial bribery committed by an employee of a given business operator will be deemed as conduct of such business operator unless evidence shows that such act is not related to such business operator’s efforts in seeking a transaction opportunity or competitive advantage.
Regulations related to business registration in Hong Kong
Our Hong Kong subsidiary, Work Medical Technology Group (China) Limited, is an investment holding company and is subject to regulations related to business registration in Hong Kong. The Business Registration Ordinance (Chapter 310 of the Laws of Hong Kong) requires every person carrying on any business to make an application to the Commissioner of Inland Revenue in the prescribed manner for the registration of that business within one month after the commencement of business. The Commissioner of Inland Revenue must register each business for which a business registration application is made and, as soon as practicable after the prescribed business registration fee and levy are paid, issue a business registration certificate or branch registration certificate for the relevant business or the relevant branch, as the case may be. Any person who fails to apply for business registration shall be guilty of an offence and shall be liable to a fine of HK$5,000 and to imprisonment for 1 year.
Except for the business registration certificate to be issued under the Business Registration Ordinance, we are not required to obtain any industry-specific license, permit, authorization or qualification for our Hong Kong subsidiary. The Commissioner of Inland Revenue issued the business registration certificate for Work Medical Technology Group (China) Limited which is valid from April 19, 2024 to April 18, 2025.
126
Table of Contents
MANAGEMENT
Directors, Director Nominees and Executive Officers
The following table sets forth information regarding our directors and officers as of the date of this prospectus. Unless otherwise stated, the business address for our directors and executive officers is that of our principal executive offices at Floor 23, No. 2 Tonghuinan Road, Xiaoshan District, Hangzhou City, Zhejiang Province, the PRC.
Name | | Age | | Position with our Company |
Shuang Wu | | 42 | | Chief Executive Officer, Director, and Chairman of the Board of Directors |
Ningfang Liang | | 52 | | Chief Financial Officer |
Baiming Yu | | 54 | | Chief Operating Officer and Director |
Xiaoyang Li | | 38 | | Independent Director |
Zhongxuan Li | | 51 | | Independent Director |
Robert Johnson | | 47 | | Independent Director |
Shuang Wu has served as our Chief Executive Officer (“CEO”) and Chairman of the Board of Directors since June 2022 and our director since March 2022. Mr. Wu has extensive managerial and financial experience. Before joining our company, from August 2019 to May 2022, he was the Chief Operating Officer of EZGO Technologies Ltd (Nasdaq: EZGO), a company focusing on sales of lithium battery and electric bicycle, and was responsible for making and enforcing business plans. From January 2018 to July 2019, he served as the vice president of Changzhou Hengmao Electricity Technology Co., Ltd., working for business operation and management. He was the General Manager of Investment of Shanghai Dafeng Investment Group Co., Ltd. from June 2015 to December 2017. From November 2011 to December 2014, he served as the assistant of General Manager of Travelex of Auckland Airport, a foreign exchange service provider. From June 2009 to November 2011, he was a financial adviser of Westpac Bank. Mr. Wu received his bachelor’s degree of finance and master’s degree of business from Massey University.
Ningfang Liang has served as our Chief Financial Officer (“CFO”) since June 2022. Mr. Liang has been in the financial field and working for U.S.-listed companies for over 15 years. From December 2008 to June 2011, he was the manager of Sirius International Insurance Group, a former Nasdaq-listed company (Nasdaq: SG), and from June 2016 to June 2022, he rejoined the company and resumed being the manager, responsible for evaluating business performance and consolidated financial results, preparing for SEC filings and recommending changes for business plan from the perspective of finance. From March 2013 to January 2016, and from June 2011 to February 2013, he worked as the Chief Financial Officer of two U.S. public companies respectively, Tantech Holdings Ltd. (Nasdaq: TANH) and China GengSheng Minerals, Inc. (OTCMKTS: CHGS). From December 2006 to December 2008, he worked as a senior accountant in American International Group Inc. (NYSE: AIG). He was a financial analyst of Celgene Corporation, a former Nasdaq-listed company (Nasdaq: CELG), from January 2005 to December 2006. Mr. Liang has a bachelor’s degree of science from Shanghai University of Finance and Economics and an MBA degree from University of Illinois, Urbana-Champaign. He is also a Certified Public Accountant of the states of New Jersey and Illinois.
Baiming Yu has served as our Chief Operating Officer (“COO”) since June 2022 and he has served as our director since August 2024. Mr. Yu has enriched experience in the field of medicine. He is the founder of Hangzhou Shanyou and has been its General Manager since January 2002, responsible for the company’s daily operations and management. Additionally, he served as the General Manager of Hangzhou Yuanqi Biotech Co., Ltd. from January 2012 to December 2018. From August 1993 to December 2001, he was a doctor of The First People’s Hospital of Xiaoshan City. Mr. Yu received his bachelor’s degree of medicine in anesthesia from the former Zhejiang Medical University, which was merged into Zhejiang University in 1998.
Xiaoyang Li has served as our director since August 2024. Dr. Li has been working in Ruijin Hospital Affiliated to Shanghai Jiao Tong University School of Medicine since July 2011. He was a Resident Physician initially and later got promoted to be an Attending Physician in June 2016. Since December 2020, he has been the Associate Chief Physician of the hospital. Dr. Li received his master’s degree of clinical medicine and doctor’s degree of internal medicine from Shanghai Jiao Tong University.
Zhongxuan Li has served as our director since August 2024. Mr. Li’s law practices cover multiple fields, including IPO, merger and acquisition, private equity, foreign direct investment, and commercial arbitration. He has been the senior partner of DeHeng Law Office (Shenzhen) since March 2008. From October 2006 to February 2008, he was
127
Table of Contents
an attorney of King & Wood Mallesons’s Shenzhen office. From July 2004 to April 2006, he was a legal manager of department of South Africa of Huawei Technologies Co., Ltd. From April 2000 to April 2003, he was a director of legal department of Shenzhen Credit Guarantee Group Co., Ltd. Mr. Li received his bachelor’s degree of law from Lanzhou University, and master’s degrees of law from both Northwest University of Political Science and Law and Washington & Lee University. He is admitted to practice as an attorney in both the PRC and State of New York.
Robert Johnson has served as our director since August 2024. Mr. Johnson has over 15 years of experience in tax and finance. He has been the Financial Controller of BAS Holdings Investments, LLC since February 2020, primarily responsible for review of periodic account and intercompany reconciliations and financial statement reporting and cash, receivables, and payables management for operating companies. From January 2017 to January 2020, he was the controller for family office of Lionstone Development, LLC. From June 2010 to June 2015, he was the controller for family office of BSL Capital, Inc. From September 2005 to June 2010, he served as a senior tax accountant of CBIZ MHM, LLC, and from January 2004 to September 2005, he was a tax accountant of Mallah Furman and Company. Mr. Johnson received his bachelor’s degrees of science in accounting and finance from University of Central Florida, and his MBA degree from University of Miami. He is both a Certified Management Accountant and Certified Public Accountant.
Board of Directors and Committees
Our board of directors consists of five directors, including three independent directors. We have established an Audit Committee, a Nominating and Corporate Governance Committee and a Compensation Committee. We have adopted a charter for each of the three committees. Each of the committees of our board of directors has the composition and responsibilities described below.
Audit Committee
Xiaoyang Li, Zhongxuan Li, and Robert Johnson serve as members of our Audit Committee with Robert Johnson serving as the chairman of the Audit Committee. All of our Audit Committee members satisfy the “independence” requirements of the Nasdaq listing rules and meet the independence standards under Rule 10A-3 under the Exchange Act. Our board of directors have determined that Robert Johnson possesses accounting or related financial management experience that qualifies him as an “audit committee financial expert” as defined by the rules and regulations of the SEC. Our Audit Committee oversees our accounting and financial reporting processes and the audits of our financial statements. Our Audit Committee performs several functions, including:
• evaluating the independence and performance of, and assesses the qualifications of, our independent auditor, and engages such independent auditor;
• approving the plan and fees for the annual audit, quarterly reviews, tax and other audit-related services, and approves in advance any non-audit service to be provided by the independent auditor;
• monitoring the independence of the independent auditor and the rotation of partners of the independent auditor on our engagement team as required by law;
• reviewing the financial statements to be included in our Annual Report on Form 20-F and Current Reports on Form 6-K and reviews with management and the independent auditors the results of the annual audit and reviews of our quarterly financial statements;
• overseeing all aspects of our systems of internal accounting control and corporate governance functions on behalf of the board;
• reviewing and approving in advance any proposed related-party transactions and report to the full Board on any approved transactions; and
• providing oversight assistance in connection with legal, ethical and risk management compliance programs established by management and our board of directors, including Sarbanes-Oxley Act implementation, and makes recommendations to our board of directors regarding corporate governance issues and policy decisions.
128
Table of Contents
Compensation Committee
Xiaoyang Li, Zhongxuan Li and Robert Johnson serve as members of our Compensation Committee with Xiaoyang Li serving as the chairman of the Compensation Committee. All of our Compensation Committee members satisfy the “independence” requirements of the Nasdaq listing rules and meet the independence standards under Rule 10A-3 under the Exchange Act. Our Compensation Committee is responsible for overseeing and making recommendations to our board of our directors regarding the salaries and other compensation of our executive officers and general employees and providing assistance and recommendations with respect to our compensation policies and practices.
Nominating and Corporate Governance Committee
Xiaoyang Li, Zhongxuan Li and Robert Johnson serve as members of our Nominating and Corporate Governance Committee, with Zhongxuan Li serving as the chairman of the Nominating and Corporate Governance Committee. All of our Nominating and Corporate Governance Committee members satisfy the “independence” requirements of the Nasdaq listing rules and meet the independence standards under Rule 10A-3 under the Exchange Act. Our Nominating and Corporate Governance Committee is responsible for identifying and proposing new potential director nominees to the board of directors for consideration and reviewing our corporate governance policies.
Code of Ethics
We have adopted a code of ethics that applies to all of our executive officers, directors and employees in accordance with the rules of Nasdaq and the SEC. The code of ethics codifies the business and ethical principles that govern all aspects of our business. You will be able to review the code of ethics by accessing our public filings at the SEC’s website at www.sec.gov.
Family Relationships
There are no family relationships, or other arrangements or understandings between or among any of the directors, director nominees, executive officers or other person pursuant to which such person was selected to serve as a director or officer.
Duties of Directors
Under Cayman Islands law, directors and officers owe the following fiduciary duties:
(i) duty to act in good faith in what the director or officer believes to be in the best interests of the company as a whole;
(ii) duty to exercise powers for the purposes for which those powers were conferred and not for a collateral purpose;
(iii) directors should not properly fetter the exercise of future discretion;
(iv) duty not to put themselves in a position in which there is a conflict between their duty to the company and their personal interests; and
(v) duty to exercise independent judgment.
In addition to the above, directors also owe a duty of care which is not fiduciary in nature. This duty has been defined as a requirement to act as a reasonably diligent person having both the general knowledge, skill and experience that may reasonably be expected of a person carrying out the same functions as are carried out by that director in relation to the company and the general knowledge skill and experience which that director has.
As set out above, directors have a duty not to put themselves in a position of conflict and this includes a duty not to engage in self-dealing, or to otherwise benefit as a result of their position. However, in some instances what would otherwise be a breach of this duty can be forgiven and/or authorized in advance by the shareholders provided that there is full disclosure by the directors. This can be done by way of permission granted in the memorandum and articles of association or alternatively by shareholder approval at general meetings.
129
Table of Contents
Accordingly, as a result of multiple business affiliations, our officers and directors may have similar legal obligations relating to presenting business opportunities meeting the above-listed criteria to multiple entities. In addition, conflicts of interest may arise when our board evaluates a particular business opportunity with respect to the above-listed criteria. We cannot assure you that any of the afore-mentioned conflicts will be resolved in our favor. Furthermore, each of our officers and directors has pre-existing fiduciary obligations to other businesses of which they are officers or directors.
Our company has the right to seek damages if a duty owed by our directors is breached. A shareholder may in certain limited exceptional circumstances have the right to seek damages in our name if a duty owed by our directors is breached. You should refer to “Description of Share Capital — Comparison of Cayman Islands Corporate Law and U.S. Corporate Law” for additional information on our standard of corporate governance under Cayman Islands law.
In fulfilling their duty of care to us, our directors must ensure compliance with our memorandum and articles of association as may be amended from time to time. Our company has a right to seek damages against any director who breaches a duty owed to us.
Our directors have all the powers necessary for managing, and for directing and supervising, our business affairs. The functions and powers of our Board of Directors include, among others:
• convening shareholders’ annual general meetings and reporting its work to shareholders at such meetings;
• declaring dividends and distributions;
• appointing officers and determining the term of office of officers;
• exercising the borrowing powers of our Company and mortgaging the property of our Company; and
• approving the transfer of shares of our Company, including the registering of such shares in our share register.
Terms of Directors and Officers
Our officers are appointed by and serve at the discretion of our board of directors and the shareholders voting by ordinary resolution. Our directors are not subject to a set term of office and hold office until the next general meeting called for the appointment of directors and until their successor is duly appointed or such time as they die, resign or are removed from office by a shareholders’ ordinary resolution. The office of a director will be vacated automatically if, among other things, such director resigns in writing, becomes bankrupt or makes any arrangement or composition with his/her creditors generally or is found to be or becomes of unsound mind.
Employment Agreements and Indemnification Agreements
We have entered into employment agreements with each of our executive officers. Under these agreements, each of our executive officers is employed for an initial term from June 1, 2022 to May 31, 2025.
The executive officers are entitled to a fixed salary, as described below under “Compensation of Directors and Officers,” and to participate in our equity incentive plans, if any and other company benefits, each as determined by the Board from time to time.
We may terminate the executive officer’s employment for cause, at any time, without notice or remuneration, for certain acts, such as conviction or plea of guilty to a felony or grossly negligent or dishonest acts to our detriment, or misconduct or a failure to perform agreed duties. In such case, the executive officer will not be entitled to receive payment of any severance benefits or other amounts by reason of the termination, and his right to all other benefits will terminate, except as required by any applicable law. We may also terminate the executive officer’s employment without cause immediately and without prior written notice upon the removal of the executive officer pursuant to the exercise of any power contained in the memorandum and articles of association of the Company or upon 30 days’ advance written notice. In such case of termination by us, we are required to provide the following severance payments and benefits to the executive officer: a cash payment of three month of base salary as of the date of such termination.
130
Table of Contents
The executive officer may terminate his or her employment at any time with 30 days’ advance written notice if there is any significant change in his or her duties and responsibilities or a material reduction in his or annual salary. In such case, the executive officer will be entitled to receive compensation equivalent to three months of his or her base salary. In addition, if we or our successor terminates the employment agreements upon a merger, consolidation, or transfer or sale of all or substantially all of our assets with or to any other individual(s) or entity, the executive officer shall be entitled to the following severance payments and benefits upon such termination: 1) a lump sum cash payment equal to three months of base salary at a rate equal to the greater of his or her annual salary in effect immediately prior to the termination, or his or her then current annual salary as of the date of such termination; 2) a lump sum cash payment equal to a pro-rated amount of target annual bonus for the fiscal year immediately preceding the termination; 3) payment of premiums for continued health benefits under our health plans for three months following the termination; and 4) immediate vesting of 100% of the then-unvested portion of any outstanding equity awards held by the executive officer. The employment agreements also contain customary restrictive covenants relating to confidentiality and non-competition. The foregoing description of the terms of the employment agreements is qualified in its entirety by reference to the provisions of the employment agreements filed as Exhibits 10.32 to 10.34 to this prospectus, which are incorporated by reference herein.
We have also entered into indemnification agreements with each of our directors and executive officers. Under these agreements, we have agreed to indemnify our directors and executive officers against certain liabilities and expenses incurred by such persons in connection with claims made by reason of their being a director or officer of our Company.
Compensation of Directors and Officers
Since June 1, 2022, we paid Baiming Yu RMB24,000 (approximately $3,529) per month for his services. As of March 31, 2024, the accumulated amount was RMB528,000 (approximately $77,638).
From June 1, 2022 to May 31, 2025, the compensation of our CEO is RMB30,000 (approximately $4,368) per month and compensation of our CFO is $2,000 per month. The Company has settled the accumulated unpaid compensation in cash in August 2024, and henceforth pays compensation to the CEO and CFO on a monthly basis.
As of the date of this prospectus, we have not set aside or accrued any amount to provide pension, retirement, or other similar benefits to our directors and executive officers.
131
Table of Contents
PRINCIPAL SHAREHOLDERS
The following table sets forth information with respect to the beneficial ownership, within the meaning of Rule 13d-3 under the Exchange Act, of our Ordinary Shares as of the date of this prospectus for:
• each of our directors and executive officers; and
• each person known to us to own beneficially more than 5% of our Ordinary Shares.
Beneficial ownership includes voting or investment power with respect to the securities. Except as indicated below, and subject to applicable community property laws, the persons named in the table have sole voting and investment power with respect to all Ordinary Shares shown as beneficially owned by them. Percentage of beneficial ownership of each listed person prior to this offering is based on 14,591,942 Ordinary Shares outstanding as of December 20, 2024. Percentage of beneficial ownership of each listed person after this offering is based on 17,091,942 Ordinary Shares outstanding immediately after the completion of this offering (based on the sale of 2,500,000 Ordinary Shares included in the Units in this offering, assuming a public offering price of $6.00 per Ordinary Unit), assuming no exercise of the Warrants.
Information with respect to beneficial ownership has been furnished by each director, officer, or beneficial owner of 5% or more of our Ordinary Shares. Beneficial ownership is determined in accordance with the rules of the SEC and generally requires that such person have voting or investment power with respect to securities. In computing the number of Ordinary Shares beneficially owned by a person listed below and the percentage ownership of such person, Ordinary Shares underlying options, warrants, or convertible securities are deemed outstanding, but are not deemed outstanding for computing the percentage ownership of any other person.
| | Ordinary Shares
Beneficially Owned
Prior to this Offering | | Ordinary Shares
Beneficially Owned
After this Offering |
| | | Number | | Percent | | Number | | Percent |
Directors and Officers:(1) | | | | | | | | | |
Shuang Wu(2) | | 717,500 | | 4.92 | % | | 717,500 | | 4.20% |
Baiming Yu(3) | | 6,250,000 | | 42.83 | % | | 6,250,000 | | 36.57% |
Ningfang Liang | | — | | — | | | — | | — |
Robert Johnson | | — | | — | | | — | | — |
Zhongxuan Li | | — | | — | | | — | | — |
Xiaoyang Li | | — | | — | | | — | | — |
All directors and officers as a group (6 persons): | | 6,967,500 | | 47.75 | % | | 6,967,500 | | 40.76% |
5% Shareholders(4): | | | | | | | | | |
LWY GROUP LTD(3) | | 6,250,000 | | 42.83 | % | | 6,250,000 | | 36.57% |
HJZ GROUP LTD(5) | | 1,435,000 | | 9.83 | % | | 1,435,000 | | 8.40% |
132
Table of Contents
RELATED PARTY TRANSACTIONS
Related Parties
The following is a list of related parties which the Group has transactions with:
No. | | Name of Related Parties | | Relationship with the Group |
a | | Baiming Yu | | COO, Director and a shareholder of the Company |
b | | Liwei Zhang | | Shareholder of the Company and the spouse of Baiming Yu |
c | | Huiyu Chuanggu (Hangzhou) Equity Investment Fund Co., Ltd. (“Huiyu Chuanggu”) | | Shuang Wu acted as the executive director and owned 30% equity interests of this related party |
d | | Baicheng Zhang | | Immediate family member of Liwei Zhang |
e | | Shuang Wu | | Chief Executive Officer of the Company |
f | | Hangzhou Shuige Technology Co., Ltd (“Hangzhou Shuige”) | | 55% equity interests owned by Baiming Yu |
g | | Hangzhou Qingniu Medical Instrument Co., Ltd (“Hangzhou Qingniu”) | | Owned by immediate family member of Baiming Yu |
h | | Hangzhou Xiaoshan Ance Building Materials Firm (“Xiaoshan Ance”) | | Owned by Liwei Zhang |
i | | Hangzhou Yuanqi Biotech Co., Ltd. (“Hangzhou Yuanqi”) | | Baiming Yu acted as director |
j | | Ming Zhao | | Shareholder of Shanghai Saitumofei |
k | | Hangzhou Yizhiying Information Consulting Management Co., Ltd. | | Shuang Wu acted as executive director |
l | | Qijia Yu | | Immediate family member of Baiming Yu |
Six Months Ended March 31, 2024 and 2023
Amounts due from related parties
Amounts due from related parties consisted of the following for the periods indicated:
| | As of
March 31,
2024 | | As of
September 30,
2023 |
Hangzhou Qingniu(1) | | $ | 234,290 | | $ | 1,294,296 |
Huiyu Chuanggu(2) | | | 214,282 | | | 1,453,583 |
Shuang Wu(3) | | | 18,376 | | | 795,243 |
Qijia Yu(3) | | | 1,315 | | | — |
Baiming Yu(4) | | | — | | | 683,376 |
Liwei Zhang(5) | | | — | | | 11,184 |
Total | | $ | 468,263 | | $ | 4,237,682 |
133
Table of Contents
Amounts due to related parties
Amounts due to related parties consisted of the following for the periods indicated:
| | As of
March 31,
2024 | | As of
September 30,
2023 |
Xiaoshan Ance(1) | | $ | 138,498 | | $ | 137,061 |
Hangzhou Shuige(1) | | | 9,001 | | | 17,818 |
Baiming Yu(2) | | | 9,354 | | | — |
Ming Zhao(2) | | | 4,089 | | | 4,048 |
Hangzhou Yuanqi(1) | | | 693 | | | 685 |
Total | | $ | 161,635 | | $ | 159,612 |
Related party transactions
Nature | | For the six months ended
March 31, |
2024 | | 2023 |
Loan to Hangzhou Shuige(1) | | $ | — | | $ | 430,040 |
Repayment from Hangzhou Shuige(1) | | | — | | | 430,040 |
Loan from Hangzhou Shuige(2) | | | 416,297 | | | — |
Repayment to Hangzhou Shuige(2) | | | 425,318 | | | — |
Loan to Liwei Zhang(3) | | | 277,531 | | | 458,709 |
Repayment from Liwei Zhang(3) | | | 288,854 | | | 458,709 |
Reimbursement from Baiming Yu(4) | | | — | | | 651,797 |
Repayment from Baiming Yu(4) | | | 691,872 | | | — |
Loan from Baiming Yu(2) | | | 9,372 | | | — |
Selling to Hangzhou Qingniu(5) | | | 233,109 | | | 429,093 |
Loan to Hangzhou Qingniu(1) | | | 2,708,432 | | | 2,072,392 |
Repayment from Hangzhou Qingniu(1&5&6) | | | 4,023,255 | | | 1,727,326 |
Rent income from Hangzhou Qingniu(6) | | | — | | | 58,373 |
Purchase from Hangzhou Qingniu(7) | | | 24,236 | | | — |
Advance from Shuang Wu(8) | | | — | | | 1,018,337 |
Repayment to Shuang Wu(8) | | | — | | | 1,653,679 |
Advance to Shuang Wu(9) | | | — | | | 1,077,790 |
Repayment from Shuang Wu(9) | | | 769,039 | | | 97,046 |
Reimbursement from Shuang Wu(9) | | | 17,681 | | | — |
Advance to Huiyu Chuanggu(9) | | | 447,796 | | | — |
Reimbursement from Huiyu Chuanggu(9) | | | 1,704,757 | | | — |
Advance to Qjia Yu(4) | | | 1,388 | | | — |
Reimbursement from Qjia Yu(4) | | | 69 | | | — |
134
Table of Contents
Years Ended September 30, 2023 and 2022
Amounts due from related parties
Amounts due from related parties consisted of the following for the periods indicated:
| | As of
September 30, |
2023 | | 2022 |
Huiyu Chuanggu(1) | | $ | 1,453,583 | | $ | — |
Hangzhou Qingniu(2) | | | 1,294,296 | | | 429,347 |
Shuang Wu(3) | | | 795,243 | | | — |
Baiming Yu(4) | | | 683,375 | | | 160,405 |
Liwei Zhang(5) | | | 11,184 | | | 70,514 |
Baicheng Zhang(5) | | | — | | | 46,012 |
Total | | $ | 4,237,682 | | $ | 706,278 |
Amounts due from related parties
Amounts due from related parties consisted of the following for the periods indicated:
| | As of
September 30, |
| | | 2023 | | 2022 |
Shuang Wu(1) | | $ | — | | $ | 623,070 |
Xiaoshan Ance(2) | | | 137,061 | | | 140,578 |
Hangzhou Shuige(2) | | | 17,818 | | | 18,275 |
Hangzhou Yuanqi(2) | | | 685 | | | — |
Hangzhou Yizhiying Information Consulting Management Co., Ltd.(2) | | | — | | | 80,812 |
Ming Zhao(3) | | | 4,048 | | | 4,151 |
Total | | $ | 159,612 | | $ | 866,886 |
135
Table of Contents
Related party transactions
Nature | | For the Years Ended
September 30, |
2023 | | 2022 |
Loan to Hangzhou Shuige(1) | | $ | 423,585 | | $ | 1,983,764 |
Repayment from Hangzhou Shuige(1) | | | 423,585 | | | 5,889,698 |
Loan to Liwei Zhang(1) | | | 303,075 | | | — |
Repayment from Liwei Zhang(1) | | | 362,377 | | | — |
Advance to Baiming Yu(2) | | | 1,187,554 | | | 17,148 |
Reimbursement from Baiming Yu(2) | | | 644,678 | | | — |
Selling to Hangzhou Qingniu(3) | | | 1,011,587 | | | 16,524 |
Loan to Hangzhou Qingniu(1) | | | 4,943,174 | | | 7,356,760 |
Rent income from Hangzhou Qingniu(4) | | | 52,749 | | | 192,636 |
Repayment from Hangzhou Qingniu(1)(3)(4) | | | 5,105,411 | | | 7,371,232 |
Loan from Xiaoshan Ance(5) | | | — | | | 152,597 |
Advance from Shuang Wu(6) | | | 2,022,703 | | | 851,143 |
Advance to Shuang Wu(6) | | | 819,227 | | | — |
Repayment to Shuang Wu(6) | | | 2,648,510 | | | 174,800 |
Advance to Huiyu Chuanggu(7) | | | 2,894,499 | | | — |
Reimbursement from Huiyu Chuanggu(7) | | | 1,397,077 | | | — |
Rent income from Hangzhou Yuanqi(4) | | | — | | | 3,815 |
Collection from Hangzhou Yuanqi(4) | | | 706 | | | 3,815 |
Advance to Ming Zhao(8) | | | 866 | | | — |
Collection from Ming Zhao(8) | | | 866 | | | 1,958 |
Advance from Hangzhou Yizhiying Information Consulting Management Co., Ltd.(9) | | | — | | | 149,606 |
Repayment to Hangzhou Yizhiying Information Consulting Management Co., Ltd.(9) | | | — | | | 61,885 |
136
Table of Contents
Years Ended September 30, 2022 and 2021
Amounts due from related parties
Amounts due from related parties consisted of the following for the periods indicated:
| | As of
September 30, |
| | | 2022 | | 2021 |
Hangzhou Shuige(1) | | $ | — | | $ | 3,952,319 |
Liwei Zhang(2) | | | 70,514 | | | 77,847 |
Hangzhou Qingniu(3) | | | 429,347 | | | — |
Baicheng Zhang(4) | | | 46,012 | | | 50,797 |
Baiming Yu(5) | | | 160,405 | | | 194,527 |
Total | | $ | 706,278 | | $ | 4,275,490 |
Amounts due to related parties
Amounts due to related parties consisted of the following for the periods indicated:
| | As of
September 30, |
2022 | | 2021 |
Shuang Wu(1) | | $ | 623,070 | | $ | — |
Xiaoshan Ance(3) | | | 140,578 | | | — |
Hangzhou Shuige(3) | | | 18,275 | | | — |
Hangzhou Yizhiying Information Consulting Management Co., Ltd(2) | | | 80,812 | | | — |
Ming Zhao(2) | | | 4,151 | | | — |
Total | | $ | 866,886 | | $ | — |
137
Table of Contents
Related party transactions
Nature | | For the years ended
September 30, |
2022 | | 2021 |
Loan to Hangzhou Shuige(1) | | $ | 1,983,764 | | $ | 11,770,039 |
Repayment from Hangzhou Shuige(1) | | | 5,889,698 | | | 8,107,731 |
Loan to Liwei Zhang(1) | | | — | | | 11,065 |
Repayment from Liwei Zhang(1) | | | — | | | 1,060,862 |
Withholding tax receivables from Liwei Zhang(6) | | | — | | | 77,084 |
Advance to Baiming Yu(2) | | | 17,148 | | | 15,368 |
Reimbursement from Baiming Yu(2) | | | — | | | 112,020 |
Withholding tax receivables from Baiming Yu(7) | | | — | | | 156,504 |
Repayment from Zhang Baicheng(1) | | | — | | | 61,470 |
Selling to Hangzhou Qingniu(3) | | | 16,524 | | | 1,270,093 |
Loan to Hangzhou Qingniu(3) | | | 7,356,760 | | | — |
Rent income from Hangzhou Qingniu(3) | | | 192,636 | | | — |
Repayment from Hangzhou Qingniu(3) | | | 7,371,232 | | | 1,427,617 |
Loan to Xiaoshan Ance(1) | | | 152,597 | | | — |
Repayment from Xiaoshan Ance(1) | | | — | | | 766,889 |
Repayment from Qijia Yu(4) | | | — | | | 888,954 |
Advance from Shuang Wu(5) | | | 851,143 | | | — |
Repayment to Shuang Wu(5) | | | 174,800 | | | — |
Rent income from Hangzhou Yuanqi(3) | | | 3,815 | | | — |
Collection from Hangzhou Yuanqi(3) | | | 3,815 | | | — |
Collection from Ming Zhao(5) | | | 1,958 | | | — |
Advance from Hangzhou Yizhiying Information Consulting Management Co., Ltd(5) | | | 149,606 | | | — |
Repayment to Hangzhou Yizhiying Information Consulting Management Co., Ltd(5) | | | 61,885 | | | — |
138
Table of Contents
Share Issuances in March 2022
On March 1, 2022, we issued the following Ordinary Shares, par value $1.00 per share, to certain founding shareholders of Work Cayman:
Purchaser | | Number of
Ordinary
Shares | | Consideration |
Tricor Services (Cayman Islands) Limited | | 1 | | $ | 1 |
LWY GROUP LTD | | 24,999 | | $ | 24,999 |
ZLW GROUP LTD | | 2,500 | | $ | 2,500 |
Share Transfer in March 2022
On March 1, 2022, Tricor Services (Cayman Islands) Limited transferred its one share of Work Cayman to LWY GROUP LTD for $1.
Change in Authorized Share Capital and Share Subdivision in April 2023
On April 6, 2023, the shareholders of the Company unanimously passed resolutions effecting the subdivision of the Company’s authorized and issued share capital and the adoption of the amended and restated memorandum of association, pursuant to which, (1) the Company effectuated a 1:2000 share subdivision, whereupon the Company’s authorized share capital was amended from US$50,000 divided into 50,000 shares of par value US$1.00 each to US$50,000 divided into 100,000,000 Ordinary Shares of a par value of $0.0005 each; and (2) immediately after the share subdivision, the shareholders voluntarily surrendered, on a pro rata basis, a total of 87,500,000 Ordinary Shares of a par value of $0.0005 each, after which, the Company had an aggregate of 12,500,000 Ordinary Shares issued and outstanding.
139
Table of Contents
DESCRIPTION OF SHARE CAPITAL
We are a Cayman Islands exempted company and our affairs are governed by our amended and restated memorandum and articles of association and the Companies Act of the Cayman Islands (the “Companies Act”).
We have adopted an amended and restated memorandum and articles of association (the “Articles”). The following is a summary of the Articles. For further details, please refer to Exhibit 3.1 and Exhibit 3.2 filed with the registration statement of which this prospectus forms a part.
Our authorized share capital is US$50,000 divided into 100,000,000 Ordinary Shares, par value $0.0005 per share. As of the date of this prospectus, 14,591,942 Ordinary Shares were issued and outstanding.
We were incorporated as an exempted company with limited liability under the Companies Act on March 1, 2022. A Cayman Islands exempted company:
• is a company that conducts its business mainly outside the Cayman Islands;
• is prohibited from trading in the Cayman Islands with any person, firm or corporation except in furtherance of the business of the exempted company carried on outside the Cayman Islands (and for this purpose can effect and conclude contracts in the Cayman Islands and exercise in the Cayman Islands all of its powers necessary for the carrying on of its business outside the Cayman Islands);
• does not have to hold an annual general meeting;
• does not have to make its register of members open to inspection by shareholders of that company;
• may obtain an undertaking against the imposition of any future taxation;
• may register by way of continuation in another jurisdiction and be deregistered in the Cayman Islands;
• may register as a limited duration company; and
• may register as a segregated portfolio company.
The following are summaries of material provisions of our Articles and the Companies Act insofar as they relate to the material terms of our Ordinary Shares.
Units Being Offered
We are offering 2,500,000 Ordinary Units at the assumed public offering price of $6.00 per Ordinary Unit, with each Ordinary Unit consisting of (i) one Ordinary Share, (ii) one Series A Warrant, and (iii) one Series B Warrant. To the extent that the purchase of Ordinary Units would cause the beneficial ownership of a purchaser in this offering, together with its affiliates, to exceed 4.99% (or, at the election of the purchaser, 9.99%) of the Ordinary Shares immediately following the consummation of this offering, the Company agrees to issue, at the election of the purchasers, a number of Pre-Funded Ordinary Units in lieu of the Ordinary Units. Each Pre-Funded Ordinary Unit consists of (i) one Pre-Funded Warrant, (ii) one Series A Warrant, and (iii) one Series B Warrant. The purchase price of each Pre-Funded Ordinary Unit will equal the price per Unit, minus $0.0005, and the exercise price of each Pre-Funded Warrant included in the Pre-Funded Ordinary Unit will be $0.0005 per share. For each Pre-Funded Warrant that we sell, the number of Ordinary Units that we are offering will be decreased on a one-for-one basis.
The Ordinary Shares and the Warrants included in the Units are being sold in this offering only as part of the Units. However, the Units will not be certificated, and the Ordinary Shares and the Warrants comprising such Units will be immediately separable and will be issued separately. Upon issuance, the Ordinary Shares and the Warrants may be transferred independent of one another, subject to applicable law and transfer restrictions.
Ordinary Shares
General
Under our Articles, the objects of our Company are unrestricted and we have the full power and authority to carry out any object not prohibited by the law of the Cayman Islands.
140
Table of Contents
All of our issued and outstanding Ordinary Shares will be fully paid up and non-assessable. Our Ordinary Shares are issued in registered form, and are issued when registered in our register of members. Unless the directors determine otherwise, each holder of our ordinary share will not receive a certificate in respect of such ordinary share. Our shareholders who are non-residents of the Cayman Islands may freely hold and vote their ordinary share. We may not issue shares or warrants to bearer.
Voting Rights
A shareholder may participate in a general meeting in person, by proxy or by telephone or other electronic means. At any general meeting a resolution put to the vote at the meeting shall be decided on a show of hands, unless a poll is (before or on the declaration of the result of the show of hands) demanded by the chairman of such meeting or by at least two shareholders having the right to vote on the resolutions or one or more shareholders present, in person or by proxy, who individually or together hold not less than ten percent of the voting rights of all those who are entitled to vote on the resolution. Unless a poll is so demanded, a declaration by the chairman as to the result of a resolution and an entry to that effect in the minutes of the meeting, shall be conclusive evidence of the outcome of a show of hands, without proof of the number or proportion of the votes recorded in favor of, or against, that resolution.
If a poll is duly demanded it shall be taken in such manner as the chairman directs and the result of the poll shall be deemed to be the resolution of the meeting at which the poll was demanded.
In the case of an equality of votes, whether on a show of hands or on a poll, the chairman of the meeting at which the show of hands takes place or at which the poll is demanded, shall not be entitled to a second or casting vote.
As a matter of Cayman Islands law, (i) an ordinary resolution requires the affirmative vote of a majority of votes cast by shareholders who, being entitled to do so, attend and vote at a general meeting of the company; and (ii) a resolution is deemed to be a special resolution where it has been approved by either (a) at least two-thirds (or any higher threshold specified in a company’s articles of association) of votes cast by shareholders who attend and vote at a general meeting for which notice specifying the intention to propose the resolution as a special resolution has been given; or (b) if so authorized by a company’s articles of association, by a unanimous written resolution of all of the company’s shareholders.
Under Cayman Islands law, some matters, such as amending the memorandum and articles of association, changing the name or resolving to be registered by way of continuation in a jurisdiction outside the Cayman Islands, require the approval of shareholders by a special resolution.
General Meetings of Shareholders and Shareholder Proposals
As a Cayman Islands exempted company, we are not obliged by the Companies Act to call shareholders’ annual general meetings. Accordingly, we may, but shall not be obliged to, in each year hold a general meeting as an annual general meeting. Any annual general meeting held shall be held at such time and place as may be determined by our board of directors. All general meetings other than annual general meetings shall be called extraordinary general meetings.
The directors may convene general meetings whenever they think fit. General meetings shall also be convened on the written requisition of one or more of the shareholders entitled to attend and vote at our general meetings who (together) hold at least ten percent of the rights to vote at such general meeting in accordance with the notice provisions in the articles, specifying the purpose of the meeting and signed by each of the shareholders making the requisition. If the directors do not convene such meeting for a date not later than 21 clear days’ after the date of receipt of the written requisition, those shareholders who requested the meeting may convene the general meeting themselves within three months after the end of such period of 21 clear days in which case reasonable expenses incurred by them as a result of the directors failing to convene a meeting shall be reimbursed by us.
At least 5 clear days’ notice of an extraordinary general meeting and 5 clear days’ notice of an annual general meeting shall be given to shareholders entitled to attend and vote at such meeting. The notice shall specify the place, the day and the hour of the meeting and the general nature of that business. In addition, if a resolution is proposed as a special resolution, the text of that resolution shall be given to all shareholders. Notice of every general meeting shall also be given to the directors and our auditors.
141
Table of Contents
Subject to the Companies Act and with the consent of the shareholders who, individually or collectively, hold at least 90 percent of the voting rights of all those who have a right to vote at a general meeting, a general meeting may be convened on shorter notice.
A quorum required for any general meeting of shareholders consists of one or more shareholders present or by proxy, holding shares which carry in aggregate not less than one-third of all votes attaching to all of our shares in issue and entitled to vote at such general meeting.
If, within 15 minutes from the time appointed for the general meeting, or at any time during the meeting, a quorum is not present, the meeting, if convened upon the requisition of shareholders, shall be cancelled. In any other case it shall stand adjourned to the same time and place seven days or to such other time or place as is determined by the directors.
The chairman may, with the consent of a meeting at which a quorum is present, adjourn the meeting. When a meeting is adjourned for seven days or more, notice of the adjourned meeting shall be given in accordance with the articles of association.
Dividends
Subject to the Companies Act and any rights and restrictions of any other class or series of shares, our board of directors may, from time to time and in accordance with our Articles, declare dividends on the shares issued and authorize payment of the dividends out of our lawfully available funds. Under the laws of the Cayman Islands, our company may pay a dividend out of either profit or the share premium account, provided that in no circumstances may a dividend be paid if this would result in our company being unable to pay its debts as they fall due in the ordinary course of business. The “share premium account,” represents the excess of the price paid to our company on the issue of its shares over the par or “nominal” value of those shares, which is similar to the U.S. concept of additional paid in capital.
Unless provided for by the rights attached to a share, no dividend or other monies payable by the us in respect of a share shall bear interest.
Transfer of Ordinary Shares
Provided that a transfer of Ordinary Shares complies with applicable rules of the SEC, Nasdaq Capital Market, and federal and state security laws of the United States, a shareholder may transfer Ordinary Shares to another person by completing an instrument of transfer in a common form or in a form prescribed by Nasdaq Capital Market or in any other form approved by the directors, executed:
(a) where the Ordinary Shares are fully paid, by or on behalf of that shareholder; and
(b) where the Ordinary Shares are partly paid, by or on behalf of that shareholder and the transferee.
Our Board of Directors may, in its absolute discretion, decline to register any transfer of any Ordinary Share.
If our directors refuse to register a transfer they shall, within three months after the date on which the instrument of transfer was lodged, send to each of the transferor and the transferee notice of such refusal.
This, however, is unlikely to affect market transactions of the Ordinary Shares purchased by investors in the public offering. Once the Ordinary Shares have been listed, the legal title to such Ordinary Shares and the registration details of those Ordinary Shares in the Company’s register of members will remain with VStock Transfer LLC. All market transactions with respect to those Ordinary Shares will then be carried out without the need for any kind of registration by the directors, as the market transactions will all be conducted through the DTC systems.
The registration of transfers may, after compliance with any notice required of the Nasdaq Capital Market, be suspended and the register closed at such times and for such periods as our Board of Directors may from time to time determine, provided, however, that the registration of transfers shall not be suspended nor the register closed for more than 30 days in any calendar year as our board may determine.
142
Table of Contents
Winding Up; Liquidation
Upon the winding up of our company, after the full amount that holders of any issued shares ranking senior to the Ordinary Shares as to distribution on liquidation or winding up are entitled to receive has been paid or set aside for payment, the holders of our Ordinary Shares are entitled to receive any remaining assets of our company available for distribution as determined by the liquidator. The shareholders may, subject to our Articles and any other sanction required by the Companies Act, pass a special resolution allowing the liquidator to do either or both of the following:
• to divide in specie among the shareholders the whole or any part of our assets and, for that purpose, to value any assets and to determine how the division shall be carried out as between the shareholders or different classes of shareholders; and
• to vest the whole or any part of the assets in trustees for the benefit of shareholders and those liable to contribute to the winding up.
The directors have the authority to present a petition for our winding up to the Grand Court of the Cayman Islands on our behalf without the sanction of a resolution passed at a general meeting.
Calls on Ordinary Shares and Forfeiture of Ordinary Shares
Our Board of Directors may from time to time make calls upon shareholders for any amounts unpaid on their Ordinary Shares in a notice served to such shareholders at least 14 clear days prior to the specified time and place of payment. Any Ordinary Shares that have been called upon and remain unpaid are subject to forfeiture. No shareholder shall be entitled to vote at any general meeting unless all calls or other sums payable by such shareholder have been paid. Our Board of Directors may deduct from a dividend or any other amount payable to a person in respect of an Ordinary Share any amount due by that person to the Company on a call or otherwise in relation to an Ordinary Share.
Redemption of Shares, Repurchase and Surrender of Shares
Subject to the Companies Act and any rights for the time being conferred on the shareholders holding a particular class of shares, we may by action of our directors:
1. issue shares that are to be redeemed or liable to be redeemed, at our option or the shareholder holding those redeemable shares, on the terms and in the manner our directors determine before the issue of those shares;
2. with the consent by special resolution of the shareholders holding shares of a particular class, vary the rights attaching to that class of shares so as to provide that those shares are to be redeemed or are liable to be redeemed at our option on the terms and in the manner which the directors determine at the time of such variation; and
3. purchase all or any of our own shares of any class including any redeemable shares on the terms and in the manner which the directors determine at the time of such purchase.
We may make a payment in respect of the redemption or purchase of its own shares in any manner authorized by the Companies Act, including out of any combination of capital, our profits and the proceeds of a fresh issue of shares.
When making a payment in respect of the redemption or purchase of shares, the directors may make the payment in cash or in specie (or partly in one and partly in the other) if so authorized by the terms of the allotment of those shares or by the terms applying to those shares, or otherwise by agreement with the shareholder holding those shares.
Under the Companies Act, the redemption or repurchase of any share may be paid out of our Company’s profits or out of the proceeds of a new issue of shares made for the purpose of such redemption or repurchase, or out of capital (including share premium account and capital redemption reserve). If the repurchase proceeds are paid out of our Company’s capital, our Company must, immediately following such payment, be able to pay its debts as they fall due in the ordinary course of business. In addition, under the Companies Act, no such share may be redeemed or repurchased (a) unless it is fully paid up, (b) if such redemption or repurchase would result in there being no shares outstanding and (c) unless the manner of purchase (if not so authorized under the articles of association) has first been authorized by a resolution of our shareholders.
143
Table of Contents
In addition, under the Companies Act, our Company may accept the surrender of any fully paid share for no consideration unless, as a result of the surrender, the surrender would result in there being no shares outstanding (other than shares held as treasury shares).
Variations of Rights of Shares
If at any time, our share capital is divided into different classes of shares, the rights attached to any class of shares (unless otherwise provided by the terms of issue of the shares of that class), whether or not our Company is being wound-up, may be varied either with the consent in writing of the holders of two-thirds of the issued shares of that class or with the sanction of a resolution passed by a majority of two-thirds of the votes cast at a separate meeting of the issued shares of that class. The rights conferred upon the holders of the shares of any class issued shall not, unless otherwise expressly provided by the terms of issue of the shares of that class, be deemed to be varied by the creation or issue of further shares ranking pari passu with such existing class of shares.
Issuance of Additional Shares
Our Articles authorize our Board of Directors to issue additional Ordinary Shares from time to time as our Board of Directors shall determine, to the extent of available authorized but unissued shares.
Inspection of Books and Records
Holders of our Ordinary Shares will have no general right under Cayman Islands law to inspect or obtain copies of our list of shareholders or our corporate records. However, we will provide our shareholders with annual audited financial statements. See “Where You Can Find Additional Information.”
Anti-Takeover Provisions
Some provisions of our Articles may discourage, delay or prevent a change of control of our Company or management that shareholders may consider favorable, including provisions that that authorize our board of directors to issue shares at such times and on such terms and conditions as the board of directors may decide without any further vote or action by our shareholders, and provisions that restrict the ability of our shareholders to call meetings and to propose special matters for consideration at shareholder meetings.
However, under Cayman Islands law, our directors may only exercise the rights and powers granted to them under our Articles for a proper purpose and for what they believe in good faith to be in the best interests of our Company.
Changes in Capital
Subject to the Companies Act, our shareholders may from time to time by ordinary resolution:
• increase the share capital by such sum, to be divided into shares of such classes and amount, as the resolution prescribes;
• consolidate and divide all or any of our share capital into shares of a larger amount than our existing shares;
• convert all or any of its paid up shares into stock and reconvert the stock into paid up shares of any denomination;
• sub-divide our existing shares, or any of them into shares of a smaller amount than that fixed by our Articles; provided that in the subdivision the proportion between the amount paid and the amount, if any, unpaid on each reduced share will be the same as it was in case of the share from which the reduced share is derived; and
• cancel any shares which, at the date of the passing of the resolution, have not been taken or agreed to be taken by any person and diminish the amount of our share capital by the amount of the shares so cancelled or, in the case of shares without nominal par value, diminish the number of shares into which our capital is divided.
144
Table of Contents
Exempted Company
We are an exempted company with limited liability under the Companies Act. The Companies Act distinguishes between ordinary resident companies and exempted companies. Any company that is registered in the Cayman Islands but conducts business mainly outside of the Cayman Islands may apply to be registered as an exempted company. The requirements for an exempted company are essentially the same as for an ordinary company except that an exempted company that does not hold a license to carry on business in the Cayman Islands:
• does not have to file an annual return of its shareholders with the Registrar of Companies;
• is not required to open its register of members for inspection;
• does not have to hold an annual general meeting;
• is prohibited from making any invitation to the public in the Cayman Islands to subscribe for any of its securities;
• may not issue negotiable or bearer shares, but may issue shares with no par value;
• may obtain an undertaking against the imposition of any future taxation (such undertakings are usually given for 20 years in the first instance);
• may register by way of continuation in another jurisdiction and be deregistered in the Cayman Islands;
• may register as an exempted limited duration company; and
• may register as a segregated portfolio company.
“Limited liability” means that the liability of each shareholder is limited to the amount unpaid by the shareholder on the shares of the company (except in exceptional circumstances, such as involving fraud, the establishment of an agency relationship or an illegal or improper purpose or other circumstances in which a court may be prepared to pierce or lift the corporate veil).
Register of Members
Under the Companies Act, we must keep a register of members and there should be entered therein:
• the names and addresses of our members, and, a statement of the shares held by each member, which:
• distinguishes each share by its number (so long as the share has a number);
• confirms the amount paid, or agreed to be considered as paid, on the shares of each member;
• confirms the number and category of shares held by each member; and
• confirms whether each relevant category of shares held by a member carries voting rights under the articles of association of the company, and if so, whether such voting rights are conditional
• the date on which the name of any person was entered on the register as a member; and
• the date on which any person ceased to be a member.
Under Cayman Islands law, the register of members of our Company is prima facie evidence of the matters set out therein (i.e., the register of members will raise a presumption of fact on the matters referred to above unless rebutted) and a member registered in the register of members is deemed as a matter of Cayman Islands law to have legal title to the shares as set against its name in the register of members. Once our register of members has been updated, the shareholders recorded in the register of members will be deemed to have legal title to the shares set against their name.
If the name of any person is incorrectly entered in or omitted from our register of members or if there is any default or unnecessary delay in entering on the register the fact of any person having ceased to be a member of our Company, the person or member aggrieved (or any member of our Company or our Company itself) may apply to the Grand Court of the Cayman Islands for an order that the register be rectified. The Court may either refuse such application or it may, if satisfied of the justice of the case, make an order for the rectification of the register.
145
Table of Contents
Warrants
The following summary of certain terms and provisions of each of the Pre-Funded Warrants, the Series A Warrants, and the Series B Warrants offered hereby is not complete and is subject to, and qualified in its entirety by, the provisions of the form of each of the Warrants, which will be filed as exhibits to the registration statement of which this prospectus forms a part. Prospective investors should carefully review the terms and provisions set forth in the applicable form of Warrants.
Exercisability and Duration. The aggregate exercise price of the Pre-Funded Warrant, except for a nominal exercise price of $0.0005 per Ordinary Share, will be pre-funded to the Company on or prior to its issuance date. The Series A Warrants will have five-year terms, will be immediately exercisable upon issuance, and have an initial exercise price equal to the actual public offering price of the Ordinary Units. Such exercise price will be reset on the Reset Date to a price that is equal to 105% of the arithmetic average of the sum of the three lowest per share VWAPs of the Ordinary Shares on the trading market for the twenty (20) trading days immediately prior to the Reset Date; provide that the exercise price shall not be lower than the Series A Floor Price. Notwithstanding the foregoing, the Company may reduce the Series A Floor Price to any amounts set forth in a written notice to the holder of the Series A Warrants; provided that such reduction shall be in compliance with applicable Nasdaq rules, irrevocable and not be subject to increase thereafter. The Series B Warrants will have five-years terms and will be immediately exercisable upon issuance at an exercise price equal to the actual public offering price of the Ordinary Units. The Series B Warrants may also be exercised in whole by means of a one-time only “alternative cashless exercise” in which the holder shall be entitled to receive a number of Ordinary Shares equal to the product of (a) the aggregate number of Ordinary Shares that would be issuable upon exercise of the Series B Warrants if such exercise were by means of a cash exercise rather than a cashless exercise; multiplied by (b) the quotient obtained by dividing (i) the exercise price minus the lowest VWAP of the Ordinary Shares over the 10 trading days immediately prior to the exercise date by (ii) 50% of the lowest VWAP of the Ordinary Shares over the 10 trading days immediately prior to the exercise date. Such alternative cashless exercise is subject to the Beneficial Ownership Limitation (as defined below), and shall only be available at a time when the applicable VWAP is lower than the then applicable exercise price. The maximum number of Ordinary Shares issuable pursuant to such alternative cashless exercise shall be equal to the quotient obtained by dividing (a) the aggregate exercise price of the Series B Warrant as of its issuance date, by (b) the Series B Floor Price. Notwithstanding the foregoing, the Company may reduce the Series B Floor Price to any amounts set forth in a written notice to the holder of the Series B Warrants; provided that such reduction shall be in compliance with applicable Nasdaq rules, irrevocable and not be subject to increase thereafter.
Beneficial Ownership Limitations. The Company shall not effect the exercise of any portion of the Warrants, and a holder shall not have the right to exercise any portion of the Warrants, pursuant to the terms and conditions of the Warrants and any such exercise shall be null and void and treated as if never made, to the extent that after giving effect to such exercise, the holder together with any other attribution parties, collectively, would beneficially own in excess of 4.99% (or, upon election by the holder prior to the issuance of the Warrants, 9.99%) of the number of Ordinary Shares outstanding immediately after giving effect to such exercise (the “Beneficial Ownership Limitations”). For purposes of the foregoing sentence, the aggregate number of Ordinary Shares beneficially owned by the holder and any other attribution parties shall include the number of Ordinary Shares held by the holder and all other attribution parties plus the number of Ordinary Shares issuable upon exercise of the Warrants with respect to which the determination of such sentence is being made, but shall exclude the number of Ordinary Shares which would be issuable upon (A) exercise of the remaining unexercised portion of the Warrants beneficially owned by the holder or any of the other attribution parties, and (B) exercise or conversion of the unexercised or unconverted portion of any other securities of the Company (including, without limitation, any other Ordinary Shares equivalents) subject to a limitation on conversion or exercise analogous to this limitation.
Dividends and Share Splits. If the Company, at any time while any of the Warrants are outstanding: (i) pays a stock dividend or otherwise makes a distribution or distributions on shares of its Ordinary Shares or any other equity or equity equivalent securities payable in Ordinary Shares, (ii) subdivides outstanding Ordinary Shares into a larger number of shares, (iii) combines (including by way of reverse share split) outstanding Ordinary Shares into a smaller number of shares, or (iv) issues by reclassification of Ordinary Shares or any other shares of the Company, then in each case the exercise price and the number of shares issuable upon exercise of the Warrant shall be proportionately adjusted. However, if the adjustment above would otherwise result in an increase in the exercise price of the Warrant, no adjustment shall be made.
146
Table of Contents
Transferability. Subject to applicable laws, the Warrants may be offered for sale, sold, transferred or assigned at the option of the holder upon surrender of the Warrants to us together with the appropriate instruments of transfer.
Exchange Listing. We do not plan on applying to list the Warrants or the Pre-Funded Warrants on the Nasdaq Capital Market, any other national securities exchange or any other nationally recognized trading system.
Fundamental Transactions. In the event of a fundamental transaction (“Fundamental Transaction”), as described in the Warrants and generally including any merger, consolidation, sale of substantially all assets, or other change of control transaction in which the Company’s shareholders immediately prior to such transaction own less than 50% of the voting power of the surviving entity, the holders of the Warrants will be entitled to receive upon exercise of the Warrants the kind and amount of securities, cash or other property that a holder of the number of Ordinary Shares for which such Warrant was exercisable immediately prior to the Fundamental Transaction would have been entitled to receive pursuant to such transaction, or at the option of the holder, the Company or successor entity shall purchase such portion of the Warrant that remains outstanding after the Fundamental Transaction for cash equal to the Black-Scholes value thereof. If the Company is not the surviving entity in the Fundamental Transaction, any successor entity shall assume the obligations under such Warrant.
Governing Law. The Warrants shall be governed by and construed and enforced in accordance with, and all questions concerning the construction, validity, interpretation and performance of the Warrants shall be governed by, the internal laws of the State of New York, without giving effect to any choice of law or conflict of law provision or rule that would cause the application of the laws of any jurisdictions other than the State of New York.
Subsequent Rights Offerings. If at any time the Company grants, issues or sells any Ordinary Share equivalents or rights to purchase stock, warrants, securities or other property pro rata to the record holders of Ordinary Share (the “Purchase Rights”), then the holder of Warrants will be entitled to acquire, upon the terms applicable to such Purchase Rights, the aggregate Purchase Rights which the holder could have acquired if the holder had held the number of Ordinary Shares acquirable upon complete exercise of the Warrants (without regard to any limitations on exercise hereof, including without limitation, the Beneficial Ownership Limitations) immediately before the date on which a record is taken for the grant, issuance or sale of such Purchase Rights, or, if no such record is taken, the date as of which the record holders of Ordinary Shares are to be determined for the grant, issue or sale of such Purchase Rights (provided, however, that, to the extent that the holder’s right to participate in any such Purchase Right would result in the holder exceeding the Beneficial Ownership Limitations, then the holder shall not be entitled to participate in such Purchase Right to such extent (or beneficial ownership of such Ordinary Shares as a result of such Purchase Right to such extent) and such Purchase Right to such extent shall be held in abeyance for the holder until such time, if ever, as its right thereto would not result in the holder exceeding the Beneficial Ownership Limitations).
Pro Rata Distributions. During such time as the Warrants are outstanding, if the Company shall declare or make any dividend or other distribution of its assets (or rights to acquire its assets) to holders of Ordinary Shares, by way of return of capital or otherwise (including, without limitation, any distribution of cash, stock or other securities, property or options by way of a dividend, spin off, reclassification, corporate rearrangement, scheme of arrangement or other similar transaction) (a “Distribution”), at any time after the issuance of the Warrants, then, in each such case, the holder of Warrants shall be entitled to participate in such Distribution to the same extent that the holder would have participated therein if the holder had held the number of Ordinary Shares acquirable upon complete exercise of the Warrants (without regard to any limitations on exercise hereof, including without limitation, the Beneficial Ownership Limitations) immediately before the date of which a record is taken for such Distribution, or, if no such record is taken, the date as of which the record holders of Ordinary Shares are to be determined for the participation in such Distribution (provided, however, that, to the extent that the holder’s right to participate in any such Distribution would result in the holder exceeding the Beneficial Ownership Limitations, then the holder shall not be entitled to participate in such Distribution to such extent (or in the beneficial ownership of any Ordinary Shares as a result of such Distribution to such extent) and the portion of such Distribution shall be held in abeyance for the benefit of the holder until such time, if ever, as its right thereto would not result in the holder exceeding the Beneficial Ownership Limitations).
Amendment and Waiver. The provisions of the Warrants may be amended or waived, and the Company may take any action, or omit to perform any act required to be performed by it, if the Company has obtained the written consent of the holders of the applicable Warrants.
147
Table of Contents
Differences in Corporate Law
Cayman Islands companies are governed by the Companies Act. The Companies Act is modelled on English law but does not follow recent English statutory enactments, and differs from laws applicable to United States corporations and their shareholders. Set forth below is a summary of the material differences between the provisions of the Companies Act applicable to us and the laws applicable to companies incorporated in the State of Delaware.
Mergers and Similar Arrangements
The Companies Act permits mergers and consolidations between Cayman Islands companies and between Cayman Islands companies and non-Cayman Islands companies. For these purposes, (a) “merger” means the merging of two or more constituent companies and the vesting of their undertaking, property and liabilities in one of such companies as the surviving company, and (b) a “consolidation” means the combination of two or more constituent companies into a consolidated company and the vesting of the undertaking, property and liabilities of such companies to the consolidated company.
In order to effect such a merger or consolidation, the directors of each constituent company must approve a written plan of merger or consolidation, which must then be authorized by (a) a special resolution of the shareholders of each constituent company, and (b) such other authorization, if any, as may be specified in such constituent company’s articles of association.
The written plan of merger or consolidation must be filed with the Registrar of Companies of the Cayman Islands together with, among other things, a declaration as to the solvency of the consolidated or surviving company, a list of the assets and liabilities of each constituent company and an undertaking that a copy of the certificate of merger or consolidation will be given to the members and creditors of each constituent company and that notification of the merger or consolidation will be published in the Cayman Islands Gazette. Court approval is not required for a merger or consolidation which is effected in compliance with these statutory procedures.
A merger between a Cayman parent company and its Cayman subsidiary or subsidiaries does not require authorization by a special resolution of that Cayman subsidiary if a copy of the plan of merger is given to every member of that Cayman subsidiary to be merged unless that member agrees otherwise. For this purpose, a company is a “parent” of a subsidiary if it holds issued shares that together represent at least ninety percent (90%) of the votes at a general meeting of the subsidiary.
The consent of each holder of a fixed or floating security interest over a constituent company is required unless this requirement is waived by a court in the Cayman Islands.
Save in certain limited circumstances, a shareholder of a Cayman constituent company who dissents from the merger or consolidation is entitled to payment of the fair value of his shares (which, if not agreed between the parties, will be determined by the Cayman Islands court) upon dissenting to the merger or consolidation, provided the dissenting shareholder complies strictly with the procedures set out in the Companies Act. The exercise of dissenter rights will preclude the exercise by the dissenting shareholder of any other rights to which he or she might otherwise be entitled by virtue of holding shares, save for the right to seek relief on the grounds that the merger or consolidation is void or unlawful.
Separate from the statutory provisions relating to mergers and consolidations, the Companies Act also contains statutory provisions that facilitate the reconstruction and amalgamation of companies by way of schemes of arrangement, provided that the arrangement is approved by a majority in number of each class of shareholders and creditors with whom the arrangement is to be made, and who must in addition represent seventy-five per cent in value of each such class of shareholders or creditors, as the case may be, that are present and voting either in person or by proxy at a meeting, or meetings, convened for that purpose. The convening of the meetings and subsequently the arrangement must be sanctioned by the Grand Court of the Cayman Islands.
While a dissenting shareholder has the right to express to the court the view that the transaction ought not to be approved, the court can be expected to approve the arrangement if it determines that:
• the statutory provisions as to the required majority vote have been met;
• the shareholders have been fairly represented at the meeting in question;
148
Table of Contents
• the arrangement is such that may be reasonably approved by an intelligent and honest man of that class acting in respect of his interest; and
• the arrangement is not one that would more properly be sanctioned under some other provision of the Companies Act.
The Companies Act also contains a statutory power of compulsory acquisition which may facilitate the “squeeze out” of dissentient minority shareholder upon a tender offer. When a tender offer is made and accepted by holders of 90% of the shares affected within four months, the offeror may, within a two-month period commencing on the expiration of such four month period, give notice in the prescribed manner to any dissenting shareholders to require them to transfer such shares to the offeror on the terms of the offer, unless an application is made by the dissenting shareholder to the Court for an order otherwise within one month from the date on which the notice was given. An objection can be made to the Grand Court of the Cayman Islands but this is unlikely to succeed in the case of an offer which has been so approved unless there is evidence of fraud, bad faith or collusion.
If an arrangement and reconstruction by way of scheme of arrangement is thus approved and sanctioned, or if a tender offer is made and accepted in accordance with the foregoing statutory procedures, a dissenting shareholder would have no rights comparable to appraisal rights, save that objectors to a takeover offer may apply to the Grand Court of the Cayman Islands for various orders that the Grand Court of the Cayman Islands has a broad discretion to make, which would otherwise ordinarily be available to dissenting shareholders of Delaware corporations, providing rights to receive payment in cash for the judicially determined value of the shares.
Squeeze-out Provisions
When a take-over offer is made and accepted by holders of not less than 90% of the shares affected within four months, the offer may, within a two-month period commencing on the expiration of such four months period, give notice to require the holders of the remaining shares to transfer such shares on the terms of the offer. An objection can be made to the Grand Court of the Cayman Islands by a dissenting shareholder within one month from the date on which the notice was given but this is unlikely to succeed unless there is evidence of fraud, bad faith or collusion.
If the arrangement and reconstruction is thus approved, the dissenting shareholder would have no rights comparable to appraisal rights, which would otherwise ordinarily be available to dissenting shareholders of United States corporations, providing rights to receive payment in cash for the judicially determined value of the shares.
When a takeover offer is made and accepted by holders of 90% of the shares to whom the offer is made within four months, the offeror may, within a two-month period commencing on the expiration of such four months period, give notice to require the dissenting shareholders to transfer such shares on the terms of the offer, unless an application is made to the Grand Court of the Cayman Islands by the dissenting shareholder within one month from the date on which the notice was given.
Further, transactions similar to a merger, reconstruction and/or an amalgamation may in some circumstances be achieved through other means than these statutory provisions, such as by way of a share capital exchange, asset acquisition or control of an operating business through contractual arrangements.
Shareholders’ Suits
In principle, we will be the proper plaintiff to sue for a wrong done to us as a company and as a general rule, a derivative action may not be brought by a minority shareholder.
However, based on English authorities, which would in all likelihood be of persuasive authority and be applied by a court in the Cayman Islands, the Cayman Islands courts can be expected (and have had occasion) to follow and apply the common law principles which laid out certain exceptions to the foregoing principle, including when:
• a company acts or proposes to act illegally or ultra vires and is therefore incapable of ratification by the shareholders;
• the act complained of, although not beyond the scope of the company’s authority, could be effected only if duly authorized by more than a simple majority vote that has not been obtained; and
• those who control the company are perpetrating a “fraud on the minority.”
149
Table of Contents
A shareholder may have a direct right of action against us where the individual rights of that shareholder have been infringed or are about to be infringed.
Indemnification of Directors and Executive Officers and Limitation of Liability
Cayman Islands law does not limit the extent to which a company’s memorandum and articles of association may provide for indemnification of officers and directors, except to the extent any such indemnification may be held by the Cayman Islands courts to be contrary to public policy, such as to provide indemnification against civil fraud or the consequences of committing a crime.
Our Articles provide that to the extent permitted by law, we shall indemnify each existing or former director (including alternate director), secretary and any of our other officers (including an investment adviser or an administrator or liquidator) and their personal representatives against:
• all actions, proceedings, costs, charges, expenses, losses, damages or liabilities incurred or sustained by the existing or former director (including alternate director), secretary or officer in or about the conduct of our business or affairs or in the execution or discharge of the existing or former director (including alternate director), secretary’s or officer’s duties, powers, authorities or discretions; and
• without limitation to paragraph (a) above, all costs, expenses, losses or liabilities incurred by the existing or former director (including alternate director), secretary or officer in defending (whether successfully or otherwise) any civil, criminal, administrative or investigative proceedings (whether threatened, pending or completed) concerning us or our affairs in any court or tribunal, whether in the Cayman Islands or elsewhere.
No such existing or former director (including alternate director), secretary or officer, however, shall be indemnified in respect of any matter arising out of their own actual fraud, willful default or willful neglect.
This standard of conduct is generally the same as is permitted under the Delaware General Corporation Law for a Delaware corporation.
In addition, we have entered into indemnification agreements with our directors and executive officers that provide such persons with additional indemnification beyond that provided in our Articles.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to our directors, officers or persons controlling us under the foregoing provisions, we have been informed that, in the opinion of the SEC, such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable.
Directors’ Fiduciary Duties
Under Delaware corporate law, a director of a Delaware corporation has a fiduciary duty to the corporation and its shareholders. This duty has two components: the duty of care and the duty of loyalty. The duty of care requires that a director act in good faith, with the care that an ordinarily prudent person would exercise under similar circumstances. Under this duty, a director must inform himself of, and disclose to shareholders, all material information reasonably available regarding a significant transaction.
The duty of loyalty requires that a director acts in a manner he or she reasonably believes to be in the best interests of the corporation. He or she must not use his corporate position for personal gain or advantage. This duty prohibits self-dealing by a director and mandates that the best interest of the corporation and its shareholders take precedence over any interest possessed by a director, officer or controlling shareholder and not shared by the shareholders generally.
In general, actions of a director are presumed to have been made on an informed basis, in good faith and in the honest belief that the action taken was in the best interests of the corporation. However, this presumption may be rebutted by evidence of a breach of one of the fiduciary duties. Should such evidence be presented concerning a transaction by a director, the director must prove the procedural fairness of the transaction and that the transaction was of fair value to the corporation.
As a matter of Cayman Islands law, a director of a Cayman Islands company owes three types of duties to the company: (i) statutory duties, (ii) fiduciary duties, and (iii) common law duties. The Companies Act imposes a number of statutory duties on a director. A Cayman Islands director’s fiduciary duties are not codified, however the courts of the
150
Table of Contents
Cayman Islands have held that a director owes the following fiduciary duties (a) a duty to act in what the director bona fide considers to be in the best interests of the company, (b) a duty to exercise their powers for the purposes they were conferred, (c) a duty to avoid fettering his or her discretion in the future and (d) a duty to avoid conflicts of interest and of duty.
The common law duties owed by a director are those to act with skill, and care and diligence that may reasonably be expected of a person carrying out the same functions as are carried out by that director in relation to the company and, also, to act with the skill, care and diligence in keeping with a standard of care commensurate with any particular skill they have which enables them to meet a higher standard than a director without those skills. It was previously considered that a director need not exhibit in the performance of his or her duties a greater degree of skill than may reasonably be expected from a person of his or her knowledge and experience. However, there are indications that English and Commonwealth courts are moving towards an objective standard with regard to the required skill and care and these authorities are likely to be followed in the Cayman Islands.
Under statute, our directors are subject to a number of statutory obligations, which provisions prescribe penalties for breach. The most serious of these involves dishonesty or the authorizing of illegal payments and carry both criminal and civil penalties. By way of example, material statutory provisions attracting penalties include where 1) the director willfully authorizes or permits any distribution or dividend in contravention of the Companies Act; 2) where the director knowingly or willfully authorizes or permits any payment out of capital by a company for a redemption or purchase of its own shares when the company is insolvent; 3) where there has been a failure to maintain the books of account, minutes of meetings, or the company’s statutory registers of members, beneficial ownership, mortgages and charges, or directors (which includes alternate directors); 4) where there has been a failure to provide information or access to documents to specified persons as required by the Companies Act; and 5) where the director makes or authorizes a false annual return to the Registrar of Companies.
Shareholder Action by Written Consent
Under the Delaware General Corporation Law, a corporation may eliminate the right of shareholders to act by written consent by amendment to its certificate of incorporation. Cayman Islands law and our Articles provide that shareholders may approve corporate matters and adopt both the ordinary resolutions and the special resolutions by way of unanimous written resolutions signed by all of the shareholders of our Company who would have been entitled to vote on such matter at a general meeting without a meeting being held.
Shareholder Proposals
Under the Delaware General Corporation Law, a shareholder has the right to put any proposal before the annual meeting of shareholders, provided it complies with the notice provisions in the governing documents. A special meeting may be called by the Board of Directors or any other person authorized to do so in the governing documents, but shareholders may be precluded from calling special meetings.
Cayman Islands law provides shareholders with only limited rights to requisition a general meeting and does not provide shareholders with any right to put any proposal before a general meeting. However, these rights may be provided in a company’s articles of association.
Our Articles allow any one or more shareholders where together hold shares which carry in aggregate not less than one-third of the total number of votes attaching to all issued and outstanding shares of our Company entitled to vote at general meetings to requisition an extraordinary general meeting of our shareholders, in which case our board is obliged to convene an extraordinary general meeting and to put the resolutions so requisitioned to a vote at such meeting. If the directors do not convene such meeting for a date not later than twenty-one clear days’ after the date of receipt of the written requisition, those shareholders who requested the meeting may convene the general meeting themselves within three months after the end of such period of twenty-one clear days in which case reasonable expenses incurred by them as a result of the directors failing to convene a meeting shall be reimbursed by us. Other than this right to requisition a shareholders’ meeting, our Articles do not provide our shareholders with any other right to put proposals before annual general meetings or extraordinary general meetings.
As a Cayman Islands exempted company, we are not obliged by the Companies Act to call shareholders’ annual general meetings.
151
Table of Contents
Cumulative Voting
Under the Delaware General Corporation Law, cumulative voting for elections of directors is not permitted unless the corporation’s certificate of incorporation specifically provides for it. Cumulative voting potentially facilitates the representation of minority shareholders on a board of directors since it permits the minority shareholder to cast all the votes to which the shareholder is entitled on a single director, which increases the shareholder’s voting power with respect to electing such director.
There are no prohibitions in relation to cumulative voting under the Companies Act but our Articles do not provide for cumulative voting.
Removal of Directors
Under the Delaware General Corporation Law, a director of a corporation with a classified board may be removed only for cause with the approval of a majority of the outstanding shares entitled to vote, unless the certificate of incorporation provides otherwise.
Subject to the provisions of our Articles (which include the removal of a director by ordinary resolution), the office of a director may be terminated forthwith if (a) he is prohibited by the laws of the Cayman Islands from acting as a director, (b) he is made bankrupt or makes an arrangement or composition with his creditors generally, (c) he resigns his office by notice to us, (d) he only held office as a director for a fixed term and such term expires, (e) in the opinion of a registered medical practitioner by whom he is being treated he becomes physically or mentally incapable of acting as a director, (f) he is given notice by the majority of the other directors (not being less than two in number) to vacate office (without prejudice to any claim for damages for breach of any agreement relating to the provision of the services of such director), (g) he is made subject to any law relating to mental health or incompetence, whether by court order or otherwise, or (h) without the consent of the other directors, he is absent from meetings of directors for continuous period of six months.
Transactions with Interested Shareholders
The Delaware General Corporation Law contains a business combination statute applicable to Delaware public corporations whereby, unless the corporation has specifically elected not to be governed by such statute by amendment to its certificate of incorporation, it is prohibited from engaging in certain business combinations with an “interested shareholder” for three years following the date that such person becomes an interested shareholder. An interested shareholder generally is a person or a group who or which owns or owned 15% or more of the target’s outstanding voting shares within the past three years.
This statute has the effect of limiting the ability of a potential acquirer to make a two-tiered bid for the target in which all shareholders would not be treated equally. The statute does not apply if, among other things, prior to the date on which such shareholder becomes an interested shareholder, the board of directors approves either the business combination or the transaction which resulted in the person becoming an interested shareholder. This encourages any potential acquirer of a Delaware corporation to negotiate the terms of any acquisition transaction with the target’s board of directors.
Cayman Islands law has no comparable statute. As a result, we cannot avail ourselves of the types of protections afforded by the Delaware business combination statute. However, although the Companies Act does not regulate transactions between a company and its significant shareholders, the directors of the company are required to comply with fiduciary duties which they owe to the company under Cayman Islands law, including the duty to ensure that, in their opinion, that such transactions must be entered into bona fide in the best interests of the company and for a proper purpose and not with the effect of constituting a fraud on the minority shareholders.
In addition, under our Articles, no director or his affiliates shall be prevented from transacting with the Company or held liable for any profit realized under any such transaction if such director discloses the nature of his interest.
Dissolution; Winding Up
Under the Delaware General Corporation Law, unless the board of directors approves the proposal to dissolve, dissolution must be approved by shareholders holding 100% of the total voting power of the corporation. Only if the dissolution is initiated by the board of directors may it be approved by a simple majority of the corporation’s outstanding shares. Delaware law allows a Delaware corporation to include in its certificate of incorporation a supermajority voting requirement in connection with dissolutions initiated by the board.
152
Table of Contents
Under the Companies Act and our Articles, the Company may be wound up by a special resolution of our shareholders, or if the winding up is initiated by our board of directors, by either a special resolution of our members or, if our company is unable to pay its debts as they fall due, by an ordinary resolution of our members. In addition, a company may be wound up by an order of the courts of the Cayman Islands. The court has authority to order winding up in a number of specified circumstances including where it is, in the opinion of the court, just and equitable to do so.
Variation of Rights of Shares
Under the Delaware General Corporation Law, a corporation may vary the rights of a class of shares with the approval of a majority of the outstanding shares of such class, unless the certificate of incorporation provides otherwise. Under the Companies Act and our Articles, if our share capital is divided into more than one class of shares, the rights attaching to any class of share (unless otherwise provided by the terms of issue of the shares of that class) may be varied either with the consent in writing of the holders of not less than two-thirds of the issued shares of that class, or with the sanction of a resolution passed by a majority of not less than two-thirds of the holders of shares of the class present in person or by proxy at a separate meeting of the holders of the shares of that class.
Amendment of Governing Documents
Under the Delaware General Corporation Law, a corporation’s certificate of incorporation may be amended only if adopted and declared advisable by the board of directors and approved by a majority of the outstanding shares entitled to vote, and the bylaws may be amended with the approval of a majority of the outstanding shares entitled to vote and may, if so provided in the certificate of incorporation, also be amended by the board of directors.
As permitted under the Companies Act and our Articles, our Articles may only be amended by special resolution of our shareholders.
Rights of Non-resident or Foreign Shareholders
Our Articles do not limit the rights of non-resident or foreign shareholders to hold or exercise voting rights on our shares. However, no person will be entitled to vote at any general meeting or at any separate meeting of the holders of the Ordinary Shares unless the person is registered as of the record date for such meeting and unless all calls or other sums presently payable by the person in respect of our Ordinary Shares have been paid. In addition, our Articles do not stipulate the ownership threshold above which shareholder ownership must be disclosed.
Inspection of Books and Records
Under the Delaware General Corporation Law, any shareholder of a corporation may for any proper purpose inspect or make copies of the corporation’s stock ledger, list of shareholders and other books and records.
Holders of our shares will have no general right under Cayman Islands law to inspect or obtain copies of our list of shareholders or our corporate records. However, we intend to provide our shareholders with annual reports containing audited financial statements. See “Where You Can Find Additional Information.”
Anti-Money Laundering — Cayman Islands
In order to comply with legislation or regulations aimed at the prevention of money laundering and terrorist financing, we are required to adopt and maintain anti-money laundering procedures and will require subscribers to provide information and evidence to verify their identity, address and source of funds. Where permitted, and subject to certain conditions, we may also delegate the maintenance of our anti-money laundering procedures (including the acquisition of due diligence information) to a suitable person.
We reserve the right to request such information and evidence as is necessary to verify the identity, address and source of funds of a subscriber.
In the event of delay or failure on the part of the subscriber in producing any information or evidence required for verification purposes, we may refuse to accept the application, in which case any funds received will be returned without interest to the account from which they were originally debited. We will not be liable for any loss suffered by a subscriber arising as a result of a refusal of, or delay in processing, an application from a subscriber if such information and documentation requested has not been provided by the subscriber in a timely manner.
153
Table of Contents
We also reserve the right to refuse to make any redemption payment to a shareholder if our directors or officers suspect or are advised that the payment of redemption proceeds to such shareholder might result in a breach of applicable anti-money laundering or other laws or regulations by any person in any relevant jurisdiction, or if such refusal is considered necessary or appropriate to ensure our compliance with any such laws or regulations in any applicable jurisdiction.
If any person resident in the Cayman Islands knows or suspects or has reasonable grounds for knowing or suspecting that another person is engaged in criminal conduct or is involved with terrorism or terrorist property and the information for that knowledge or suspicion came to their attention in the course of their business in the regulated sector, or other trade, profession, business or employment, the person will be required to report such knowledge or suspicion to (i) a nominated officer (appointed in accordance with the Proceeds of Crime Act (Revised) of the Cayman Islands) or the Financial Reporting Authority of the Cayman Islands, pursuant to the Proceeds of Crime Act (Revised), if the disclosure relates to criminal conduct or money laundering or (ii) to the Financial Reporting Authority or a police constable or a nominated officer (pursuant to the Terrorism Act (Revised) of the Cayman Islands), if the disclosure relates to involvement with terrorism or terrorist financing and terrorist property. Such a report shall not be treated as a breach of confidence or of any restriction upon the disclosure of information imposed by any enactment or otherwise.
By subscribing for shares, the subscriber consents to the disclosure of any information about them to regulators and others upon request in connection with money laundering and similar matters both in the Cayman Islands and in other jurisdictions.
Data Protection — Cayman Islands
We have certain duties under the Data Protection Act (Revised) of the Cayman Islands (the “DPA”) based on internationally accepted principles of data privacy.
Privacy Notice
Introduction
This privacy notice puts our shareholders on notice that through your investment in the Company you will provide us with certain personal information which constitutes personal data within the meaning of the DPA (“personal data”).
In the following discussion, the “Company” refers to us and our affiliates and/or delegates, except where the context requires otherwise.
Investor Data
We will collect, use, disclose, retain and secure personal data to the extent reasonably required only and within the parameters that could be reasonably expected during the normal course of business. We will only process, disclose, transfer or retain personal data to the extent legitimately required to conduct our activities of on an ongoing basis or to comply with legal and regulatory obligations to which we are subject. We will only transfer personal data in accordance with the requirements of the DPA and will apply appropriate technical and organizational information security measures designed to protect against unauthorized or unlawful processing of the personal data and against the accidental loss, destruction or damage to the personal data.
In our use of this personal data, we will be characterized as a “data controller” for the purposes of the DPA, while our affiliates and service providers who may receive this personal data from us in the conduct of our activities may either act as our “data processors” for the purposes of the DPA or may process personal information for their own lawful purposes in connection with services provided to us.
We may also obtain personal data from other public sources. Personal data includes, without limitation, the following information relating to a shareholder and/or any individuals connected with a shareholder as an investor: name, residential address, email address, contact details, corporate contact information, signature, nationality, place of birth, date of birth, tax identification, credit history, correspondence records, passport number, bank account details, source of funds details and details relating to the shareholder’s investment activity.
154
Table of Contents
Who this Affects
If you are a natural person, this will affect you directly. If you are a corporate investor (including, for these purposes, legal arrangements such as trusts or exempted limited partnerships) that provides us with personal data on individuals connected to you for any reason in relation your investment in the Company, this will be relevant for those individuals and you should transmit the content of this Privacy Notice to such individuals or otherwise advise them of its content.
How the Company May Use a Shareholder’s Personal Data
The Company, as the data controller, may collect, store and use personal data for lawful purposes, including, in particular:
(i) where this is necessary for the performance of our rights and obligations under any purchase agreements;
(ii) where this is necessary for compliance with a legal and regulatory obligation to which we are subject (such as compliance with anti-money laundering and FATCA/CRS requirements); and/or
(iii) where this is necessary for the purposes of our legitimate interests and such interests are not overridden by your interests, fundamental rights or freedoms.
Should we wish to use personal data for other specific purposes (including, if applicable, any purpose that requires your consent), we will contact you.
Why We May Transfer Your Personal Data
In certain circumstances we may be legally obliged to share personal data and other information with respect to your shareholding with the relevant regulatory authorities such as the Cayman Islands Monetary Authority or the Tax Information Authority. They, in turn, may exchange this information with foreign authorities, including tax authorities.
We anticipate disclosing personal data to persons who provide services to us and their respective affiliates (which may include certain entities located outside the United States, the Cayman Islands or the European Economic Area), who will process your personal data on our behalf.
The Data Protection Measures We Take
Any transfer of personal data by us or our duly authorized affiliates and/or delegates outside of the Cayman Islands shall be in accordance with the requirements of the DPA.
We and our duly authorized affiliates and/or delegates shall apply appropriate technical and organizational information security measures designed to protect against unauthorized or unlawful processing of personal data, and against accidental loss or destruction of, or damage to, personal data.
We shall notify you of any personal data breach that is reasonably likely to result in a risk to your interests, fundamental rights or freedoms or those data subjects to whom the relevant personal data relates.
Listing
Our Ordinary Shares are listed on the Nasdaq Capital Market under the symbol “WOK.”
Transfer Agent and Registrar
The transfer agent and registrar for our Ordinary Shares is VStock Transfer LLC. Vstock Transfer LLC’s address is 18 Lafayette Pl, Woodmere, NY 11598.
155
Table of Contents
MATERIAL INCOME TAX CONSIDERATIONS
The following discussion of material Cayman Islands, PRC, and United States federal income tax consequences of an investment in our Ordinary Shares is based upon laws and relevant interpretations thereof in effect as of the date of this prospectus, all of which are subject to change. This discussion does not deal with all possible tax consequences relating to an investment in our Ordinary Shares, such as the tax consequences under state, local, and other tax laws.
This summary does not apply to the acquisition, holding, and disposition of the Warrants by holders. Such holders should consult their own tax advisors with respect to an investment in the Warrants.
WE URGE POTENTIAL PURCHASERS OF OUR ORDINARY SHARES TO CONSULT THEIR OWN TAX ADVISORS CONCERNING THE U.S. FEDERAL, STATE, LOCAL, AND NON-U.S. TAX CONSEQUENCES OF PURCHASING, OWNING AND DISPOSING OF OUR ORDINARY SHARES.
People’s Republic of China Taxation
We are a holding company incorporated in the Cayman Islands and we gain substantial income by way of dividends paid to us from the PRC subsidiaries. The EIT Law and its implementation rules provide that PRC-sourced income of foreign enterprises, such as dividends paid by a subsidiary in mainland China to its equity holders that are non-resident enterprises, will normally be subject to PRC withholding tax at a rate of 10%, unless any such foreign investor’s jurisdiction of incorporation has a tax treaty with China that provides for a preferential tax rate or a tax exemption.
Under the EIT Law, an enterprise established outside of China with a “de facto management body” within China is considered a “resident enterprise,” which means that it is treated in a manner similar to a PRC domestic enterprise for enterprise income tax purposes. Although the implementation rules of the EIT Law define “de facto management body” as a managing body that actually, comprehensively manage and control the production and operation, staff, accounting, property, and other aspects of an enterprise, the only official guidance for this definition currently available is set forth in SAT Circular 82, which provides guidance on the determination of the tax residence status of a PRC-controlled offshore incorporated enterprise, defined as an enterprise that is incorporated under the laws of a foreign country or territory and that has a PRC enterprise or enterprise group as its primary controlling shareholder. In spite of further to SAT Circular 82, the SAT issued the SAT Bulletin 45, which took effect in September 2011 and was amended in June 2018, but SAT Bulletin 45 only provides for procedures and administration details of determination on resident status and administration on post-determination matters, did not define “de facto management body.” Although Work Cayman does not have a PRC enterprise or enterprise group as our primary controlling shareholder and is therefore not a PRC-controlled offshore incorporated enterprise within the meaning of SAT Circular 82, in the absence of guidance specifically applicable to us, we have applied the guidance set forth in SAT Circular 82 to evaluate the tax residence status of Work Cayman and its subsidiaries organized outside of mainland China.
According to SAT Circular 82, a PRC-controlled offshore incorporated enterprise will be regarded as a PRC tax resident by virtue of having a “de facto management body” in China and will be subject to PRC enterprise income tax on its worldwide income only if all of the following criteria are met: (i) the places where senior management and senior management departments that are responsible for daily production, operation and management of the enterprise perform their duties are mainly located within the territory of mainland China; (ii) financial decisions (such as money borrowing, lending, financing and financial risk management) and personnel decisions (such as appointment, dismissal and salary and wages) are decided or need to be decided by organizations or persons located within the territory of mainland China; (iii) main property, accounting books, corporate seal, the board of directors and files of the minutes of shareholders’ meetings of the enterprise are located or preserved within the territory of mainland China; and (iv) one half (or more) of the directors or senior management staff having the right to vote habitually reside within the territory of mainland China.
We believe that we do not meet some of the conditions outlined in the immediately preceding paragraph. For example, the key assets and records of Work Cayman, including the resolutions and meeting minutes of our board of directors and the resolutions and meeting minutes of our shareholders, are located and maintained outside mainland China. In addition, we are not aware of any offshore holding companies with a corporate structure similar to ours that has been deemed a PRC “resident enterprise” by the PRC tax authorities. Accordingly, we believe that Work Cayman and its offshore subsidiary should not be treated as a “resident enterprise” for PRC tax purposes if the criteria for “de facto management body” as set forth in SAT Circular 82 were deemed applicable to us. As the tax residency status
156
Table of Contents
of an enterprise is subject to determination by the PRC tax authorities and uncertainties remain with respect to the interpretation of the term “de facto management body” as applicable to our offshore entities, however, we will continue to monitor our tax status.
If the PRC tax authorities determine that Work Cayman is a PRC resident enterprise for enterprise income tax purposes, we may be required to withhold a 10% withholding tax from any dividends we pay to our shareholders that are non- resident enterprises. In addition, non-resident enterprise shareholders may be subject to a 10% PRC withholding tax on gains realized on the sale or other disposition of our Ordinary Shares, if such income is treated as sourced from within mainland China. It is unclear whether our non-PRC individual shareholders would be subject to any PRC tax on dividends or gains obtained by such non-PRC individual shareholders in the event we are determined to be a PRC resident enterprise. If any PRC tax were to apply to dividends or gains realized by non-PRC individuals, it would generally apply at a rate of 20% unless a reduced rate is available under an applicable tax treaty. It is also unclear, however, whether our non-PRC shareholders would be able to claim the benefits of any tax treaties between their country of tax residence and the PRC in the event that we are treated as a PRC resident enterprise. There is no guidance from the PRC government to indicate whether or not any tax treaties between mainland China and other countries would apply in circumstances where a non-PRC company was deemed to be a PRC tax resident, and thus there is no basis for expecting how tax treaty between mainland China and other countries may impact non-resident enterprises. See “Risk Factors — Risks Related to Doing Business in the PRC — We may be treated as a resident enterprise for PRC tax purposes under the PRC Enterprise Income Tax Law, and we may therefore be subject to PRC income tax on our global income.” on page 27.
As a Cayman Islands holding company, we may receive dividends from the PRC subsidiary through offshore subsidiaries. The EIT Law and its implementing rules provide that dividends paid by a PRC entity to a non-resident enterprise for income tax purposes is subject to PRC withholding tax at a rate of 10%, subject to reduction by an applicable tax treaty with China. Pursuant to the Arrangement between Mainland China and the Hong Kong Special Administrative Region for the Avoidance of Double Taxation and Tax Evasion on Income, the withholding tax rate in respect to the payment of dividends by a PRC enterprise to a Hong Kong enterprise may be reduced to 5% from a standard rate of 10% if the Hong Kong enterprise directly holds at least 25% of the PRC enterprise. Pursuant to the Notice of the State Administration of Taxation on the Issues concerning the Application of the Dividend Clauses of Tax Agreements, or SAT Circular 81, a Hong Kong resident enterprise must meet the following conditions, among others, in order to apply the reduced withholding tax rate: (i) it must be a company; (ii) it must directly own the required percentage of equity interests and voting rights in the PRC resident enterprise; and (iii) it must have directly owned such required percentage in the PRC resident enterprise throughout the 12 months prior to receiving the dividends. In October 2019, the SAT promulgated the Administrative Measures for Entitlement to Treaty Benefits for Non-resident Taxpayers, or SAT Circular 35, which became effective on January 1, 2020. SAT Circular 35 provides that non-resident enterprises are not required to obtain pre-approval from the relevant tax authority in order to enjoy the reduced withholding tax. Instead, non-resident enterprises and their withholding agents may, by self-assessment and on confirmation that the prescribed criteria to enjoy the tax treaty benefits are met, directly apply the reduced withholding tax rate, and file necessary forms and supporting documents when performing tax filings, which will be subject to post-tax filing examinations by the relevant tax authorities. Accordingly, we may be able to benefit from the 5% withholding tax rate for the dividends it receives from our operating entities established in mainland China, if we satisfy the conditions prescribed under SAT Circular 82 and other relevant tax rules and regulations. However, according to SAT Circular 82 and SAT Circular 35, if the relevant tax authorities consider the transactions or arrangements we have are for the primary purpose of enjoying a favorable tax treatment, the relevant tax authorities may adjust the favorable withholding tax in the future.
Provided that Work Cayman is not deemed to be a PRC resident enterprise, holders of our Ordinary Shares who are not PRC residents will not be subject to PRC income tax on dividends distributed by us or gains realized from the sale or other disposition of our shares. However, under SAT Bulletin 7, where a non-resident enterprise conducts an “indirect transfer” by transferring taxable assets, including, in particular, equity interests in a PRC resident enterprise, indirectly by disposing of the equity interests of an overseas holding company, the non-resident enterprise, being the transferor, or the transferee or the PRC entity which directly owned such taxable assets may report to the relevant tax authority such indirect transfer. Using a “substance over form” principle, the PRC tax authority may disregard the existence of the overseas holding company if it lacks a reasonable commercial purpose and was established for the purpose of reducing, avoiding, or deferring PRC tax. As a result, gains derived from such indirect transfer may be subject to PRC enterprise income tax, and the transferee or other person who is obligated to pay for the transfer is obligated to withhold the applicable taxes, currently at a rate of 10% for the transfer of equity interests in a PRC resident enterprise.
157
Table of Contents
We and our non-PRC resident investors may be at risk of being required to file a return and being taxed under SAT Bulletin 7, and we may be required to expend valuable resources to comply with SAT Bulletin 7, or to establish that we should not be taxed under this Bulletin. See “Risk Factors — Risks Related to Doing Business in China — We and our shareholders face uncertainties with respect to indirect transfers of equity interests in PRC resident enterprises by their non-PRC holding companies.” on page 28.
Hong Kong Taxation
According to Tax (Amendment) (No. 3) Ordinance 2018 published by Hong Kong government, since September 1, 2018, under the two-tiered profits tax rates regime, the profits tax rate for the first HKD2 million of assessable profits has been lowered to 8.25% (half of the rate specified in Schedule 8 to the Inland Revenue Ordinance (IRO)) for corporations. Work HK was not subject to Hong Kong profit tax for any period presented as it did not have assessable profit during the periods presented.
Cayman Islands Taxation
The Cayman Islands currently levies no taxes on individuals or corporations based upon profits, income, gains, or appreciation and there is no taxation in the nature of inheritance tax or estate duty. There are no other taxes likely to be material to us levied by the Government of the Cayman Islands except for stamp duties which may be applicable on instruments executed in, or, after execution, brought within the jurisdiction of the Cayman Islands. No stamp duty is payable in the Cayman Islands on the issue of shares by, or any transfers of shares of, Cayman Islands companies (except those which hold interests in land in the Cayman Islands). There are no exchange control regulations or currency restrictions in the Cayman Islands.
Payments of dividends and capital in respect of our Ordinary Shares will not be subject to taxation in the Cayman Islands and no withholding will be required on the payment of a dividend or capital to any holder of our Ordinary Shares, as the case may be, nor will gains derived from the disposal of our Ordinary Shares be subject to Cayman Islands income or corporation tax.
158
Table of Contents
UNITED STATES FEDERAL INCOME TAX CONSIDERATIONS
THE FOLLOWING BRIEF SUMMARY IS INCLUDED HEREIN FOR GENERAL INFORMATION AND IS NOT INTENDED TO BE, AND SHOULD NOT BE CONSIDERED TO BE, LEGAL OR TAX ADVICE. EACH U.S. HOLDER SHOULD CONSULT WITH HIS OR HER OWN TAX ADVISOR AS TO THE PARTICULAR U.S. FEDERAL INCOME TAX CONSEQUENCES OF THE PURCHASE, OWNERSHIP, AND SALE OF ORDINARY SHARES, THE WARRANTS, AND THE ORDINARY SHARES ISSUED OR ISSUABLE UPON EXERCISE OF THE WARRANTS, INCLUDING THE EFFECTS OF APPLICABLE STATE, LOCAL, FOREIGN OR OTHER TAX LAWS AND POSSIBLE CHANGES IN THE TAX LAWS.
Subject to the limitations described in the next paragraph, the following discussion summarizes the material U.S. federal income tax consequences to a “U.S. Holder” arising from the purchase, ownership and sale of the Ordinary Shares, the Warrants, and the Ordinary Shares issued or issuable upon exercise of the Warrants, or collectively, the “Securities.” For this purpose, a “U.S. Holder” is a holder of Securities that is: (1) an individual citizen or resident of the United States, including an alien individual who is a lawful permanent resident of the United States or meets the substantial presence residency test under U.S. federal income tax laws; (2) a corporation (or entity treated as a corporation for U.S. federal income tax purposes) or a partnership (other than a partnership that is not treated as a U.S. person under any applicable U.S. Treasury regulations) created or organized under the laws of the United States or the District of Columbia or any political subdivision thereof; (3) an estate, the income of which is includable in gross income for U.S. federal income tax purposes regardless of source; (4) a trust if a court within the United States is able to exercise primary supervision over the administration of the trust and one or more U.S. persons have authority to control all substantial decisions of the trust; or (5) a trust that has a valid election in effect to be treated as a U.S. person to the extent provided in U.S. Treasury regulations.
This brief summary is for general information purposes only and does not purport to be a comprehensive description of all of the U.S. federal income tax considerations that may be relevant to a decision to purchase our Securities. This brief summary generally considers only U.S. Holders that will own our Securities as capital assets. Except to the limited extent discussed below, this summary does not consider the U.S. federal tax consequences to a person that is not a U.S. Holder, nor does it describe the rules applicable to determine a taxpayer’s status as a U.S. Holder. This summary is based on the provisions of the Internal Revenue Code of 1986, as amended, or the Code, final, temporary and proposed U.S. Treasury regulations promulgated thereunder, and administrative and judicial interpretations thereof, (including with respect to the Tax Cuts and Jobs Act of 2017), all as in effect as of the date hereof and all of which are subject to change, possibly on a retroactive basis, and all of which are open to differing interpretations. We will not seek a ruling from the IRS with regard to the U.S. federal income tax treatment of an investment in our Securities by U.S. Holders and, therefore, can provide no assurances that the IRS will agree with the conclusions set forth below.
This discussion does not address all of the aspects of U.S. federal income taxation that may be relevant to a particular U.S. Holder based on such holder’s particular circumstances and in particular does not discuss any estate, gift, generation-skipping, transfer, state, local, excise or foreign tax considerations. In addition, this discussion does not address the U.S. federal income tax treatment of a U.S. Holder who is: (1) a bank, life insurance company, regulated investment company, or other financial institution or “financial services entity;” (2) a broker or dealer in securities or foreign currency; (3) a person who acquired our Securities in connection with employment or other performance of services; (4) a U.S. Holder that is subject to the U.S. alternative minimum tax; (5) a U.S. Holder that holds our Securities as a hedge or as part of a hedging, straddle, conversion or constructive sale transaction or other risk-reduction transaction for U.S. federal income tax purposes; (6) a tax-exempt entity; (7) real estate investment trusts or grantor trusts; (8) a U.S. Holder that expatriates out of the United States or a former long-term resident of the United States; or (9) a person having a functional currency other than the U.S. dollar. This discussion does not address the U.S. federal income tax treatment of a U.S. Holder that owns, directly or constructively, at any time, Securities representing 10% or more of our voting power. Additionally, the U.S. federal income tax treatment of partnerships (or other pass-through entities) or persons who hold securities through a partnership or other pass-through entity are not addressed.
Each prospective investor is advised to consult his or her own tax adviser for the specific tax consequences to that investor of purchasing, holding or disposing of our Securities, including the effects of applicable state, local, foreign or other tax laws and possible changes in the tax laws.
159
Table of Contents
Tax Treatment of the Pre-Funded Warrants
We intend to treat our Pre-Funded Warrants as a class of our Ordinary Shares for U.S. federal income tax purposes. However, our position is not binding on the IRS and the IRS may treat the Pre-Funded Warrants as warrants to acquire our Ordinary Shares. Accordingly, you should consult your tax adviser regarding the U.S. federal tax consequences of an investment in the Pre-Funded Warrants. The following discussion assumes our Pre-Funded Warrants are properly treated as a class of our Ordinary Shares.
Exercise or Expiry of Warrants
No gain or loss will be realized on the exercise of a Warrant. When a Warrant is exercised, the U.S. Holder’s cost of the Ordinary Share acquired thereby will be equal to the U.S. Holder’s adjusted cost basis of the Warrant plus the exercise price paid for the Ordinary Share. The expiration of an unexercised Warrant will generally give rise to a capital loss equal to the adjusted cost basis to the U.S. Holder of the Warrant. The Pre-Funded Warrants do not expire. The holding period of the Ordinary Shares acquired by the exercise of a Warrant includes the holding period of the Warrant.
Taxation of Dividends Paid on Securities
Subject to the PFIC rules discussed below, the gross amount of distributions made by us to you with respect to the Ordinary Shares (including the amount of any taxes withheld therefrom) will generally be includable in your gross income as dividend income on the date of receipt by you, but only to the extent that the distribution is paid out of our current or accumulated earnings and profits (as determined under U.S. federal income tax principles). With respect to corporate U.S. Holders, the dividends will not be eligible for the dividends-received deduction allowed to corporations in respect of dividends received from other U.S. corporations.
With respect to non-corporate U.S. Holders, including individual U.S. Holders, dividends will be taxed at the lower capital gains rate applicable to qualified dividend income, provided that (1) the Ordinary Shares are readily tradable on an established securities market in the United States, or we are eligible for the benefits of an approved qualifying income tax treaty with the United States that includes an exchange of information program, (2) we are not a PFIC for either our taxable year in which the dividend is paid or the preceding taxable year, and (3) certain holding period requirements are met. Because there is no income tax treaty between the United States and the Cayman Islands, clause (1) above can be satisfied only if the Ordinary Shares are readily tradable on an established securities market in the United States. Under U.S. Internal Revenue Service authority, Ordinary Shares are considered for purpose of clause (1) above to be readily tradable on an established securities market in the United States if they are listed on certain exchanges, which presently include the Nasdaq Stock Market. You are urged to consult your tax advisors regarding the availability of the lower rate for dividends paid with respect to our Ordinary Shares, including the effects of any change in law after the date of this prospectus.
Dividends will constitute foreign source income for foreign tax credit limitation purposes. If the dividends are taxed as qualified dividend income (as discussed above), the amount of the dividend taken into account for purposes of calculating the foreign tax credit limitation will be limited to the gross amount of the dividend, multiplied by the reduced rate divided by the highest rate of tax normally applicable to dividends. The limitation on foreign taxes eligible for credit is calculated separately with respect to specific classes of income. For this purpose, dividends distributed by us with respect to our Ordinary Shares will constitute “passive category income” but could, in the case of certain U.S. Holders, constitute “general category income.”
To the extent that the amount of the distribution exceeds our current and accumulated earnings and profits (as determined under U.S. federal income tax principles), it will be treated first as a tax-free return of your tax basis in your Ordinary Shares, and to the extent the amount of the distribution exceeds your tax basis, the excess will be taxed as capital gain. We do not intend to calculate our earnings and profits under U.S. federal income tax principles. Therefore, a U.S. Holder should expect that a distribution will be treated as a dividend even if that distribution would otherwise be treated as a non-taxable return of capital or as capital gain under the rules described above.
160
Table of Contents
Taxation of the Disposition of Securities
Except as provided under the PFIC rules described below under “Passive Foreign Investment Companies,” upon the sale, exchange or other disposition of our Securities, a U.S. Holder will recognize capital gain or loss in an amount equal to the difference between such U.S. Holder’s tax basis for the securities in U.S. dollars and the amount realized on the disposition in U.S. dollar (or its U.S. dollar equivalent determined by reference to the spot rate of exchange on the date of disposition, if the amount realized is denominated in a foreign currency). The gain or loss realized on the sale, exchange or other disposition of securities will be long-term capital gain or loss if the U.S. Holder has a holding period of more than one year at the time of the disposition. Individuals who recognize long-term capital gains may be taxed on such gains at reduced rates of tax. The deduction of capital losses is subject to various limitations.
Passive Foreign Investment Companies
Special U.S. federal income tax laws apply to U.S. taxpayers who own shares of a corporation that is a PFIC. We will be treated as a PFIC for U.S. federal income tax purposes for any taxable year that either:
• 75% or more of our gross income (including our pro rata share of gross income for any company, in which we are considered to own 25% or more of the shares by value), in a taxable year is passive; or
• At least 50% of our assets, averaged over the year and generally determined based upon fair market value (including our pro rata share of the assets of any company in which we are considered to own 25% or more of the shares by value) are held for the production of, or produce, passive income.
For this purpose, passive income generally consists of dividends, interest, rents, royalties, annuities and income from certain commodities transactions and from notional principal contracts. Cash is treated as generating passive income.
The tests for determining PFIC status are applied annually, and it is difficult to make accurate projections of future income and assets which are relevant to this determination. In addition, our PFIC status may depend in part on the market value of our Securities and how quickly we dispose of the cash raised in this offering. Accordingly, there can be no assurance that we will not become a PFIC. The Company has no obligation to take measures to avoid becoming a PFIC.
If we currently are or become a PFIC, each U.S. Holder who has not elected to mark the shares to market (as discussed below), would, upon receipt of certain distributions by us and upon disposition of our Securities at a gain: (1) have such distribution or gain allocated ratably over the U.S. Holder’s holding period for the securities, as the case may be; (2) the amount allocated to the current taxable year and any period prior to the first day of the first taxable year in which we were a PFIC would be taxed as ordinary income; and (3) the amount allocated to each of the other taxable years would be subject to tax at the highest rate of tax in effect for the applicable class of taxpayer for that year, and an interest charge for the deemed deferral benefit would be imposed with respect to the resulting tax attributable to each such other taxable year. In addition, when shares of a PFIC are acquired by reason of death from a decedent that was a U.S. Holder, the tax basis of such shares would not receive a step-up to fair market value as of the date of the decedent’s death, but instead would be equal to the decedent’s basis if lower, unless all gain were recognized by the decedent. Indirect investments in a PFIC may also be subject to these special U.S. federal income tax rules.
The PFIC rules described above would not apply to a U.S. Holder who makes a QEF election for all taxable years that such U.S. Holder has held the Securities while we are a PFIC, provided that we comply with specified reporting requirements. Instead, each U.S. Holder who has made such a QEF election is required for each taxable year that we are a PFIC to include in income such U.S. Holder’s pro rata share of our ordinary earnings as ordinary income and such U.S. Holder’s pro rata share of our net capital gains as long-term capital gain, regardless of whether we make any distributions of such earnings or gain. In general, a QEF election is effective only if we make available certain required information. The QEF election is made on a shareholder-by-shareholder basis and generally may be revoked only with the consent of the IRS. We do not intend to notify U.S. Holders if we believe we will be treated as a PFIC for any tax year. In addition, we do not intend to furnish U.S. Holders annually with information needed in order to complete IRS Form 8621 and to make and maintain a valid QEF election for any year in which we or any of our Subsidiaries are a PFIC. Therefore, the QEF election will not be available with respect to our Securities.
161
Table of Contents
In addition, the PFIC rules described above would not apply if we were a PFIC and a U.S. Holder made a mark-to-market election. A U.S. Holder of our Securities which are regularly traded on a qualifying exchange, including the Nasdaq Capital Market, can elect to mark the securities to market annually, recognizing as ordinary income or loss each year an amount equal to the difference as of the close of the taxable year between the fair market value of the securities and the U.S. Holder’s adjusted tax basis in the securities. Losses are allowed only to the extent of net mark-to-market gain previously included income by the U.S. Holder under the election for prior taxable years.
U.S. Holders who hold our Securities during a period when we are a PFIC will be subject to the foregoing rules, even if we cease to be a PFIC. U.S. Holders are strongly urged to consult their tax advisors about the PFIC rules.
Tax on Net Investment Income
U.S. Holders who are individuals, estates or trusts will generally be required to pay a 3.8% Medicare tax on their net investment income (including dividends on and gains from the sale or other disposition of our Securities), or in the case of estates and trusts on their net investment income that is not distributed. In each case, the 3.8% Medicare tax applies only to the extent the U.S. Holder’s total adjusted income exceeds applicable thresholds.
Tax Consequences for Non-U.S. Holders of Securities
Except as provided below, an individual, corporation, estate or trust that is not a U.S. Holder referred to below as a non-U.S. Holder, generally will not be subject to U.S. federal income or withholding tax on the payment of dividends on, and the proceeds from the disposition of, our Securities.
A non-U.S. Holder may be subject to U.S. federal income tax on a dividend paid on our Securities or gain from the disposition of our Securities if: (1) such item is effectively connected with the conduct by the non-U.S. Holder of a trade or business in the United States and, if required by an applicable income tax treaty is attributable to a permanent establishment or fixed place of business in the United States; or (2) in the case of a disposition of our Securities, the individual non-U.S. Holder is present in the United States for 183 days or more in the taxable year of the disposition and other specified conditions are met.
In general, non-U.S. Holders will not be subject to backup withholding with respect to the payment of dividends on our Securities if payment is made through a paying agent, or office of a foreign broker outside the United States. However, if payment is made in the United States or by a U.S. related person, non-U.S. Holders may be subject to backup withholding, unless the non-U.S. Holder provides an applicable IRS Form W-8 (or a substantially similar form) certifying its foreign status, or otherwise establishes an exemption.
The amount of any backup withholding from a payment to a non-U.S. Holder will be allowed as a credit against such holder’s U.S. federal income tax liability and may entitle such holder to a refund, provided that the required information is timely furnished to the IRS.
Information Reporting and Withholding
A U.S. Holder may be subject to backup withholding at a rate of 24% with respect to cash dividends and proceeds from a disposition of securities. In general, backup withholding will apply only if a U.S. Holder fails to comply with specified identification procedures. Backup withholding will not apply with respect to payments made to designated exempt recipients, such as corporations and tax-exempt organizations. Backup withholding is not an additional tax and may be claimed as a credit against the U.S. federal income tax liability of a U.S. Holder, provided that the required information is timely furnished to the IRS.
Pursuant to recently enacted legislation, a U.S. Holder with interests in “specified foreign financial assets” (including, among other assets, our Securities, unless such securities are held on such U.S. Holder’s behalf through a financial institution) may be required to file an information report with the IRS if the aggregate value of all such assets exceeds $50,000 on the last day of the taxable year or $75,000 at any time during the taxable year (or such higher dollar amount as may be prescribed by applicable IRS guidance); and may be required to file a Report of Foreign Bank and Financial Accounts, if the aggregate value of the foreign financial accounts exceeds $10,000 at any time during the calendar year. You should consult your own tax advisor as to the possible obligation to file such information report.
162
Table of Contents
UNDERWRITING
We intend to enter into an underwriting agreement with Univest Securities, LLC, as representative of the underwriters named below (the “Representative”) with respect to the Ordinary Units and Pre-Funded Ordinary Units in this offering. The Representative may retain other brokers or dealers to act as sub-agents on its behalf in connection with this offering. Under the terms and subject to the conditions contained in the underwriting agreement, we will agree to issue and sell to the underwriters the number of Ordinary Units and Pre-Funded Ordinary Units as indicated below.
Underwriters | | Number of
Ordinary Units | | Number of
Pre-Funded
Ordinary Units |
Univest Securities, LLC | | | | |
Total | | | | |
The underwriting agreement will provide that the underwriters must buy all of the Ordinary Units (or if applicable, Pre-Funded Ordinary Units) if they buy any of them, which underwriter obligations to pay for and accept delivery of such Units offered by us in this prospectus are subject to various representations and warranties and other customary conditions specified in the underwriting agreement, such as receipt by the underwriters of officers’ certificates and legal opinions.
The Units are offered subject to a number of conditions, including:
• receipt and acceptance of the Units by the underwriters; and
• the underwriters’ right to reject orders in whole or in part.
We have been advised by the Representative that the underwriters intend to make a market in our Ordinary Shares and Warrants but that they are not obligated to do so and may discontinue making a market at any time without notice.
In connection with this offering, certain of the underwriters or securities dealers may distribute prospectuses electronically.
Underwriting Discounts and Expenses
Units sold by the underwriters to the public will initially be offered at the initial offering price set forth on the cover of this prospectus. Any Units sold by the underwriters to securities dealers may be sold at a discount of up to $ per Unit from the public offering price. The underwriters may offer the Units through one or more of their affiliates or selling agents. If all the Units are not sold at the public offering price, the underwriters may change the offering price and the other selling terms. Upon execution of the underwriting agreement, the underwriters will be obligated to purchase the Units at the prices and upon the terms stated therein.
The underwriting discount is equal to the public offering price per Unit, less the amount paid by the underwriters to us per Unit. The underwriting discount was determined through an arms’ length negotiation between us and the underwriters. We have agreed to sell the Units to the underwriters at the offering price of per Unit, which represents the public offering price of our Units set forth on the cover page of this prospectus less a 7% underwriting discount.
The following table shows the public offering price, underwriting discount, and proceeds, before expenses, to us:
| | Per Unit | | Per
Pre-Funded
Ordinary
Unit | | Total |
Public offering price | | $ | | | $ | | | $ | |
Underwriters’ discounts(1) | | $ | | | $ | | | $ | |
Non-accountable expense allowance(2) | | | | | | | | | |
Proceeds to our company before expenses(3) | | $ | | | $ | | | $ | [•] |
163
Table of Contents
We intend to reimburse the Representative for all reasonable travel and other accountable out-of-pocket expenses, including the reasonable fees, costs and disbursements of its legal counsel, in an amount not to exceed an aggregate of $150,000. We also intend to reimburse the Representative for all fees and expenses of the clearing firm, escrow agent, registrar or transfer agent, as applicable, provided that the fees and expenses of the clearing firm or escrow agent shall not exceed $12,900.
We estimate that the total expenses of the offering payable by us, not including the underwriting discount, will be approximately $ .
Tail Financing
We have agreed that the Representative shall be entitled to compensation commensurate with that to be received in this offering from the sale of any equity, debt and/or equity derivative instruments to any investor actually introduced by the Representative to us during the period between the date of that certain engagement agreement by and between the Company and the Representative, dated October 31, 2024 (the “Engagement Agreement”), and the closing of the offering, in connection with any public or private financing or capital raise (each a “Tail Financing”), and such Tail Financing is consummated any time within the twelve (12) month period following the closing date of this offering, provided that the Representative shall have furnished the Company with a comprehensive list of up to ten (10) prospective investors, or all prospective investors it sourced during the term of the Engagement Agreement on the Company’s behalf who signed a letter of intent, non-disclosure agreement, or any other instrument demonstrating an indication of interest with the Representative..
Right of First Refusal
Following the closing of this offering, we have agreed, provided that this offering is completed, that until twelve (12) months from the commencement of sales for this offering, the Representative shall have a right of first refusal to act as sole investment banker, sole book-runner, and/or sole placement agent at its sole discretion, for each and every future public and private equity and debt offering, including all equity linked financings (each a “Subject Transaction”), during such twelve (12) month period, of our Company, or any successor to or any current or future subsidiary of our Company, provided, however, that such right shall be subject to FINRA Rule 5110(g), including that the right of first refusal may be terminated by the Company for cause in case of the Representative’s material failure to provide the services contemplated in the underwriting agreement. During such twelve (12)-month period, the Representative shall have the sole right to determine whether any other broker dealer shall have the right to participate in a Subject Transaction and the economic terms of such participation, and we shall not retain, engage or solicit any additional investment banker, book-runner, financial advisor, underwriter and/or placement agent in a Subject Transaction without the express written consent of the Representative.
Lock-Up Agreements
Our directors, officers and holders of five percent (5%) or more of our outstanding Ordinary Shares as of the effective date of the registration statement of which this prospectus forms a part will enter into customary “lock-up” agreements in favor of the Representative pursuant to which such persons and entities shall agree, for a period of one hundred eighty (180) days following the closing of the offering of the securities offered hereby, that they shall neither offer, issue, sell, contract to sell, encumber, grant any option for the sale of or otherwise dispose of any of our securities without the Representative’s prior written consent, including the issuance of Ordinary Shares upon the exercise of currently outstanding convertible securities.
The Company, on behalf of itself and any successor entity, will not, without the prior written consent of the Representatives, for a period of thirty (30) days from the closing of this offering, (i) offer, pledge, announce the intention to sell, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase, lend, or otherwise transfer or dispose of, directly or indirectly, or file with the SEC a registration statement under the Securities Act relating to, any Ordinary Shares or any securities convertible into or exercisable or exchangeable for Ordinary Shares, (ii) enter into any swap or other agreement that transfers, in whole or in part, any of the economic consequences of ownership of the Ordinary Shares or any such other securities, whether any such transaction described in clause (i), or (ii) above is to be settled by delivery of Ordinary Shares or such other securities, in cash or otherwise, except to the underwriters, or (iii) repurchase any Ordinary Shares.
164
Table of Contents
Indemnification
We have agreed to indemnify the underwriters against certain liabilities, including liabilities under the Securities Act. If we are unable to provide this indemnification, we will contribute to payments that the underwriters may be required to make for these liabilities.
Pricing of the Offering
The offering price of the Ordinary Units (and Pre-Funded Ordinary Units, as applicable) is based on the last reported sale price of our Ordinary Shares on Nasdaq immediately prior to effectiveness of the registration statement of which this prospectus forms a part.
Electronic Offer, Sale, and Distribution of Securities
A prospectus in electronic format may be made available on the websites maintained by the underwriters. In addition, securities may be sold by the underwriters to securities dealers who resell securities to online brokerage account holders. The securities to be sold pursuant to Internet distributions will be allocated on the same basis as other allocations. Other than the prospectus in electronic format, the information on these websites is not part of, nor incorporated by reference into, this prospectus or the registration statement of which this prospectus forms a part, has not been approved or endorsed by us or the underwriters, and should not be relied upon by investors.
Price Stabilization, Short Positions, and Penalty Bids
In connection with this offering, the underwriters may engage in transactions that stabilize, maintain, or otherwise affect the price of our securities. Specifically, the underwriters may sell more securities than they are obligated to purchase under the underwriting agreement, creating a short position. A short sale is covered if the short position is no greater than the number of securities available for purchase by the underwriters under option to purchase additional securities. The underwriters can close out a covered short sale by exercising the option to purchase additional securities or purchasing securities in the open market. In determining the source of securities to close out a covered short sale, the underwriters will consider, among other things, the open market price of securities compared to the price available under the option to purchase additional securities. The underwriters may also sell securities in excess of the option to purchase additional securities, creating a naked short position. The underwriters must close out any naked short position by purchasing securities in the open market. A naked short position is more likely to be created if the underwriters are concerned that there may be downward pressure on the price of the securities in the open market after pricing that could adversely affect investors who purchase in the offering.
The underwriters may also impose a penalty bid. This occurs when a particular underwriter or dealer repays selling concessions allowed to it for distributing our securities in this offering because such underwriter repurchases those securities in stabilizing or short covering transactions.
Finally, the underwriters may bid for, and purchase, our securities in market making transactions, including “passive” market making transactions as described below.
These activities may stabilize or maintain the market price of our securities at a price that is higher than the price that might otherwise exist in the absence of these activities. The underwriters are not required to engage in these activities, and may discontinue any of these activities at any time without notice. These transactions may be effected on the Nasdaq Capital Market, in the over-the-counter market, or otherwise.
Passive Market Making
In connection with this offering, the underwriters may engage in passive market making transactions in our securities on the Nasdaq Capital Market in accordance with Rule 103 of Regulation M under the Exchange Act, during a period before the commencement of offers or sales of the securities and extending through the completion of the distribution. A passive market maker must display its bid at a price not in excess of the highest independent bid of that security. However, if all independent bids are lowered below the passive market maker’s bid, then that bid must then be lowered when specified purchase limits are exceeded.
165
Table of Contents
Potential Conflicts of Interest
The underwriters and their affiliates may, from time to time, engage in transactions with and perform services for us in the ordinary course of their business for which they may receive customary fees and reimbursement of expenses. In the ordinary course of their various business activities, the underwriters and their affiliates may make or hold a broad array of investments and actively trade debt and equity securities (or related derivative securities) and financial instruments (including bank loans) for their own accounts and for the accounts of their customers and such investment and securities activities may involve securities and/or instruments of our Company. The underwriters and their affiliates may also make investment recommendations and/or publish or express independent research views in respect of such securities or instruments and may at any time hold, or recommend to clients that they acquire, long and/or short positions in such securities and instruments.
Other Relationships
The underwriters and certain of their affiliates are full service financial institutions engaged in various activities, which may include securities trading, commercial and investment banking, financial advisory, investment management, investment research, principal investment, hedging, financing, and brokerage activities. The underwriters and certain of their affiliates may in the future engage in investment banking and other commercial dealings in the ordinary course of business with us and our affiliates, for which they may in the future receive customary fees, commissions, and expenses.
In addition, in the ordinary course of their business activities, the underwriters and their affiliates may make or hold a broad array of investments and actively trade debt and equity securities (or related derivative securities) and financial instruments (including bank loans) for their own account and for the accounts of their customers. Such investments and securities activities may involve securities and/or instruments of ours or our affiliates. The underwriters and their affiliates may also make investment recommendations and/or publish or express independent research views in respect of such securities or financial instruments and may hold, or recommend to clients that they acquire, long, and/or short positions in such securities and instruments.
Stamp Taxes
If you purchase Units offered in this prospectus, you may be required to pay stamp taxes and other charges under the laws and practices of the country of purchase, in addition to the offering price listed on the cover page of this prospectus.
Selling Restrictions
No action has been taken in any jurisdiction (except in the United States) that would permit a public offering of the Units, or the possession, circulation or distribution of this prospectus or any other material relating to us or the Ordinary Shares, where action for that purpose is required. Accordingly, the Ordinary Shares may not be offered or sold, directly or indirectly, and neither this prospectus nor any other offering material or advertisements in connection with the Ordinary Shares may be distributed or published, in or from any country or jurisdiction except in compliance with any applicable rules and regulations of any such country or jurisdiction.
Canada. The Ordinary Shares may not be offered, sold, or distributed, directly or indirectly, in any province or territory of Canada other than the provinces of Ontario and Quebec or to or for the benefit of any resident of any province or territory of Canada other than the provinces of Ontario and Quebec, and only on a basis that is pursuant to an exemption from the requirement to file a prospectus in such province, and only through a dealer duly registered under the applicable securities laws of such province or in accordance with an exemption from the applicable registered dealer requirements.
Cayman Islands. This prospectus does not constitute a public offer of the Ordinary Shares, whether by way of sale or subscription, in the Cayman Islands. The underwriters have represented and agreed that it has not offered or sold, and will not offer or sell, directly or indirectly, any Ordinary Shares to any member of the public in the Cayman Islands.
Hong Kong. The Ordinary Shares may not be offered or sold in Hong Kong by means of this prospectus or any other document other than (i) in circumstances that do not constitute an offer or invitation to the public within the meaning of the Companies (Cap.32, Laws of Hong Kong) or the Securities and Futures Ordinance (Cap.571, Laws of Hong Kong), or (ii) to “professional investors” within the meaning of the Securities and Futures Ordinance (Cap.571, Laws of Hong Kong)
166
Table of Contents
and any rules made thereunder, or (iii) in other circumstances that do not result in the document being a “prospectus” within the meaning of the Companies Ordinance (Cap.32, Laws of Hong Kong), and no advertisement, invitation or document relating to the Ordinary Shares may be issued or may be in the possession of any person for the purpose of issue (in each case whether in Hong Kong or elsewhere), that is directed at, or the contents of which are likely to be accessed or read by, the public in Hong Kong (except if permitted to do so under the laws of Hong Kong) other than with respect to Ordinary Shares which are or are intended to be disposed of only to persons outside Hong Kong or only to “professional investors” within the meaning of the Securities and Futures Ordinance (Cap.571, Laws of Hong Kong) and any rules made thereunder.
Malaysia. The shares have not been and may not be approved by the securities commission Malaysia, or SC, and this document has not been and will not be registered as a prospectus with the SC under the Malaysian capital markets and services act of 2007, or CMSA. Accordingly, no securities or offer for subscription or purchase of securities or invitation to subscribe for or purchase securities are being made to any person in or from within Malaysia under this document except to persons falling within any of paragraphs 2(g)(i) to (xi) of schedule 5 of the CMSA and distributed only by a holder of a capital markets services license who carries on the business of dealing in securities and subject to the issuer having lodged this prospectus with the SC within seven days from the date of the distribution of this prospectus in Malaysia. The distribution in Malaysia of this document is subject to Malaysian laws. Save as aforementioned, no action has been taken in Malaysia under its securities laws in respect of this document. This document does not constitute and may not be used for the purpose of a public offering or an issue, offer for subscription or purchase, invitation to subscribe for or purchase any securities requiring the approval of the SC or the registration of a prospectus with the SC under the CMSA.
People’s Republic of China. This prospectus may not be circulated or distributed in the PRC, and the Ordinary Shares may not be offered or sold, and will not be offered or sold to any person for re-offering or resale, directly or indirectly, to any resident of the PRC except pursuant to applicable laws and regulations of the PRC. For the purpose of this paragraph, PRC does not include Taiwan and the special administrative regions of Hong Kong and Macau.
Singapore. This prospectus has not been registered as a prospectus with the Monetary Authority of Singapore. Accordingly, this prospectus and any other document or material in connection with the offer or sale, or invitation for subscription or purchase, of our Ordinary Shares may not be circulated or distributed, nor may our Ordinary Shares be offered or sold, or be made the subject of an invitation for subscription or purchase, whether directly or indirectly, to persons in Singapore other than (i) to an institutional investor under Section 274 of the Securities and Futures Act, Chapter 289 of Singapore, or SFA, (ii) to a relevant person or any person pursuant to Section 275(1A), and in accordance with the conditions specified in Section 275 of the SFA, and in accordance with the conditions specified in Section 275 of the SFA or (iii) otherwise pursuant to, and in accordance with the conditions of, any other applicable provision of the SFA, in each case subject to compliance with conditions set forth in the SFA.
Where our Ordinary Shares are subscribed or purchased under Section 275 by a relevant person which is: (a) a corporation (which is not an accredited investor as defined in Section 4A of the SFA) the sole business of which is to hold investments and the entire share capital of which is owned by one or more individuals, each of whom is an accredited investor; or (b) a trust (where the trustee is not an accredited investor) whose sole purpose is to hold investments and each beneficiary of the trust is an individual who is an accredited investor; shares, debentures and units of shares and debentures of that corporation or the beneficiaries’ rights and interest (howsoever described) in that trust shall not be transferred within six months after that corporation or that trust has acquired the Ordinary Shares under Section 275 of the SFA, except: (1) to an institutional investor (for corporations under Section 274 of the SFA) or to a relevant person defined in Section 275(2) of the SFA, or to any person pursuant to an offer that is made on terms that such shares, debentures and units of shares and debentures of that corporation or such rights and interest in that trust are acquired at a consideration of not less than S$200,000 (or its equivalent in a foreign currency) for each transaction, whether such amount is to be paid for in cash or by exchange of securities or other assets, and further for corporations, in accordance with the conditions, specified in Section 275 of the SFA; (2) where no consideration is or will be given for the transfer; or (3) where the transfer is by operation of law.
Taiwan The Ordinary Shares have not been and will not be registered or filed with, or approved by, the Financial Supervisory Commission of Taiwan pursuant to relevant securities laws and regulations and may not be offered or sold in Taiwan through a public offering or in circumstances which constitute an offer within the meaning of the Securities and Exchange Act of Taiwan or relevant laws and regulations that require a registration, filing, or approval of the Financial Supervisory Commission of Taiwan. No person or entity in Taiwan has been authorized to offer or sell the Ordinary Shares in Taiwan.
167
Table of Contents
EXPENSES RELATING TO THIS OFFERING
Set forth below is an itemization of the total expenses, excluding underwriting discounts. With the exception of the SEC registration fee and the FINRA filing fee, all amounts are estimates.
SEC Registration Fee | | $ | 6,889.50 |
FINRA Filing Fee | | | 2,300.00 |
Legal Fees and Expenses | | | 72,200.00 |
Printing Expenses | | | 3,000.00 |
Transfer Agent Fee | | | 3,000.00 |
Miscellaneous Expenses | | | 331.92 |
Total | | $ | 87,721.42 |
These expenses will be borne by us. Underwriting discounts will be borne by us in proportion to the numbers of Ordinary Shares and accompanying warrants sold in the offering.
168
Table of Contents
LEGAL MATTERS
We are being represented by Hunter Taubman Fischer & Li LLC with respect to certain legal matters as to United States federal securities and New York State law. The validity of the Ordinary Shares included in the Units, offered in this offering and certain other legal matters as to Cayman Islands law will be passed upon for us by Ogier (Cayman) LLP, our counsel as to Cayman Islands law. Legal matters as to PRC law will be passed upon for us by AllBright Law Offices (Fuzhou). Certain legal matters with respect to the United States federal securities and New York law in connection with this offering will be passed upon for the underwriters by Sullivan & Worcester LLP.
EXPERTS
The consolidated financial statements of our company as of September 30, 2023 and 2022, and for each of the fiscal years in the period then ended included in this prospectus have been so included in reliance on the report of WWC, P.C., an independent registered public accounting firm, given on the authority of said firm as experts in auditing and accounting. The office of WWC, P.C. is located at 2010 Pioneer Court, San Mateo, CA 94403.
WHERE YOU CAN FIND ADDITIONAL INFORMATION
We have filed with the SEC a registration statement on Form F-1, including relevant exhibits and schedules under the Securities Act, covering the securities offered by this prospectus. You should refer to our registration statements and their exhibits and schedules if you would like to find out more about us and about the securities offered hereby. This prospectus summarizes material provisions of contracts and other documents that we refer you to. Since the prospectus may not contain all the information that you may find important, you should review the full text of these documents.
We are subject to periodic reporting and other informational requirements of the Exchange Act, as applicable to foreign private issuers. Accordingly, we are required to file reports, including annual reports on Form 20-F, and other information with the SEC. As a foreign private issuer, we are exempt from the rules of the Exchange Act prescribing the furnishing and content of proxy statements to shareholders under the federal proxy rules contained in Sections 14(a), (b) and (c) of the Exchange Act, and our executive officers, directors and principal shareholders are exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act.
The SEC maintains a website that contains reports, proxy statements, and other information about issuers, such as us, who file electronically with the SEC. The address of that website is http://www.sec.gov. The information on that website is not a part of this prospectus.
169
Table of Contents
WORK MEDICAL TECHNOLOGY GROUP LTD
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
F-1
Table of Contents

REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To: The Board of Directors and Shareholders of
Work Medical Technology Group Ltd
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Work Medical Technology Group Ltd and its subsidiaries (collectively the “Company”) as of September 30, 2023 and 2022, and the related consolidated statements of income and comprehensive loss, changes in equity, and cash flows in each of the years in the two-year period ended September 30, 2023, and the related notes (collectively referred to as the financial statements). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of September 30, 2023 and 2022, and the results of its operations and its cash flows in each of the years in the two period ended September 30, 2023, in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.

WWC, P.C.
Certified Public Accountants
PCAOB ID No.1171
We have served as the Company’s auditor since December 2021.
San Mateo, California
February 9, 2024
F-2
Table of Contents
WORK MEDICAL TECHNOLOGY GROUP LTD
CONSOLIDATED BALANCE SHEETS
(In U.S. dollars, except for otherwise noted)
| | As of September 30, |
| | | 2023 | | 2022 |
Assets | | | | | | | | |
Current assets: | | | | | | | | |
Cash and cash equivalents | | $ | 1,596,096 | | | $ | 731,178 | |
Restricted cash | | | 41,187 | | | | 42,244 | |
Accounts receivable | | | 3,332,322 | | | | 3,287,817 | |
Inventories, net | | | 4,620,191 | | | | 4,358,171 | |
Amounts due from related parties | | | 4,237,682 | | | | 706,278 | |
Advance to suppliers | | | 3,469,819 | | | | 4,688,114 | |
Prepaid expenses and other current assets | | | 4,855,381 | | | | 2,796,992 | |
Total current assets | | | 22,152,678 | | | | 16,610,794 | |
| | | | | | | | | |
Property, plant and equipment, net | | | 6,626,774 | | | | 7,888,415 | |
Intangible assets, net | | | 998,450 | | | | 949,955 | |
Right-of-use assets, net | | | 114,127 | | | | 92,305 | |
Deferred tax assets | | | 66,872 | | | | — | |
Total non-current assets | | | 7,806,223 | | | | 8,930,675 | |
TOTAL ASSETS | | $ | 29,958,901 | | | $ | 25,541,469 | |
| | | | | | | | | |
Liabilities | | | | | | | | |
Current liabilities: | | | | | | | | |
Short-term bank borrowings | | $ | 8,840,461 | | | $ | 5,974,555 | |
Accounts payable | | | 3,961,827 | | | | 3,202,664 | |
Deferred revenue | | | 776,210 | | | | 744,107 | |
Amount due to related parties | | | 159,612 | | | | 866,886 | |
Accrued expenses and other liabilities | | | 4,398,596 | | | | 2,830,456 | |
Lease liabilities, current | | | 49,560 | | | | 18,589 | |
Income tax payable | | | 736,590 | | | | 619,576 | |
Total current liabilities | | | 18,922,856 | | | | 14,256,833 | |
| | | | | | | | | |
Lease liabilities, non-current | | | 50,130 | | | | 68,504 | |
Deferred tax liability | | | — | | | | 25,532 | |
Total non-current liabilities | | | 50,130 | | | | 94,036 | |
TOTAL LIABILITIES | | $ | 18,972,986 | | | $ | 14,350,869 | |
| | | | | | | | | |
Shareholders’ equity | | | | | | | | |
Ordinary Shares (par value of $0.0005 per share; 100,000,000 shares authorized as of September 30, 2023 and 2022; 12,500,000 shares issued and outstanding as of September 30, 2023 and 2022, respectively)* | | $ | 6,250 | | | $ | 6,250 | |
Subscription receivable | | | (6,250 | ) | | | (6,250 | ) |
Additional paid-in capital | | | 85,012 | | | | 85,012 | |
Statutory reserve | | | 887,482 | | | | 850,690 | |
Retained earnings | | | 9,580,585 | | | | 9,505,347 | |
Accumulated other comprehensive loss | | | (676,828 | ) | | | (440,278 | ) |
Total shareholders’ equity | | | 9,876,251 | | | | 10,000,771 | |
Non-controlling interests | | | 1,109,664 | | | | 1,189,829 | |
Total equity | | | 10,985,915 | | | | 11,190,600 | |
TOTAL LIABILITIES AND EQUITY | | $ | 29,958,901 | | | $ | 25,541,469 | |
The accompanying notes are an integral part of these consolidated financial statements.
F-3
Table of Contents
WORK MEDICAL TECHNOLOGY GROUP LTD
CONSOLIDATED STATEMENTS OF INCOME AND COMPREHENSIVE LOSS
(In U.S. dollars, except for otherwise noted)
| | For the Years Ended
September 30, |
| | | 2023 | | 2022 |
Net revenue from third parties | | $ | 12,501,615 | | | $ | 19,498,315 | |
Net revenue from related party | | | 1,064,336 | | | | 212,975 | |
Cost of revenue | | | (9,422,967 | ) | | | (15,292,498 | ) |
Gross profit | | | 4,142,984 | | | | 4,418,792 | |
| | | | | | | | | |
Operating expenses: | | | | | | | | |
Selling expenses | | | (1,567,385 | ) | | | (1,032,685 | ) |
General and administrative expenses | | | (1,861,728 | ) | | | (2,563,879 | ) |
Research and development expenses | | | (301,644 | ) | | | (183,900 | ) |
Total operating expenses | | | (3,730,757 | ) | | | (3,780,464 | ) |
| | | | | | | | | |
Other (loss) income: | | | | | | | | |
Interest expense | | | (367,323 | ) | | | (350,999 | ) |
Government subsidies | | | 74,232 | | | | 1,186,641 | |
Other expenses, net | | | (8,747 | ) | | | (360,409 | ) |
Total other (loss) income | | | (301,838 | ) | | | 475,233 | |
| | | | | | | | | |
Income before income tax expense | | | 110,389 | | | | 1,113,561 | |
Income tax expense | | | (47,006 | ) | | | (169,435 | ) |
Net income | | $ | 63,383 | | | $ | 944,126 | |
| | | | | | | | | |
Net (loss) income attributable to non-controlling interest | | | (48,647 | ) | | | 78,916 | |
Net income attributable to ordinary shareholders | | $ | 112,030 | | | $ | 865,210 | |
Other comprehensive loss: | | | | | | | | |
Foreign currency translation adjustment | | | (268,068 | ) | | | (1,147,710 | ) |
Total comprehensive loss | | | (204,685 | ) | | | (203,584 | ) |
| | | | | | | | | |
Total comprehensive loss attributable to non-controlling interests | | | (80,165 | ) | | | (164,329 | ) |
Total comprehensive loss attributable to shareholders | | $ | (124,520 | ) | | $ | (39,255 | ) |
| | | | | | | | | |
Weighted average number of Ordinary Shares – basic and diluted* | | | 12,500,000 | | | | 12,500,000 | |
| | | | | | | | | |
Basic and diluted earnings per ordinary share | | $ | 0.01 | | | $ | 0.07 | |
The accompanying notes are an integral part of these consolidated financial statements.
F-4
Table of Contents
WORK MEDICAL TECHNOLOGY GROUP LTD
CONSOLIDATED STATEMENTS OF CHANGES IN EQUITY
(In U.S. dollars, except for share and per share data, or otherwise noted)
| |
Ordinary Shares*
| | Subscription
receivable | | Additional
paid-in
capital | | Statutory
reserves | | Retained
earnings | | Accumulated
other
comprehensive
income (loss) | | Total
shareholder’s
equity | | Non-
controlling
interest | | Total
equity |
Share | | Amount | |
Balance as of September 30, 2021 | | 12,500,000 | | $ | 6,250 | | $ | (6,250 | ) | | $ | 85,012 | | $ | 748,529 | | $ | 8,742,298 | | | $ | 464,187 | | | $ | 10,040,026 | | | $ | 1,354,158 | | | $ | 11,394,184 | |
Net income | | — | | | — | | | — | | | | — | | | — | | | 865,210 | | | | — | | | | 865,210 | | | | 78,916 | | | | 944,126 | |
Provision for statutory reserve | | — | | | — | | | — | | | | — | | | 102,161 | | | (102,161 | ) | | | — | | | | — | | | | — | | | | — | |
Foreign currency translation adjustment | | — | | | — | | | — | | | | — | | | — | | | — | | | | (904,465 | ) | | | (904,465 | ) | | | (243,245 | ) | | | (1,147,710 | ) |
Balance as of September 30, 2022 | | 12,500,000 | | $ | 6,250 | | $ | (6,250 | ) | | $ | 85,012 | | $ | 850,690 | | $ | 9,505,347 | | | $ | (440,278 | ) | | $ | 10,000,771 | | | $ | 1,189,829 | | | $ | 11,190,600 | |
Net income | | — | | | — | | | — | | | | — | | | — | | | 112,030 | | | | — | | | | 112,030 | | | | (48,647 | ) | | | 63,383 | |
Provision for statutory reserve | | — | | | — | | | — | | | | — | | | 36,792 | | | (36,792 | ) | | | — | | | | — | | | | — | | | | — | |
Foreign currency translation adjustment | | — | | | — | | | — | | | | — | | | — | | | — | | | | (236,550 | ) | | | (236,550 | ) | | | (31,518 | ) | | | (268,068 | ) |
Balance as of September 30, 2023 | | 12,500,000 | | $ | 6,250 | | $ | (6,250 | ) | | $ | 85,012 | | $ | 887,482 | | $ | 9,580,585 | | | $ | (676,828 | ) | | $ | 9,876,251 | | | $ | 1,109,664 | | | $ | 10,985,915 | |
The accompanying notes are an integral part of these consolidated financial statements.
F-5
Table of Contents
WORK MEDICAL TECHNOLOGY GROUP LTD
CONSOLIDATED STATEMENTS OF CASH FLOWS
(In U.S. dollars, except for share and per share data, or otherwise noted)
| | For the Years Ended
September 30, |
| | | 2023 | | 2022 |
CASH FLOWS FROM OPERATING ACTIVITIES: | | | | | | | | |
Net income | | $ | 63,383 | | | $ | 944,126 | |
Adjustments to reconcile net loss to net cash used in operating activities: | | | | | | | | |
Depreciation and amortization | | | 1,603,129 | | | | 3,258,267 | |
Inventories write-down | | | 18,320 | | | | (117,327 | ) |
Gain (loss) on disposal of equipment | | | 8,089 | | | | (10,871 | ) |
Allowance for doubtful accounts | | | 149,503 | | | | 1,108,999 | |
Deferred tax benefits | | | (94,533 | ) | | | (314,008 | ) |
Changes in assets and liabilities: | | | | | | | | |
Accounts receivable – third parties, net | | | (131,950 | ) | | | (4,234,997 | ) |
Accounts receivable – related party, net | | | — | | | | 275,993 | |
Advance to suppliers | | | 977,318 | | | | (191,774 | ) |
Amount due (from) to related parties | | | (3,964,077 | ) | | | 743,490 | |
Inventories | | | (400,544 | ) | | | 2,053,675 | |
Operating lease liabilities | | | (51,755 | ) | | | (5,212 | ) |
Prepaid expenses and other current assets | | | 1,291,145 | | | | (194,118 | ) |
Accounts payable | | | 864,585 | | | | (5,822,547 | ) |
Income taxes payable | | | 136,508 | | | | 81,428 | |
Deferred revenue | | | 52,245 | | | | (751,375 | ) |
Accrued expenses and other liabilities | | | 1,688,370 | | | | 917,303 | |
Net cash provided by (used in) operating activities | | | 2,209,736 | | | | (2,258,948 | ) |
| | | | | | | | | |
CASH FLOWS FROM INVESTING ACTIVITIES: | | | | | | | | |
Purchase of intangible asset | | | (127,913 | ) | | | — | |
Purchase of property and equipment | | | (455,391 | ) | | | (2,012,546 | ) |
Proceed from disposal of property and equipment | | | — | | | | 601,760 | |
Cash acquired from Shanghai Saitumofei | | | — | | | | 63,621 | |
Net cash used in investing activities | | | (583,304 | ) | | | (1,347,165 | ) |
| | | | | | | | | |
CASH FLOWS FROM FINANCING ACTIVITIES: | | | | | | | | |
Proceeds from short-term borrowings | | | 9,107,082 | | | | 7,248,367 | |
Repayments of short-term borrowings | | | (6,000,791 | ) | | | (7,248,367 | ) |
Loan to related parties | | | (5,669,834 | ) | | | (9,493,121 | ) |
Repayment from related parties | | | 5,891,373 | | | | 13,260,930 | |
Interest-free loans to third party | | | (41,229 | ) | | | (1,030,628 | ) |
Repayment from interest-free loans to third party | | | — | | | | 829,042 | |
Payment of offering cost | | | (1,356,176 | ) | | | — | |
Interest-bearing loans to third party | | | (2,682,706 | ) | | | — | |
Repayment of interest-bearing loans from third party | | | 23,562 | | | | — | |
Net cash (used in) provided by financing activities | | | (728,719 | ) | | | 3,566,223 | |
| | | | | | | | | |
Effect of exchange rate changes | | | (33,852 | ) | | | (59,819 | ) |
| | | | | | | | | |
Net increase (decrease) in cash and cash equivalents and restricted cash | | | 863,861 | | | | (99,709 | ) |
Cash, cash equivalents and restricted cash, at beginning of year | | | 773,422 | | | | 873,131 | |
Cash, cash equivalents and restricted cash, at end of year | | $ | 1,637,283 | | | $ | 773,422 | |
| | | | | | | | | |
SUPPLEMENTAL DISCLOSURE OF CASH FLOW INFORMATION: | | | | | | | | |
Income taxes paid | | $ | 160,162 | | | $ | 340,024 | |
Interest paid | | $ | 312,035 | | | $ | 312,282 | |
The accompanying notes are an integral part of these consolidated financial statements.
F-6
Table of Contents
WORK MEDICAL TECHNOLOGY GROUP LTD
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED SEPTEMBER 30, 2023 AND 2022
(In U.S. dollars, except share and per share data)
1. ORGANIZATION AND PRINCIPAL ACTIVITIES
WORK Medical Technology Group LTD (the “Company”, “Work Cayman” or “WORK”) was incorporated under the law of the Cayman Islands on March 1, 2022 as an exempted company with limited liability. The Company, together with its subsidiaries, is engaged in manufacturing and selling medical consumables through its wholly owned subsidiaries in the People’s Republic of China (the “PRC” or “China”).
History of the Group and Reorganization
The Company conducts its operations through its PRC subsidiary Hangzhou Shanyou Medical Equipment Co., Ltd. (“Hangzhou Shanyou”) and its subsidiaries, since 2002.
In preparation for its initial public offering, the Group completed a reorganization on May 6, 2022 (the “Reorganization”), which involved the following steps:
• on November 10, 2021, Work (Hangzhou) Medical Treatment Technology Co., Ltd. (“Work Hangzhou”) was established by Baiming Yu and his spouse, Liwei Zhang, who were the ultimate shareholders of Work Hangzhou;
• on January 17, 2022, Hangzhou Shanyou newly issued 95% of equity interest to Work Hangzhou. The remaining shareholders of Hangzhou Shanyou are Baiming Yu, with 3.35% of equity interest, and Liwei Zhang, with 1.65% of equity interest;
• on March 1, 2022, Work Cayman was incorporated and (indirectly) issued Ordinary Shares at par value $1.00 per share to certain founding shareholders. Baiming Yu (“LWY GROUP LTD”) and Liwei Zhang (“ZLW GROUP LTD”), who, following transfer of the initial one subscriber share from Tricor Services (Cayman Islands) Limited to LWY GROUP LTD, indirectly held a 50% and 5% equity interest of Work Cayman, respectively. Certain third parties, as strategic investors, acquired 45% equity shares of the PRC subsidiaries at fair value from Baiming Yu and Liwei Zhang. In exchange, Work Cayman issued the remainder of its 45% Ordinary Shares at par value $1.00 per share to these strategic investors on the day of its incorporation.
• on March 15, 2022, Work Medical Technology Group Limited (“Work BVI”) was incorporated in the British Virgin Islands as a wholly owned subsidiary of the Company;
• on April 19, 2022, Work Medical Technology Group (China) Limited (“Work Medical Technology” or “Work HK”) was incorporated in Hong Kong as a wholly owned subsidiary of Work BVI;
• on April 28, 2022, Work Age (Hangzhou) Medical Treatment Technology Co., Ltd. (“WFOE” or “Work Age”) was established as a wholly owned subsidiary of Work HK in the PRC; and
• on May 6, 2022, WFOE acquired 100% equity interest of Work Hangzhou.
On February 21, 2022, Hangzhou Shanyou entered into a share purchase agreement to purchase 60% equity shares of Hangzhou Hanshi Medical Equipment Co., Ltd. (“Hangzhou Hanshi”) from Baiming Yu. Since both Hangzhou Shanyou and Hangzhou Hanshi are under the common control immediately before and after the merger, this transaction was accounted for as a common control merger, and using merger accounting as if the Reorganization had been consummated at the beginning of the earliest period presented, and no gain or loss is recognized. All the assets and liabilities of Hangzhou Hanshi are recorded at carrying value.
Immediately before and after share issuances and transfer of Work Cayman, Work Hangzhou acquired Hangzhou Shanyou, and WFOE acquired Work Hangzhou. The ultimate shareholders in these entities, who are Baiming Yu and his spouse, Liwei Zhang, did not change. Accordingly, the Reorganization has been treated as a corporate restructuring of entities under common control. Thus, the current capital structure has been retroactively presented in prior periods as if such structure existed at that time, and the entities are presented on a combined basis for all periods to which such
F-7
Table of Contents
WORK MEDICAL TECHNOLOGY GROUP LTD
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED SEPTEMBER 30, 2023 AND 2022
(In U.S. dollars, except share and per share data)
1. ORGANIZATION AND PRINCIPAL ACTIVITIES (cont.)
entities were under common control. Thus, the results of these subsidiaries are included in the financial statements for years ended September 30, 2023 and 2022. The discussion and presentation of financial statements herein assumes the completion of the Reorganization, which is accounted for retroactively as if it occurred on October 1, 2019, and the equity has been restated to reflect the change, as well.
The consolidated financial statements reflect the activities of the Company and each of the following entities:
Name | | Date of
Incorporation | | Place of
incorporation | | Percentage of
effective ownership | | Principal Activities |
Subsidiaries | | | | | | | | |
Work BVI | | March 15, 2022 | | BVI | | 100% | | Investment holding |
Work Medical Technology | | April 19, 2022 | | Hong Kong | | 100% | | Investment holding |
WFOE | | April 28, 2022 | | PRC | | 100% | | Investment holding |
Work Hangzhou | | November 10, 2021 | | PRC | | 100% | | Investment holding |
Hangzhou Shanyou | | April 29, 2002 | | PRC | | 95% | | Produce and sale of medical consumables |
Hangzhou Hanshi | | July 22, 2019 | | PRC | | 60% owned by Hangzhou Shanyou | | Sale of medical consumables |
Shanghai Saitumofei Medical Treatment Technology Co., Ltd. (“Shanghai Saitumofei”) | | May 15, 2019 | | PRC | | 51% | | Sale of medical consumable |
Hunan Saitumofei Medical Treatment Technology Co., Ltd (“Hunan Saitumofei”) | | April 02, 2022 | | PRC | | 51% | | Sale of medical consumables |
Hangzhou Woli Medical Treatment Technology Co., Ltd (“Hangzhou Woli”) | | July 22, 2022 | | PRC | | 100% | | Sale of medical consumables |
Shanghai Chuqiang Medical Equipment Co., Ltd. (“Shanghai Chuqiang”) | | March 12, 2018 | | PRC | | 100% owned by Hangzhou Shanyou | | Sale of medical consumables |
Hangzhou Youshunhe Technology Co., Ltd. (“Hangzhou Youshunhe”) | | February 27, 2023 | | PRC | | 51% owned by Hangzhou Shanyou | | Sale of medical consumables |
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
(a) Basis of presentation and principles of consolidation
The accompanying consolidated financial statements are prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”) and pursuant to the rules and regulations of the Securities and Exchange Commission (the “SEC”). The accompanying consolidated financial statements include the financial statements of the Company and its subsidiaries. All inter-company balances and transactions are eliminated upon consolidation. For consolidated subsidiaries where the Group’s ownership in the subsidiary is less than 100%, the equity interest not held by the Group is shown as noncontrolling interests.
F-8
Table of Contents
WORK MEDICAL TECHNOLOGY GROUP LTD
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED SEPTEMBER 30, 2023 AND 2022
(In U.S. dollars, except share and per share data)
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.)
(b) Use of estimates
The preparation of the consolidated financial statements in accordance with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, related disclosures of contingent assets and liabilities at the balance sheet date, and the reported revenue and expenses during the reported periods in the consolidated financial statements and accompanying notes. Significant accounting estimates include, but not limited to, the allowance for receivable, useful lives of property, plant and equipment and intangible assets, inventories write-downs and the recoverability of long-lived assets. Changes in facts and circumstances may result in revised estimates. Actual results could differ from those estimates, and as such, differences may be material to the consolidated financial statements.
(c) Cash and cash equivalents
Cash and cash equivalents consist of cash on hand. The Group’s demand deposits are held at financial institutions, which have original maturities of less than three months and are unrestricted as to withdrawal and use. Deposits at financial institutions in mainland China and Hong Kong are not FDIC insured, however, those financial institutions are highly solvent and the risk of loss is remote.
(d) Restricted cash
Cash that is deposited in the bank but restricted for a specific purpose and therefore not available for immediate and general use by the Group is classified as restricted cash, which is presented separately from cash and cash equivalents on the consolidated balance sheets. As of September 30, 2023 and 2022, restricted cash represented cash preserved for guarantee deposit and litigation as disclosed in the notes to the accompanying financial statements.
(e) Leases
On October 1, 2021, the Group adopted Accounting Standards Update (“ASU”) 2016-02, Lease (FASB ASC Topic 842). The adoption of Topic 842 resulted in the presentation of operating lease right-of-use (“ROU”) assets and operating lease liabilities on the consolidated balance sheet. The Group has elected the package of practical expedients, which allows the Group not to reassess (1) whether any expired or existing contracts as of the adoption date are or contain a lease; (2) lease classification for any expired or existing leases as of the adoption date; and (3) initial direct costs for any expired or existing leases as of the adoption date. Lastly, the Group elected the short-term lease exemption for all contracts with lease terms of 12 months or less.
At inception of a contract, the Group assesses whether a contract is, or contains, a lease. A contract is or contains a lease if it conveys the right to control the use of an identified asset for a period of time in exchange for a consideration. To assess whether a contract is or contains a lease, the Group assess whether the contract involves the use of an identified asset, whether it has the right to obtain substantially all the economic benefits from the use of the asset and whether it has the right to control the use of the asset.
The right-of-use assets and related lease liabilities are recognized at the lease commencement date. The Group recognizes operating lease expenses for lease payments on a straight-line basis over the lease term.
Operating lease right-of-use of assets
The right-of-use of asset is initially measured at cost, which comprises the initial amount of the lease liability adjusted for any lease payments made at or before the commencement date, plus any initial direct costs incurred and less any lease incentive received.
F-9
Table of Contents
WORK MEDICAL TECHNOLOGY GROUP LTD
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED SEPTEMBER 30, 2023 AND 2022
(In U.S. dollars, except share and per share data)
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.)
Lease liabilities
Lease liability is initially measured at the present value of the outstanding lease payments at the commencement date, discounted using the Group’s incremental borrowing rate. Lease payments included in the measurement of the lease liability comprise fixed lease payments, variable lease payments that depend on an index or a rate, amounts expected to be payable under a residual value guarantee and any exercise price under a purchase option that the Group is reasonably certain to exercise.
Lease liability is measured at amortized cost using the effective interest rate method. It is re-measured when there is a change in future lease payments, if there is a change in the estimate of the amount expected to be payable under a residual value guarantee, or if there is any change in the Group assessment of option purchases, contract extensions or termination options.
(f) Accounts receivable, net
Accounts receivable are stated at the original amount less an allowance for doubtful receivable.
Accounts receivable is recognized in the period when the Group has provided services to its customers and when its right to consideration is unconditional. On January 1, 2023, the Group adopted ASU 2016-13, “Financial Instruments — Credit Losses (Accounting Standards Codification (“ASC”) Topic 326): Measurement on Credit Losses on Financial Instruments”, including certain subsequent amendments, transitional guidance and other interpretive guidance within ASU 2018-19, ASU 2019-04, ASU 2019-05, ASU 2019-11, ASU 2020-02 and ASU 2020-03 (collectively, including ASU 2016-13, “ASC 326”). ASC 326 introduces an approach based on expected losses to estimate the allowance for doubtful accounts, which replaces the previous incurred loss impairment model. The Group’s estimation of allowance for credit losses considers factors such as historical credit loss experience, age of receivable balances, current market conditions, reasonable and supportable forecasts of future economic conditions, as well as an assessment of receivables due from specific identifiable counterparties to determine whether these receivables are considered at risk or uncollectible.
The Group evaluates its accounts receivable for expected credit losses on a regular basis. The Group maintains an estimated allowance for credit losses to reduce its accounts receivable to the amount that it believes will be collected. The Group considers factors in assessing the collectability of its receivables, such as the age of the amounts due, the customer’s payment history, credit-worthiness and other specific circumstances related to the accounts. The Group adjusts the allowance percentage periodically when there are significant differences between estimated bad debts and actual bad debts. If there is strong evidence indicating that the accounts receivable is likely to be unrecoverable, the Group also makes specific allowance in the period in which a loss is determined to be probable. Accounts receivable balances are written off after all collection efforts have been exhausted.
(g) Inventories
Inventories, primarily consisting of the raw materials purchased by the Group for the production of medical consumables, are stated at the lower of cost or net realizable value. Cost of inventory is determined using weighted-average method. Where there is evidence that the utility of inventories, in their disposal in the ordinary course of business, will be less than cost, whether due to physical deterioration, obsolescence, changes in price levels, or other causes, the inventories are written down to net realizable value.
F-10
Table of Contents
WORK MEDICAL TECHNOLOGY GROUP LTD
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED SEPTEMBER 30, 2023 AND 2022
(In U.S. dollars, except share and per share data)
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.)
(h) Advance to suppliers
Advances to suppliers refer to advances for purchase of materials or other service agreements, which are applied against accounts payable when the materials or services are received. The Group reviews a supplier’s credit history and background information before advancing a payment. If the financial condition of its suppliers were to deteriorate, resulting in an impairment of their ability to deliver goods or provide services, the Group would provide allowance for such amount in the period when it is considered impaired. Advances to suppliers as of September 30, 2023 and 2022 primarily consisted of prepayments for purchasing medical consumables.
(i) Property, plant and equipment, net
Property, plant and equipment, except mask production machine, are stated at cost less accumulated depreciation and impairment, if any, and depreciated on a straight-line basis over the estimated useful lives of the assets. Mask production machine is stated at cost less accumulated depreciation and impairment, if any, and depreciated on a double declining balance method over 5 years. Cost represents the purchase price of the asset and other costs incurred to bring the asset into its intended use. Estimated useful lives are as follows:
Category | | Estimated useful lives |
Property and buildings | | 10 – 20 years |
Machinery and equipment | | 3 – 10 years |
Leasehold improvement | | Lesser of useful life and lease term |
Vehicle | | 4 – 5 years |
Office and electric equipment | | 3 – 5 years |
Repair and maintenance costs are charged to expenses as incurred, whereas the cost of renewals and betterment that extends the useful lives of property, plant and equipment are capitalized as additions to the related assets. Retirements, sales and disposals of assets are recorded by removing the costs, accumulated depreciation and impairment with any resulting gain or loss recognized in the consolidated statements of income.
(j) Intangible assets, net
Intangible assets are non-monetary assets without physical substance. These items are initially measured at cost and subsequently carried at cost less any accumulated amortization and impairment losses. Intangible assets with finite useful lives are amortized on a straight-line basis over their estimated useful lives, which is as follows:
Category | | Estimated useful lives |
Land use rights | | 36 years |
Patent | | 1 – 10 years |
Digital factory operation management system | | 10 years |
Mask customization system | | 5 years |
(k) Impairment of long-lived assets
The Group reviews its long-lived assets for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may no longer be recoverable. When these events occur, the Group measures impairment by comparing the carrying value of the long-lived assets to the estimated undiscounted future cash flows expected to result from the use of the assets and their eventual disposition. If the sum of the expected undiscounted cash flow is less than the carrying amount of the assets, the Group would recognize an impairment loss, which is the excess of carrying amount over the fair value of the assets, using the expected future discounted cash flows.
F-11
Table of Contents
WORK MEDICAL TECHNOLOGY GROUP LTD
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED SEPTEMBER 30, 2023 AND 2022
(In U.S. dollars, except share and per share data)
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.)
(l) Fair value measurement
Fair value is defined as the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. A three-level fair value hierarchy prioritizes the inputs used to measure fair value. The hierarchy requires entities to maximize the use of observable inputs and minimize the use of unobservable inputs. The three levels of inputs used to measure fair value are as follows:
• Level 1 — inputs to the valuation methodology are quoted prices (unadjusted) for identical assets or liabilities in active markets.
• Level 2 — inputs to the valuation methodology include quoted prices for similar assets and liabilities in active markets, quoted market prices for identical or similar assets in markets that are not active, inputs other than quoted prices that are observable and inputs derived from or corroborated by observable market data.
• Level 3 — inputs to the valuation methodology are unobservable.
The level in the fair value hierarchy within which a fair value measurement in its entirety falls is based on the lowest level input that is significant to the fair value measurement in its entirety. In situations where there is little, if any, market activity for the asset or liability at the measurement date, the fair value measurement reflects management’s own judgments about the assumptions that market participants would use in pricing the asset or liability. Those judgments are developed by management based on the best information available in the circumstances.
The carrying amounts of the Group’s financial instruments approximate their fair values because of their short-term nature.
(m) Revenue recognition
On October 1, 2019, the Group adopted Accounting Standards Codification (“ASC”) 606 using the modified retrospective approach.
The core principle of the new revenue standard is that a company should recognize revenue to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which the company expects to be entitled in exchange for those goods or services. The following five steps are applied to achieve that core principle:
Step 1: Identify the contract with the customer
Step 2: Identify the performance obligations in the contract
Step 3: Determine the transaction price
Step 4: Allocate the transaction price to the performance obligations in the contract
Step 5: Recognize revenue when the company satisfies a performance obligation
These criteria as they relate to each of the following major revenue generating activities are described below. Revenue is presented net of value added taxes (“VAT”) and surcharges. VAT tax to be collected from customers, net of VAT paid for purchases, is recorded as a liability in the consolidated balance sheets until it is paid to the tax authorities.
F-12
Table of Contents
WORK MEDICAL TECHNOLOGY GROUP LTD
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED SEPTEMBER 30, 2023 AND 2022
(In U.S. dollars, except share and per share data)
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.)
Revenue from sales of self-manufactured masks and medical devices other than masks
The Group sells products to different distributor customers and direct-end user customers, primarily of sales of self-manufactured masks and medical devices other than masks. The Group does not accept returns of products or offer refunds for its customers after the expiration of quality objection periods. The Group usually offers a seven-day quality objection period and any quality deficiencies are determined by the testing of a third-party institution.
The Group grants credit sales for customers and the credit period varies among different customers. The Group normally grants the customers 30 to 180 days to complete their payments after the credit sales, and the length of the credit period is dependent on the customer’s creditworthiness and its transaction experience with the Group. The Group makes its judgment taking all of the facts and circumstances into account, including the PRC subsidiaries’ customary business practices and the knowledge of the customer, in determining whether it is probable that the PRC subsidiaries will collect substantially all of the consideration to which they will be entitled in exchange for the goods or services that the PRC subsidiaries expect to transfer to the customer.
Control of a product is transferred to a distributor customer or direct-end user customer upon delivery of the product to the designated place. The revenue is recognized at a point in time when the Group satisfies the performance obligation by transferring the promised product to distributor customers or direct-end user customers upon acceptance by them. The Group presents the revenue generated from its sales of products on a gross basis as the Group acts as a principal.
Revenue from commodity trading
Revenue from commodity trading is recognized on a net basis or gross basis based on whether the Group arranges the provision of products through third parties and control the specified products provided by the third parties before that products are transferred to the customers. The revenue is recognized at a point in time when the Group satisfies performance obligations by arranging the transfer of a promised product to a customer. When the Group acted as an agent, the revenue is measured at fixed consideration which is determined as the difference between the sales price that the Group expects to receive in exchange for arranging promised products to the customer and the settlement price with the third-party suppliers. When the Group acted as a principal, the revenue is measured at fixed consideration which is the sales price that the Group expects to receive in exchange for arranging promised products.
Shipping and handling activities are considered to be fulfilment activities rather than promised services and are not, therefore, considered to be separate performance obligations. The Group’s sales terms provide no right of return outside of a standard quality policy and returns are generally not significant.
Geographic information
The following table disaggregates the Group’s revenue by geographic market for the years ended September 30, 2023 and 2022:
| | For the Year Ended
September 30, |
| | | 2023 | | 2022 |
Overseas market | | $ | 967,690 | | $ | 1,419,763 |
China domestic market | | | 12,598,261 | | | 18,291,527 |
Net revenue | | $ | 13,565,951 | | $ | 19,711,290 |
F-13
Table of Contents
WORK MEDICAL TECHNOLOGY GROUP LTD
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED SEPTEMBER 30, 2023 AND 2022
(In U.S. dollars, except share and per share data)
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.)
Revenue by product categories
The following table disaggregates the Group’s revenue product categories for the years ended September 30, 2023 and 2022:
| | For the Year Ended
September 30, |
| | | 2023 | | 2022 |
Masks | | $ | 5,091,331 | | $ | 10,619,035 |
Medical devices other than masks(1) | | | 7,997,540 | | | 8,747,886 |
Commodity trading(2) | | | 325,429 | | | 106,643 |
Others | | | 151,651 | | | 237,726 |
Net revenue | | $ | 13,565,951 | | $ | 19,711,290 |
Contract balances
Payment terms are established on the Group’s pre-established credit requirements based upon an evaluation of customers’ credit. Contract assets are recognized for in related accounts receivable.
The contract liabilities consist of deferred revenue, which represents the billings or cash received for services in advance of revenue recognition and is recognized as revenue when all the Group’s revenue recognition criteria are met. The Group’s deferred revenue was $776,210 and $744,107 as of September 30, 2023 and 2022, respectively.
Other than accounts receivable and deferred revenue, the Group had no other material contract assets, contract liabilities or deferred contract costs recorded on its consolidated balance sheets as of September 30, 2023 and 2022.
Contract costs
For the years ended September 30, 2023 and 2022, the Group did not have any significant incremental costs of obtaining contracts with customers incurred and/or costs incurred in fulfilling contracts with customers within the scope of ASC Topic 606, that shall be recognized as an asset and amortized to expenses in a pattern that matches the timing of the revenue recognition of the related contract.
(n) Government subsidies
Government subsidies are recognized as other income when received and all the conditions for their receipt have been met. The government subsidies were paid by cash and have no defined rules and regulations to govern the criteria necessary for the Group to enjoy the benefits.
For the years ended September 30, 2023 and 2022, the Company received government subsidies of $74,232 and $1,186,641, respectively.
(o) Costs of revenue
Cost of revenue mainly comprised of raw material costs, depreciation, labor cost, and other overhead expenses as well as inventories write-down.
(p) Income taxes
The Group accounts for income taxes under ASC 740. Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the consolidated financial statement carrying amounts of existing assets and liabilities and their respective tax bases.
F-14
Table of Contents
WORK MEDICAL TECHNOLOGY GROUP LTD
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED SEPTEMBER 30, 2023 AND 2022
(In U.S. dollars, except share and per share data)
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.)
Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period including the enactment date. Valuation allowances are established, when necessary, to reduce deferred tax assets to the amount expected to be realized. Current income taxes are provided for in accordance with the laws of the relevant taxing authorities.
The provisions of ASC 740-10-25, “Accounting for Uncertainty in Income Taxes,” prescribe a more-likely-than-not threshold for consolidated financial statement recognition and measurement of a tax position taken (or expected to be taken) in a tax return. This interpretation also provides guidance on the recognition of income tax assets and liabilities, classification of current and deferred income tax assets and liabilities, accounting for interest and penalties associated with tax positions, and related disclosures. The Group’s operating subsidiaries in the PRC are subject to examination by the relevant tax authorities. According to the PRC Tax Administration and Collection Law, the Law of the PRC on Enterprise Income Tax and other relevant laws and regulations, the statute of limitations is three years if the underpayment of taxes is due to computational errors made by the taxpayer or the withholding agent. The statute of limitations is extended to five years under special circumstances, where the underpayment of taxes is more than RMB0.1 million ($15,368). In the case of transfer pricing issues, the statute of limitation is ten years. There is no statute of limitation in the case of tax evasion. Penalties and interest incurred related to underpayment of income tax are classified as income tax expense in the period incurred.
The Group did not accrue any liability, interest or penalties related to uncertain tax positions in its provision for income taxes line of its consolidated statements of income for the years ended September 30, 2023 and 2022, respectively.
The Group does not expect that its assessment regarding unrecognized tax positions will materially change over the next 12 months.
(q) Value added tax (“VAT”)
The Group is subject to VAT and related surcharges on revenue generated from sales of products, facilitation services and platform services. The Group records revenue net of VAT. This VAT may be offset by qualified input VAT. Net VAT balance between input VAT and output VAT is recorded in the line item of other current assets on the consolidated balance sheets.
The VAT rate is 13% for taxpayers selling consumer products.
(r) Non-controlling Interest
A non-controlling interest in a subsidiary of the Group represents the portion of the equity (net assets) in the subsidiary not directly or indirectly attributable to the Group. Non-controlling interests are presented as a separate component of equity on the consolidated balance sheets, and net income and other comprehensive loss attributable to non-controlling shareholders are presented as a separate component on the consolidated statements of operations.
(s) Foreign currency transactions and translations
The Group’s principal country of operations is the PRC. The financial position and results of its operations are determined using RMB, the local currency, as the functional currency. The Group’s financial statements are reported using U.S. Dollars (“$”). The results of operations and the consolidated statements of cash flows denominated in foreign currency are translated at the average rate of exchange during the reporting period.
F-15
Table of Contents
WORK MEDICAL TECHNOLOGY GROUP LTD
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED SEPTEMBER 30, 2023 AND 2022
(In U.S. dollars, except share and per share data)
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.)
Assets and liabilities denominated in foreign currencies at the balance sheet date are translated at the applicable rates of exchange in effect at that date. The equity denominated in the functional currency is translated at the historical rate of exchange at the time of capital contribution. Because cash flows are translated based on the average translation rate, amounts related to assets and liabilities reported on the consolidated statements of cash flows will not necessarily agree with changes in the corresponding balances on the consolidated balance sheets. Translation adjustments arising from the use of different exchange rates from period to period are included as a separate component of accumulated other comprehensive loss included in consolidated statements of changes in equity. Gains and losses from foreign currency transactions are included in the results of operations.
The value of RMB against US$ and other currencies may fluctuate and is affected by, among other things, changes in the PRC’s political and economic conditions. Any significant revaluation of RMB may materially affect the Group’s financial condition in terms of US$ reporting. The following table outlines the currency exchange rates that were used in creating the consolidated financial statements:
| | As of September 30, |
| | | 2023 | | 2022 |
Balance sheet items, except for equity accounts | | 7.2960 | | 7.1135 |
| | For the Years Ended
September 30, |
| | | 2023 | | 2022 |
Items in the statements of income and comprehensive loss, and statements of cash flows | | 7.0824 | | 6.5532 |
No representation is made that the RMB amounts could have been, or could be, converted into U.S. dollars at the rates used in translation.
(t) Comprehensive loss
Comprehensive loss consists of two components, net income and other comprehensive loss. The foreign currency translation gain or loss resulting from translation of the financial statements expressed in RMB to USD is reported in other comprehensive loss in the consolidated statements of income and comprehensive loss.
(u) Earnings per share
Earnings per Ordinary Share is calculated in accordance with ASC 260, Earnings per Share. Basic earnings per Ordinary Share is computed by dividing the net income attributable to shareholders of the Company by the weighted average number of Ordinary Shares outstanding during the year. Diluted earnings per Ordinary Share is computed in accordance with the treasury stock method and based on the weighted average number of Ordinary Shares and dilutive Ordinary Share equivalents. Dilutive Ordinary Share equivalents are excluded from the computation of diluted earnings per Ordinary Share if their effects would be anti-dilutive. There is no Ordinary Share equivalent issued to date.
(v) Segment reporting
The Group uses the management approach in determining its operating segments. The Group’s chief operating decision maker (“CODM”) identified as the Group’s Chief Executive Officer, relies upon the consolidated results of operations as a whole when making decisions about allocating resources and assessing the performance of the Group. As a result of the assessment made by CODM, the Group has only one reportable segment. The Group does not distinguish between markets or segments for the purpose of internal reporting. As substantially all of the Group’s long-lived assets are located in the PRC, no geographical segments are presented.
F-16
Table of Contents
WORK MEDICAL TECHNOLOGY GROUP LTD
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED SEPTEMBER 30, 2023 AND 2022
(In U.S. dollars, except share and per share data)
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.)
(w) Commitments and contingencies
Liabilities for loss contingencies arising from claims, assessments, litigation, fines, and penalties and other sources are recorded when it is probable that a liability has been incurred and the amount can be reasonably estimated. If a potential material loss contingency is not probable but is reasonably possible, or is probable but cannot be estimated, then the nature of the contingent liability, together with an estimate of the range of possible loss if determinable and material, is disclosed. Legal costs incurred in connection with loss contingencies are expensed as incurred.
(x) Recent accounting pronouncements
The Company is an “emerging growth company” (“EGC”) as defined in the Jumpstart Our Business Startups Act of 2012 (the “JOBS Act”). Under the JOBS Act, EGC can delay adopting new or revised accounting standards issued subsequent to the enactment of the JOBS Act until such time as those standards apply to private companies.
Recent accounting standards that have been issued by FASB that do not require adoption until a future date are not expected to have a material impact on the consolidated financial statements upon adoption. The Group does not discuss recent standards that are not anticipated to have an impact on or are unrelated to its consolidated financial position, results of operations, cash flows or disclosures.
3. ACCOUNTS RECEIVABLE, NET
Accounts receivable, net consisted of the following:
| | As of September 30, |
| | | 2023 | | 2022 |
Accounts receivable | | $ | 4,343,923 | | | $ | 4,632,297 | |
Allowance for doubtful accounts | | | (1,011,601 | ) | | | (1,344,480 | ) |
Accounts receivable, net | | $ | 3,332,322 | | | $ | 3,287,817 | |
The Group recorded reversal of bad debt expense of $134,846 for the years ended September 30, 2023; and recorded bad debt expense of $136,228 and $1,258,039 for the years ended September 30, 2023 and 2022, respectively.
As of date of issuance this financial statement, the Group has collected $2,584,990 of outstanding balance as of September 30, 2023.
| | As of September 30, |
| | | 2023 | | 2022 |
Balance at the beginning of the year | | $ | 1,344,480 | | | $ | 204,826 | |
Current period addition | | | 136,228 | | | | 1,258,039 | |
Write-off | | | (309,656 | ) | | | — | |
Reversal | | | (134,846 | ) | | | — | |
Foreign currency translation adjustment | | | (24,605 | ) | | | (118,385 | ) |
Balance at the end of the year | | $ | 1,011,601 | | | $ | 1,344,480 | |
F-17
Table of Contents
WORK MEDICAL TECHNOLOGY GROUP LTD
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED SEPTEMBER 30, 2023 AND 2022
(In U.S. dollars, except share and per share data)
4. INVENTORIES, NET
Inventories consisted of the following:
| | As of September 30, |
| | | 2023 | | 2022 |
Raw materials | | $ | 741,988 | | $ | 629,672 |
Work in progress | | | 2,030,108 | | | 1,747,142 |
Finished goods | | | 2,089,383 | | | 2,210,596 |
Less: impairment | | | 241,288 | | | 229,239 |
Inventories, net | | $ | 4,620,191 | | $ | 4,358,171 |
Impairment provided for the inventories was $18,320 and $102,092 for the years ended September 30, 2023 and 2022, respectively.
| | As of September 30, |
| | | 2023 | | 2022 |
Balance at the beginning of the year | | $ | 229,239 | | | $ | 372,405 | |
Current period addition | | | 18,320 | | | | 102,092 | |
Reduction | | | — | | | | (222,465 | ) |
Foreign currency translation adjustment | | | (6,271 | ) | | | (22,793 | ) |
Balance at the end of the year | | $ | 241,288 | | | $ | 229,239 | |
5. PREPAID EXPENSES AND OTHER CURRENT ASSETS
Prepaid expenses and other current assets consisted of the following:
| | As of September 30, |
| | | 2023 | | 2022 |
Interest-bearing loans to third parties(1) | | $ | 2,604,167 | | | $ | — | |
Deferred offering costs | | | 1,356,176 | | | | 619,837 | |
Interest-free loans to third parties(2) | | | 550,706 | | | | 2,032,473 | |
Prepaid expenses | | | 24,152 | | | | 13,643 | |
Receivable from third party | | | 613,580 | | | | 492,888 | |
Others | | | 59,535 | | | | 8,897 | |
Allowance for doubtful accounts | | | (352,935 | ) | | | (370,746 | ) |
Total prepaid expenses and other current assets | | $ | 4,855,381 | | | $ | 2,796,992 | |
F-18
Table of Contents
WORK MEDICAL TECHNOLOGY GROUP LTD
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED SEPTEMBER 30, 2023 AND 2022
(In U.S. dollars, except share and per share data)
6. PROPERTY, PLANT AND EQUIPMENT, NET
Property, plant and equipment, net, consisted of the following:
| | As of September 30, |
| | | 2023 | | 2022 |
Property and buildings | | $ | 5,397,008 | | $ | 5,533,254 |
Machinery and equipment | | | 12,084,239 | | | 12,835,325 |
Vehicle | | | 156,119 | | | 130,760 |
Office and electric equipment | | | 137,050 | | | 121,535 |
Subtotal | | | 17,774,416 | | | 18,620,874 |
Less: impairment | | | 2,648,945 | | | 3,004,490 |
Less: accumulated depreciation | | | 8,498,697 | | | 7,727,969 |
Property, plant and equipment, net | | $ | 6,626,774 | | $ | 7,888,415 |
Depreciation expense were $1,507,792 and $3,209,585 for the years ended September 30, 2023 and 2022, respectively.
The Group did not record any impairment charge for the years ended September 30, 2023 and 2022.
Written-off of impairment due to disposal of related assets were $22,327 and $1,531,190 for the years ended September 30, 2023 and 2022, respectively.
7. INTANGIBLE ASSETS, NET
Intangible assets consisted of the following:
| | As of September 30, |
| | | 2023 | | 2022 |
Land use rights | | $ | 978,539 | | $ | 1,003,644 |
Others | | | 277,778 | | | 157,805 |
Subtotal | | | 1,256,317 | | | 1,161,449 |
Less: accumulated amortization | | | 257,867 | | | 211,494 |
Intangible assets, net | | $ | 998,450 | | $ | 949,955 |
Amortization expenses were $53,220 and $48,682 for the years ended September 30, 2023 and 2022, respectively.
The following table presents future amortization as of September 30, 2023:
Year ended September 30, | | Amount |
2024 | | $ | 62,162 |
2025 | | | 62,162 |
2026 | | | 62,162 |
2027 | | | 47,830 |
2028 | | | 38,834 |
Thereafter | | | 725,300 |
| | | $ | 998,450 |
F-19
Table of Contents
WORK MEDICAL TECHNOLOGY GROUP LTD
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED SEPTEMBER 30, 2023 AND 2022
(In U.S. dollars, except share and per share data)
8. ACCRUED EXPENSES AND OTHER LIABILITIES
Accrued expenses and other liabilities consisted of the following:
| | As of September 30, |
| | | 2023 | | 2022 |
VAT payable | | $ | 1,388,754 | | $ | 1,103,504 |
Payroll payable | | | 625,802 | | | 508,567 |
Other payable | | | 2,384,040 | | | 1,218,385 |
Total accrued expenses and other liabilities | | $ | 4,398,596 | | $ | 2,830,456 |
9. LEASES
The Group has two operating lease for its office space.
Operating lease right-of-use assets and liabilities are recognized at commencement date based on the present value of lease payments over the lease term. The discount rate used to calculate present value is incremental borrowing rate or, if available, the rate which is implicit in the lease. The Group determines the incremental borrowing rate for each lease based primarily on its lease term in PRC, which is approximately 4.75%.
Operating lease expenses were $42,118 and $19,330 for the years ended September 30, 2023 and 2022, respectively.
The undiscounted future minimum lease payment schedule as follows:
For the Years Ended September 30 | | Lease
Payment |
2024 | | 53,269 | |
2025 | | 25,621 | |
2026 | | 26,902 | |
Total lease payments | | 105,792 | |
Interest | | (6,102 | ) |
Total | | 99,690 | |
10. TAXATION
Cayman Islands
The Company is incorporated in the Cayman Islands. Under the current laws of the Cayman Islands, the Company is not subject to income or capital gains taxes. In addition, dividend payments are not subject to withholdings tax in the Cayman Islands.
Hong Kong
According to Tax (Amendment) (No. 3) Ordinance 2018 published by Hong Kong government, since September 1, 2018, under the two-tiered profits tax rate regime, the profits tax rate for the first HKD2 million of assessable profits has been lowered to 8.25% (half of the rate specified in Schedule 8 to the Inland Revenue Ordinance (IRO)) for corporations. Work HK was not subject to Hong Kong profit tax for any period presented as it did not have assessable profit during the periods presented.
F-20
Table of Contents
WORK MEDICAL TECHNOLOGY GROUP LTD
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED SEPTEMBER 30, 2023 AND 2022
(In U.S. dollars, except share and per share data)
10. TAXATION (cont.)
PRC
The Group’s subsidiaries in mainland China are all subject to PRC Enterprise Income Tax (“EIT”) on the taxable income in accordance with the relevant PRC income tax laws and regulations. The EIT rate for the Group’s operation in mainland China was 25% with the following exceptions.
Hangzhou Shanyou was qualified as a “high and new technology enterprise strongly supported by the State” in November 2017 and renewed in December 2023, and entitled to an EIT rate of 15%, expiring on December 8, 2026.
The income tax provision consisted of the following components:
| | For the Years Ended
September 30, |
| | | 2023 | | 2022 |
Current income tax expenses | | $ | 141,539 | | | $ | 483,443 | |
Deferred income tax benefit | | | (94,533 | ) | | | (314,008 | ) |
Total income tax expenses | | $ | 47,006 | | | $ | 169,435 | |
A reconciliation between the Group’s actual provision for income taxes and the provision at the PRC, mainland statutory rate is as follows:
| | For the Years Ended
September 30, |
| | | 2023 | | 2022 |
Income before income taxes | | $ | 110,389 | | | $ | 1,113,561 | |
Income tax expenses computed at statutory EIT rate of 25% | | | 27,597 | | | | 278,390 | |
Reconciling items: | | | | | | | | |
Effect of preferred tax rate | | | (14,264 | ) | | | (102,578 | ) |
Additional deduction for R&D expenses | | | (45,247 | ) | | | (27,585 | ) |
Change in valuation allowance | | | 53,964 | | | | 13,660 | |
Tax effect of non-deductible items | | | 24,956 | | | | 7,548 | |
Income tax expenses | | $ | 47,006 | | | | 169,435 | |
Effective tax rates | | | 42.6 | % | | | 15.2 | % |
F-21
Table of Contents
WORK MEDICAL TECHNOLOGY GROUP LTD
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED SEPTEMBER 30, 2023 AND 2022
(In U.S. dollars, except share and per share data)
10. TAXATION (cont.)
Deferred tax assets/liability
As of September 30, 2023 and 2022, the significant components of the deferred tax assets and deferred tax liabilities are summarized below:
| | As of September 30, |
| | | 2023 | | 2022 |
Deferred tax assets: | | | | | | | | |
Allowance for doubtful accounts | | $ | 510,542 | | | $ | 493,598 | |
Inventories write-down | | | 36,193 | | | | 34,386 | |
Impairment of equipment | | | 33,768 | | | | 74,438 | |
Net operating loss carry forwards | | | 52,359 | | | | 12,584 | |
Deferred tax assets, gross | | | 632,862 | | | | 615,006 | |
Less: valuation allowance | | | (59,879 | ) | | | (12,584 | ) |
Deferred tax assets, net | | $ | 572,983 | | | $ | 602,422 | |
Deferred tax liability: | | | | | | | | |
Depreciation* | | | 506,111 | | | | 627,954 | |
Deferred tax liability, net | | | 506,111 | | | | 627,954 | |
Net deferred tax asset (liability): | | $ | 66,872 | | | $ | (25,532 | ) |
Uncertain tax positions
The Group evaluates each uncertain tax position (including the potential application of interest and penalties) based on the technical merits, and measure the unrecognized benefits associated with the tax positions. As of September 30, 2023 and 2022, the Group did not have any significant unrecognized uncertain tax positions.
11. BORROWINGS
As of September 30, 2023 and 2022, summary of the borrowings is as following:
| | Annual
Interest Rate | | Maturity
date | | September 30,
2023 | | September 30,
2022 |
Bank of Jiangsu | | 4.05 | % | | March 2024 | | $ | 411,184 | | $ | — |
| | | | | | Subtotal | | $ | 411,184 | | $ | — |
Xiaoshan Rural Commercial Bank | | 4.40 | % – 4.70% | | August 2024 | | $ | 4,454,496 | | $ | 4,568,778 |
| | | 4.35 | % – 4.50% | | August 2024 | | | 1,370,614 | | | 1,405,777 |
| | | | | | Subtotal | | $ | 5,825,110 | | $ | 5,974,555 |
Bank of Beijing | | 3.55 | % | | June 2024 | | $ | 1,370,614 | | $ | — |
| | | | | | Subtotal | | $ | 1,370,614 | | $ | — |
China Citic Bank | | 3.90 | % | | June 2024 | | | 1,233,553 | | | — |
| | | | | | Subtotal | | $ | 1,233,553 | | $ | — |
| | | | | | Total | | $ | 8,840,461 | | $ | 5,974,555 |
Interest expenses were $312,035 and $319,045 for the years ended September 30, 2023 and 2022, respectively.
The borrowings from Xiaoshan Rural Commercial Bank were secured by the Group’s buildings and guarantee by Huiyu Holdings Group Co., Ltd. The borrowing from China Citic Bank was secured by Baiming Yu.
F-22
Table of Contents
WORK MEDICAL TECHNOLOGY GROUP LTD
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED SEPTEMBER 30, 2023 AND 2022
(In U.S. dollars, except share and per share data)
12. RELATED PARTY TRANSACTIONS
The following is a list of related parties which the Group has transactions with:
No. | | Name of Related Parties | | Relationship with the Group |
a | | Baiming Yu | | COO, Director and a shareholder of the Company |
b | | Liwei Zhang | | Shareholder of the Company and the spouse of
Baiming Yu |
c | | Huiyu Chuanggu (Hangzhou) Equity Investment Fund Co., Ltd. (“Huiyu Chuanggu”) | | Shuang Wu acted as the executive director and owned 30% equity interests of this related party |
d | | Baicheng Zhang | | Immediate family member of Liwei Zhang |
e | | Shuang Wu | | Chief Executive Officer of the Company |
f | | Hangzhou Shuige Technology Co., Ltd (“Hangzhou Shuige”) | | 55% equity interests owned by Baiming Yu |
g | | Hangzhou Qingniu Medical Instrument Co., Ltd (“Hangzhou Qingniu”) | | Owned by immediate family member of Baiming Yu |
h | | Hangzhou Xiaoshan Ance Building Materials Firm (“Xiaoshan Ance”) | | Owned by Liwei Zhang |
i | | Hangzhou Yuanqi Biotech Co., Ltd. (“Hangzhou Yuanqi”) | | Baiming Yu acted as director |
j | | Ming Zhao | | Shareholder of Shanghai Saitumofei |
k | | Hangzhou Yizhiying Information Consulting Management Co., Ltd | | Shuang Wu acted as executive director |
Amounts due from related parties
Amounts due from related parties consisted of the following for the periods indicated:
| | As of September 30, |
| | | 2023 | | 2022 |
Huiyu Chuanggu(1) | | $ | 1,453,583 | | $ | — |
Hangzhou Qingniu(2) | | | 1,294,296 | | | 429,347 |
Shuang Wu(3) | | | 795,243 | | | — |
Baiming Yu(4) | | | 683,376 | | | 160,405 |
Liwei Zhang(5) | | | 11,184 | | | 70,514 |
Baicheng Zhang(5) | | | — | | | 46,012 |
Total | | $ | 4,237,682 | | $ | 706,278 |
F-23
Table of Contents
WORK MEDICAL TECHNOLOGY GROUP LTD
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED SEPTEMBER 30, 2023 AND 2022
(In U.S. dollars, except share and per share data)
12. RELATED PARTY TRANSACTIONS (cont.)
Amounts due to related parties
Amounts due from related parties consisted of the following for the periods indicated:
| | As of September 30, |
| | | 2023 | | 2022 |
Shuang Wu(1) | | $ | — | | $ | 623,070 |
Xiaoshan Ance(2) | | | 137,061 | | | 140,578 |
Hangzhou Shuige(2) | | | 17,818 | | | 18,275 |
Hangzhou Yuanqi(2) | | | 685 | | | — |
Hangzhou Yizhiying Information Consulting Management Co., Ltd(2) | | | — | | | 80,812 |
Ming Zhao(3) | | | 4,048 | | | 4,151 |
Total | | $ | 159,612 | | $ | 866,886 |
Related party transactions
| | For the Years Ended
September 30, |
Nature | | 2023 | | 2022 |
Loan to Hangzhou Shuige(1) | | $ | 423,585 | | $ | 1,983,764 |
Repayment from Hangzhou Shuige(1) | | | 423,585 | | | 5,889,698 |
Loan to Liwei Zhang(1) | | | 303,075 | | | — |
Repayment from Liwei Zhang(1) | | | 362,377 | | | — |
Advance to Baiming Yu(2) | | | 1,187,554 | | | 17,148 |
Reimbursement from Baiming Yu(2) | | | 644,678 | | | — |
Selling to Hangzhou Qingniu(3) | | | 1,011,587 | | | 16,524 |
Loan to Hangzhou Qingniu(1) | | | 4,943,174 | | | 7,356,760 |
Rent income from Hangzhou Qingniu(4) | | | 52,749 | | | 192,636 |
Repayment from Hangzhou Qingniu(1)(3)(4) | | | 5,105,411 | | | 7,371,232 |
Loan from Xiaoshan Ance(5) | | | — | | | 152,597 |
Advance from Shuang Wu(6) | | | 2,022,703 | | | 851,143 |
Advance to Shuang Wu(6) | | | 819,227 | | | — |
Repayment to Shuang Wu(6) | | | 2,648,510 | | | 174,800 |
Advance to Huiyu Chuanggu(7) | | | 2,894,499 | | | — |
Reimbursement from Huiyu Chuanggu(7) | | | 1,397,077 | | | — |
Rent income from Hangzhou Yuanqi(4) | | | — | | | 3,815 |
F-24
Table of Contents
WORK MEDICAL TECHNOLOGY GROUP LTD
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED SEPTEMBER 30, 2023 AND 2022
(In U.S. dollars, except share and per share data)
12. RELATED PARTY TRANSACTIONS (cont.)
| | For the Years Ended
September 30, |
Nature | | 2023 | | 2022 |
Collection from Hangzhou Yuanqi(4) | | 706 | | 3,815 |
Advance to Ming Zhao(8) | | 866 | | — |
Collection from Ming Zhao(8) | | 866 | | 1,958 |
Advance from Hangzhou Yizhiying Information Consulting Management Co., Ltd(9) | | — | | 149,606 |
Repayment to Hangzhou Yizhiying Information Consulting Management Co., Ltd(9) | | — | | 61,885 |
13. ORDINARY SHARES
Ordinary Shares
The Company was established as an exempted company under the laws of Cayman Islands on March 1, 2022. The authorized number of Ordinary Shares was 50,000 with par value of $1 per share. The Company issued 50,000 shares to the shareholders at par value $1 per share.
On April 6, 2023, the shareholders of the Company unanimously passed resolutions effecting the subdivision of the Company’s authorized and issued share capital and the adoption of the amended and restated memorandum of association, pursuant to which, (1) the Company effectuated a 1:2000 share subdivision, whereupon the Company’s authorized share capital was amended from US$50,000 divided into 50,000 shares of par value US$1.00 each to US$50,000 divided into 100,000,000 Ordinary Shares of a par value of $0.0005 each; and (2) immediately after the share subdivision, the shareholders voluntarily surrendered, on a pro rata basis, a total of 87,500,000 Ordinary Shares of a par value of $0.0005 each, after which, the Company had an aggregate of 12,500,000 Ordinary Shares issued and outstanding.
Subscription receivable
As of September 30, 2023 and 2022, subscription receivable on the consolidated balance sheets represented the unrecovered consideration of the 12,500,000 ordinary shares issued by the Company.
F-25
Table of Contents
WORK MEDICAL TECHNOLOGY GROUP LTD
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED SEPTEMBER 30, 2023 AND 2022
(In U.S. dollars, except share and per share data)
14. STATUTORY SURPLUS RESERVES AND RESTRICTED NET ASSETS
A significant portion of the Group’s operations are conducted through its PRC (excluding Hong Kong) subsidiaries, the Company’s ability to pay dividends is primarily dependent on receiving distributions of funds from our subsidiaries. Relevant PRC statutory laws and regulations permit payments of dividends by our subsidiaries only out of their retained earnings, if any, as determined in accordance with PRC accounting standards and regulations, and after it has met the PRC requirements for appropriation to statutory reserves. The Group is required to make appropriations to certain reserve funds, comprising the statutory surplus reserve and the discretionary surplus reserve, based on after-tax net income determined in accordance with generally accepted accounting principles of the PRC (“PRC GAAP”). Appropriations to the statutory surplus reserve are required to be at least 10% of the after-tax net income determined in accordance with PRC GAAP until the reserve is equal to 50% of the entity’s registered capital. Appropriations to the surplus reserve are made at the discretion of the Board of Directors. Paid-in capital of our subsidiaries included in the Company’s consolidated net assets are also non-distributable for dividend purposes.
As a result of these PRC laws and regulations, the Company’s PRC subsidiaries are restricted in their ability to transfer a portion of their net assets to the Company. As of September 30, 2023 and 2022, net assets restricted in the aggregate, which include paid-in capital and statutory reserve funds of the Company’s subsidiaries, that are included in the consolidated net assets were $972,494 and $935,702, respectively.
15. CONCENTRATION OF CREDIT RISK
Financial instruments that potentially expose the Group to concentrations of credit risk consist primarily of accounts receivable. The Group conducts credit evaluations of its customers, and generally does not require collateral or other security from them. The Group evaluates its collection experience and long outstanding balances to determine the need for an allowance for doubtful accounts. The Group conducts periodic reviews of the financial condition and payment practices of its customers to minimize collection risk on accounts receivable.
The following table sets forth a summary of single customers who represented 10% or more of the Group’s total accounts receivable:
| | As of September 30, |
| | | 2023 | | 2022 |
Percentage of the Group’s accounts receivable | | | | | |
Customer A | | 19 | % | | * |
Customer B | | 14 | % | | * |
Customer C | | 13 | % | | * |
The following table sets forth a summary of single suppliers who represented 10% or more of the Group’s total purchases:
| | For the Years Ended
September 30, |
| | | 2023 | | 2022 |
Percentage of the Group’s purchase | | | | | | |
Supplier A | | 13 | % | | * | |
Supplier B | | 11 | % | | 11 | % |
Supplier C | | * | | | 16 | % |
F-26
Table of Contents
WORK MEDICAL TECHNOLOGY GROUP LTD
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED SEPTEMBER 30, 2023 AND 2022
(In U.S. dollars, except share and per share data)
15. CONCENTRATION OF CREDIT RISK (cont.)
The following table sets forth a summary of single suppliers who represented 10% or more of the Group’s total advance to suppliers:
| | As of September 30, |
| | | 2023 | | 2022 |
Percentage of the Group’s advance to | | | | | | |
Supplier A | | * | | | 43 | % |
Supplier D | | 40 | % | | — | |
Supplier E | | 20 | % | | — | |
Supplier F | | 10 | % | | * | |
Supplier G | | — | | | 33 | % |
16. COMMITMENTS AND CONTINGENCIES
Commitments
As of September 30, 2023 and 2022, the Group has no material purchase commitments or significant leases.
Contingencies
In the ordinary course of business, the Group may be subject to legal proceedings regarding contractual and employment relationships and a variety of other matters. The Group records contingent liabilities resulting from such claims, when a loss is assessed to be probable, and the amount of the loss is reasonably estimable. In the opinion of management, there were no material pending or threatened claims and litigation as of the issuance date of these consolidated financial statements.
17. CONDENSED FINANCIAL INFORMATION OF THE PARENT COMPANY
The condensed financial information of the parent company, WORK Medical Technology Group LTD, has been prepared using the same accounting policies as set out in the Group’s consolidated financial statements except that the parent company has used equity method to account for its investment in its subsidiaries.
The Company and its subsidiaries are included in the consolidated financial statements where the inter-company balances and transactions are eliminated upon consolidation. For the purpose of the Company’s stand-alone financial statements, its investments in subsidiaries are reported using the equity method of accounting. The Company’s share of income from its subsidiaries is reported as earnings from subsidiaries in the accompanying condensed financial information of parent company.
F-27
Table of Contents
WORK MEDICAL TECHNOLOGY GROUP LTD
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED SEPTEMBER 30, 2023 AND 2022
(In U.S. dollars, except share and per share data)
17. CONDENSED FINANCIAL INFORMATION OF THE PARENT COMPANY (cont.)
The Company did not have significant capital and other commitments, long-term obligations, or guarantees as of September 30, 2023 and 2022.
PARENT COMPANY BALANCE SHEETS
| | As of September 30, |
| | | 2023 | | 2022 |
ASSETS | | | | | | | | |
Current Assets | | | | | | | | |
Prepaid expenses and other current assets | | $ | — | | | $ | 619,837 | |
Total current assets | | | — | | | | 619,837 | |
| | | | | | | | | |
Non-current Assets | | | | | | | | |
Long term investment | | | 9,876,251 | | | | 10,000,771 | |
Total non-current assets | | | 9,876,251 | | | | 10,000,771 | |
Total assets | | | 9,876,251 | | | | 10,620,608 | |
| | | | | | | | | |
LIABILITIES AND SHAREHOLDERS’ EQUITY | | | | | | | | |
Liabilities | | | | | | | | |
Amounts due to related parties | | | — | | | | 619,837 | |
Current Liabilities | | | — | | | | 619,837 | |
Total liabilities | | | — | | | | 619,837 | |
| | | | | | | | | |
Shareholders’ equity | | | | | | | | |
Ordinary Shares | | | 6,250 | | | | 6,250 | |
Subscription receivable | | | (6,250 | ) | | | (6,250 | ) |
Additional paid-in capital | | | 85,012 | | | | 85,012 | |
Statutory reserve | | | 887,482 | | | | 850,690 | |
Retained earnings | | | 9,580,585 | | | | 9,505,347 | |
Accumulated other comprehensive (loss) income | | | (676,828 | ) | | | (440,278 | ) |
Total shareholders’ equity | | | 9,876,251 | | | | 10,000,771 | |
Total liabilities and shareholders’ equity | | $ | 9,876,251 | | | $ | 10,620,608 | |
PARENT COMPANY STATEMENTS OF INCOME AND COMPREHENSIVE LOSS
| | As of September 30, |
| | | 2023 | | 2022 |
Other income: | | | | | | | | |
Equity in earnings of subsidiaries | | $ | 112,030 | | | $ | 865,210 | |
Income before income tax expense | | | 112,030 | | | | 865,210 | |
Income tax expense | | | — | | | | — | |
Net income | | $ | 112,030 | | | $ | 865,210 | |
Other comprehensive loss: | | | | | | | | |
Foreign currency translation loss, net of nil income taxes | | | (236,550 | ) | | | (904,465 | ) |
Total comprehensive loss | | $ | (124,520 | ) | | $ | (39,255 | ) |
F-28
Table of Contents
WORK MEDICAL TECHNOLOGY GROUP LTD
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED SEPTEMBER 30, 2023 AND 2022
(In U.S. dollars, except share and per share data)
17. CONDENSED FINANCIAL INFORMATION OF THE PARENT COMPANY (cont.)
PARENT COMPANY STATEMENTS OF CASH FLOWS
| | For the Years Ended
September 30, |
| | | 2023 | | 2022 |
Cash flows from operating activities | | $ | — | | $ | — |
Cash flows from investing activities | | | — | | | — |
Cash flows from financing activities | | | — | | | — |
Effect of exchange rate changes | | | — | | | — |
Net increase in cash, cash equivalents and restricted cash | | | — | | | — |
Cash, cash equivalents and restricted cash, at the beginning of the year | | | — | | | — |
Cash, cash equivalents and restricted cash, at the end of the year | | $ | — | | $ | — |
18. SUBSEQUENT EVENTS
The Group has evaluated subsequent events from September 30, 2023 and through the date of issuance of the consolidated financial statements which is February 9, 2024, and did not identify any subsequent events with material financial impact or that required adjustment of the Group’s consolidated financial statements.
F-29
Table of Contents

REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To: | | The Board of Directors and Shareholders of
Work Medical Technology Group Ltd |
Results of Review Interim Financial Information
We have reviewed the accompanying condensed consolidated balance sheets of Work Medical Technology Group Ltd and its subsidiaries (collectively the “Company”) as of March 31, 2024, and the related condensed consolidated statements of income and comprehensive income, changes in equity, and cash flows for the six-month periods ended March 31, 2024 and 2023, and the related notes (collectively referred to as the interim financial statements). Based on our reviews, we are not aware of any material modifications that should be made to the accompanying interim financial statements for them to be in conformity with accounting principles generally accepted in the United States of America.
We have previously audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the consolidated balance sheets of the Company as of September 30, 2023 and 2022, and the related statements of income and comprehensive income, changes in equity and cash flows in each of the years for the two-year period ended September 30, 2023; and in our report dated February 9, 2024, we expressed an unqualified opinion on those financial statements. In our opinion, the information set forth in the accompanying condensed consolidated balance sheet as of September 30, 2023, is fairly stated, in all material respects, in relation to the balance sheet from which it has been derived.
Basis for Review Results
These interim financial statements are the responsibility of the Company’s management. We conducted our review in accordance with the standards of the PCAOB. A review of interim financial information consists principally of applying analytical procedures and making inquiries of persons responsible for financial and accounting matters. It is substantially less in scope than an audit conducted in accordance with standards of the PCAOB, the objective of which is the expression of an opinion regarding the financial statements taken as a whole. Accordingly, we do not express such an opinion. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
/s/ WWC, P.C.
WWC, P.C.
Certified Public Accountants
PCAOB ID No.1171
We have served as the Company’s auditor since 2021.
San Mateo, California
July 5, 2024 except for the subsequent events discussed in Note 19 as to which the date is December 20, 2024.

F-30
Table of Contents
WORK MEDICAL TECHNOLOGY GROUP LTD
UNAUDITED CONDENSED CONCONSOLIDATED BALANCE SHEETS
(In U.S. dollars, except for otherwise noted)
| | As of
March 31,
2024 | | As of
September 30,
2023 |
| | | (unaudited) | | |
Assets | | | | | | | | |
Current assets: | | | | | | | | |
Cash and cash equivalents | | $ | 1,039,020 | | | $ | 1,596,096 | |
Restricted cash | | | 41,619 | | | | 41,187 | |
Accounts receivable, net | | | 1,247,640 | | | | 3,332,322 | |
Inventories, net | | | 4,339,283 | | | | 4,620,191 | |
Amounts due from related parties | | | 468,263 | | | | 4,237,682 | |
Advance to suppliers | | | 7,335,849 | | | | 3,469,819 | |
Prepaid expenses and other current assets | | | 4,977,351 | | | | 4,855,381 | |
Total current assets | | | 19,449,025 | | | | 22,152,678 | |
| | | | | | | | | |
Non-current assets: | | | | | | | | |
Property, plant and equipment, net | | | 6,063,787 | | | | 6,626,774 | |
Intangible assets, net | | | 979,684 | | | | 998,450 | |
Right-of-use assets, net | | | 89,747 | | | | 114,127 | |
Deferred tax assets | | | 95,304 | | | | 66,872 | |
Other non-current asset | | | 5,816,933 | | | | — | |
Total non-current assets | | | 13,045,455 | | | | 7,806,223 | |
TOTAL ASSETS | | $ | 32,494,480 | | | $ | 29,958,901 | |
| | | | | | | | | |
Liabilities | | | | | | | | |
Current liabilities: | | | | | | | | |
Short-term bank borrowings | | $ | 14,057,588 | | | $ | 8,840,461 | |
Accounts payable | | | 2,643,488 | | | | 3,961,827 | |
Deferred revenue | | | 703,209 | | | | 776,210 | |
Amount due to related parties | | | 161,635 | | | | 159,612 | |
Accrued expenses and other liabilities | | | 2,742,375 | | | | 4,398,596 | |
Lease liabilities, current | | | 59,277 | | | | 49,560 | |
Income tax payable | | | 791,412 | | | | 736,590 | |
Total current liabilities | | | 21,158,984 | | | | 18,922,856 | |
Lease liabilities, non-current | | $ | — | | | $ | 50,130 | |
Total non-current liabilities | | | — | | | | 50,130 | |
TOTAL LIABILITIES | | $ | 21,158,984 | | | $ | 18,972,986 | |
| | | | | | | | | |
Shareholders’ equity | | | | | | | | |
Ordinary Shares (par value of $0.0005 per share; 100,000,000 shares authorized as of March 31, 2024 and September 30, 2023; 12,500,000 shares issued and outstanding as of March 31, 2024 and September 30, 2023, respectively)* | | $ | 6,250 | | | $ | 6,250 | |
Subscription receivable | | | (6,250 | ) | | | (6,250 | ) |
Additional paid-in capital | | | 85,012 | | | | 85,012 | |
Statutory reserve | | | 887,482 | | | | 887,482 | |
Retained earnings | | | 9,807,110 | | | | 9,580,585 | |
Accumulated other comprehensive loss | | | (565,263 | ) | | | (676,828 | ) |
Total shareholders’ equity | | | 10,214,341 | | | | 9,876,251 | |
Non-controlling interests | | | 1,121,155 | | | | 1,109,664 | |
Total equity | | | 11,335,496 | | | | 10,985,915 | |
TOTAL LIABILITIES AND EQUITY | | $ | 32,494,480 | | | $ | 29,958,901 | |
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
F-31
Table of Contents
WORK MEDICAL TECHNOLOGY GROUP LTD
UNAUDITED CONDENSED CONSOLIDATED STATEMENTS OF INCOME AND
COMPREHENSIVE INCOME
(In U.S. dollars, except for otherwise noted)
| | For the six months ended
March 31, |
| | | 2024 | | 2023 |
Net revenue from third parties | | $ | 5,075,986 | | | $ | 8,523,938 | |
Net revenue from related party | | | 233,109 | | | | 487,466 | |
Cost of revenue | | | (3,766,116 | ) | | | (5,111,338 | ) |
Gross profit | | | 1,542,979 | | | | 3,900,066 | |
| | | | | | | | | |
Operating expenses: | | | | | | | | |
Selling expenses | | | (1,094,833 | ) | | | (619,763 | ) |
General and administrative expenses | | | (777,056 | ) | | | (1,116,964 | ) |
Research and development expenses | | | (178,783 | ) | | | (372,276 | ) |
Total operating expenses | | | (2,050,672 | ) | | | (2,109,003 | ) |
| | | | | | | | | |
Other income: | | | | | | | | |
Interest expense | | | (252,485 | ) | | | (146,079 | ) |
Government subsidies | | | 345,245 | | | | — | |
Other income, net | | | 669,837 | | | | 9,052 | |
Total other income (loss) | | | 762,597 | | | | (137,027 | ) |
| | | | | | | | | |
Income before income tax expense | | | 254,904 | | | | 1,654,036 | |
Income tax expense | | | (20,050 | ) | | | (220,671 | ) |
Net income | | $ | 234,854 | | | $ | 1,433,365 | |
| | | | | | | | | |
Net income (loss) attributable to non-controlling interest | | | 8,329 | | | | (42,369 | ) |
Net income attributable to ordinary shareholders | | $ | 226,525 | | | $ | 1,475,734 | |
Other comprehensive income: | | | | | | | | |
Foreign currency translation adjustment | | | 114,727 | | | | 423,333 | |
Total comprehensive income | | | 349,581 | | | | 1,856,698 | |
| | | | | | | | | |
Total comprehensive income (loss) attributable to non-controlling interests | | | 11,491 | | | | (19,630 | ) |
Total comprehensive income attributable to shareholders | | $ | 338,090 | | | $ | 1,876,328 | |
| | | | | | | | | |
Weighted average number of Ordinary Shares – basic and diluted* | | | 12,500,000 | | | | 12,500,000 | |
| | | | | | | | | |
Basic and diluted earnings per ordinary share | | $ | 0.02 | | | $ | 0.12 | |
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
F-32
Table of Contents
WORK MEDICAL TECHNOLOGY GROUP LTD
UNAUDITED CONDENSED CONSOLIDATED STATEMENTS OF CHANGES IN EQUITY
(In U.S. dollars, except for share and per share data, or otherwise noted)
| |
Ordinary Shares*
| | Subscription
receivable | | Additional
paid-in
capital | | Statutory
reserves | | Retained
earnings | | Accumulated
other
comprehensive
income (loss) | | Total
shareholder’s
equity | | Non-
controlling
interest | | Total
equity |
| | | Share | | Amount | |
Balance as of September 30, 2022 | | 12,500,000 | | $ | 6,250 | | $ | (6,250 | ) | | $ | 85,012 | | $ | 850,690 | | $ | 9,505,347 | | $ | (440,278 | ) | | $ | 10,000,771 | | $ | 1,189,829 | | | $ | 11,190,600 |
Net income | | — | | | — | | | — | | | | — | | | — | | | 1,475,734 | | | — | | | | 1,475,734 | | | (42,369 | ) | | | 1,433,365 |
Foreign currency translation adjustment | | — | | | — | | | — | | | | — | | | — | | | — | | | 400,594 | | | | 400,594 | | | 22,739 | | | | 423,333 |
Balance as of March 31, 2023 | | 12,500,000 | | $ | 6,250 | | $ | (6,250 | ) | | $ | 85,012 | | $ | 850,690 | | $ | 10,981,081 | | $ | (39,684 | ) | | $ | 11,877,099 | | $ | 1,170,199 | | | $ | 13,047,298 |
| |
Ordinary Shares*
| | Subscription
receivable | | Additional
paid-in
capital | | Statutory
reserves | | Retained
earnings | | Accumulated
other
comprehensive
income (loss) | | Total
shareholder’s
equity | | Non-
controlling
interest | | Total
equity |
| | | Share | | Amount | |
Balance as of September 30, 2023 | | 12,500,000 | | $ | 6,250 | | $ | (6,250 | ) | | $ | 85,012 | | $ | 887,482 | | $ | 9,580,585 | | $ | (676,828 | ) | | $ | 9,876,251 | | $ | 1,109,664 | | $ | 10,985,915 |
Net income | | — | | | — | | | — | | | | — | | | — | | | 226,525 | | | — | | | | 226,525 | | | 8,329 | | | 234,854 |
Foreign currency translation adjustment | | — | | | — | | | — | | | | — | | | — | | | — | | | 111,565 | | | | 111,565 | | | 3,162 | | | 114,727 |
Balance as of March 31, 2024 | | 12,500,000 | | $ | 6,250 | | $ | (6,250 | ) | | $ | 85,012 | | $ | 887,482 | | $ | 9,807,110 | | $ | (565,263 | ) | | $ | 10,214,341 | | $ | 1,121,155 | | $ | 11,335,496 |
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
F-33
Table of Contents
WORK MEDICAL TECHNOLOGY GROUP LTD
UNAUDITED CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
(In U.S. dollars, except for share and per share data, or otherwise noted)
| | For the six months ended
March 31, |
| | | 2024 | | 2023 |
CASH FLOWS FROM OPERATING ACTIVITIES: | | | | | | | | |
Net cash (used in) provided by operating activities | | | (1,264,684 | ) | | | 719,455 | |
| | | | | | | | | |
CASH FLOWS FROM INVESTING ACTIVITIES: | | | | | | | | |
Purchase of intangible asset | | | — | | | | (137,369 | ) |
Purchase of property, plant and equipment | | | (28,783 | ) | | | (538,628 | ) |
Disposal of property and equipment | | | — | | | | 153,510 | |
Purchase of other non-current asset | | | (5,828,153 | ) | | | — | |
Net cash used in investing activities | | | (5,856,936 | ) | | | (522,487 | ) |
| | | | | | | | | |
CASH FLOWS FROM FINANCING ACTIVITIES: | | | | | | | | |
Proceeds from short-term borrowings | | | 6,244,449 | | | | 650,758 | |
Repayments of short-term borrowings | | | (1,110,124 | ) | | | — | |
Loan to related parties | | | (2,985,963 | ) | | | (2,961,141 | ) |
Repayment from related parties | | | 5,773,020 | | | | 2,616,075 | |
Interest-free loans from related parties | | | 425,669 | | | | — | |
Repayment of interest-free loans to related parties | | | (425,318 | ) | | | — | |
Interests-free loans to third parties | | | (624,647 | ) | | | (392,800 | ) |
Repayment from interest-free loans to third party | | | 466,699 | | | | — | |
Payment of offering cost | | | (1,256,961 | ) | | | — | |
Interest-bearing loan to third party | | | (2,636,545 | ) | | | — | |
Repayment from interest-bearing loan to third party | | | 2,686,425 | | | | — | |
Net cash provided by (used in) financing activities | | | 6,556,704 | | | | (87,108 | ) |
| | | | | | | | | |
Effect of exchange rate changes | | | 8,272 | | | | (44,829 | ) |
| | | | | | | | | |
Net (decrease)/increase in cash and cash equivalents and restricted cash | | | (556,644 | ) | | | 65,031 | |
Cash, cash equivalents and restricted cash, at beginning of year | | | 1,637,283 | | | | 773,422 | |
Cash, cash equivalents and restricted cash, at end of year | | $ | 1,080,639 | | | $ | 838,453 | |
| | | | | | | | | |
SUPPLEMENTAL DISCLOSURE OF CASH FLOW INFORMATION: | | | | | | | | |
Income taxes paid | | | 624 | | | | — | |
Interest paid | | | 253,691 | | | | 141,440 | |
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
F-34
Table of Contents
WORK MEDICAL TECHNOLOGY GROUP LTD
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(In U.S. dollars, except share and per share data)
1. ORGANIZATION AND PRINCIPAL ACTIVITIES
WORK Medical Technology Group LTD (the “Company”, “Work Cayman” or “WORK”) was incorporated under the law of the Cayman Islands on March 1, 2022 as an exempted company with limited liability. The Company, together with its subsidiaries, is engaged in manufacturing and selling medical consumables through its wholly owned subsidiaries in the People’s Republic of China (the “PRC” or “China”).
History of the Group and Reorganization
The Company conducts its operations through its PRC subsidiary Hangzhou Shanyou Medical Equipment Co., Ltd. (“Hangzhou Shanyou”) and its subsidiaries, since 2002.
In preparation for its initial public offering, the Group completed a reorganization on May 6, 2022 (the “Reorganization”), which involved the following steps:
• on November 10, 2021, Work (Hangzhou) Medical Treatment Technology Co., Ltd. (“Work Hangzhou”) was established by Baiming Yu and his spouse, Liwei Zhang, who were the ultimate shareholders of Work Hangzhou;
• on January 17, 2022, Hangzhou Shanyou newly issued 95% of equity interest to Work Hangzhou. The remaining shareholders of Hangzhou Shanyou are Baiming Yu, with 3.35% of equity interest, and Liwei Zhang, with 1.65% of equity interest;
• on March 1, 2022, Work Cayman was incorporated and (indirectly) issued Ordinary Shares at par value $1.00 per share to certain founding shareholders. Baiming Yu (“LWY GROUP LTD”) and Liwei Zhang (“ZLW GROUP LTD”), who, following transfer of the initial one subscriber share from Tricor Services (Cayman Islands) Limited to LWY GROUP LTD, indirectly held a 50% and 5% equity interest of Work Cayman, respectively. Certain third parties, as strategic investors, acquired 45% equity shares of the PRC subsidiaries at fair value from Baiming Yu and Liwei Zhang. In exchange, Work Cayman issued the remainder of its 45% Ordinary Shares at par value $1.00 per share to these strategic investors on the day of its incorporation.
• on March 15, 2022, Work Medical Technology Group Limited (“Work BVI”) was incorporated in the British Virgin Islands as a wholly owned subsidiary of the Company;
• on April 19, 2022, Work Medical Technology Group (China) Limited (“Work Medical Technology” or “Work HK”) was incorporated in Hong Kong as a wholly owned subsidiary of Work BVI;
• on April 28, 2022, Work Age (Hangzhou) Medical Treatment Technology Co., Ltd. (“WFOE” or “Work Age”) was established as a wholly owned subsidiary of Work HK in the PRC; and
• on May 6, 2022, WFOE acquired 100% equity interest of Work Hangzhou.
On February 21, 2022, Hangzhou Shanyou entered into a share purchase agreement to purchase 60% equity shares of Hangzhou Hanshi Medical Equipment Co., Ltd. (“Hangzhou Hanshi”) from Baiming Yu. Since both Hangzhou Shanyou and Hangzhou Hanshi are under the common control immediately before and after the merger, this transaction was accounted for as a common control merger, and using merger accounting as if the Reorganization had been consummated at the beginning of the earliest period presented, and no gain or loss is recognized. All the assets and liabilities of Hangzhou Hanshi are recorded at carrying value.
Immediately before and after share issuances and transfer of Work Cayman, Work Hangzhou acquired Hangzhou Shanyou, and WFOE acquired Work Hangzhou. The ultimate shareholders in these entities, who are Baiming Yu and his spouse, Liwei Zhang, did not change. Accordingly, the Reorganization has been treated as a corporate restructuring of entities under common control. Thus, the current capital structure has been retroactively presented in prior periods as if such structure existed at that time, and the entities are presented on a combined basis for all periods to which such entities were under common control. Thus, the results of these subsidiaries are included in the financial statements
F-35
Table of Contents
WORK MEDICAL TECHNOLOGY GROUP LTD
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(In U.S. dollars, except share and per share data)
1. ORGANIZATION AND PRINCIPAL ACTIVITIES (cont.)
for the year ended September 30, 2023 and six months ended March 31, 2024. The discussion and presentation of financial statements herein assumes the completion of the Reorganization, which is accounted for retroactively as if it occurred on October 1, 2019, and the equity has been restated to reflect the change, as well.
The unaudited condensed consolidated financial statements reflect the activities of the Company and each of the following entities:
Name | | Date of
Incorporation | | Place of
incorporation | | Percentage of
effective ownership | | Principal
Activities |
Subsidiaries | | | | | | | | |
Work BVI | | March 15, 2022 | | BVI | | 100% | | Investment holding |
Work Medical Technology | | April 19, 2022 | | Hong Kong | | 100% | | Investment holding |
WFOE | | April 28, 2022 | | PRC | | 100% | | Investment holding |
Work Hangzhou | | November 10, 2021 | | PRC | | 100% | | Investment holding |
Hangzhou Shanyou | | April 29, 2002 | | PRC | | 95% | | Produce and sale of medical consumables |
Hangzhou Hanshi | | July 22, 2019 | | PRC | | 60% owned by Hangzhou Shanyou | | Sale of medical consumables |
Shanghai Saitumofei Medical Treatment Technology Co., Ltd. (“Shanghai Saitumofei”) | | May 15, 2019 | | PRC | | 51% | | Sale of medical consumable |
Hunan Saitumofei Medical Treatment Technology Co., Ltd (“Hunan Saitumofei”) | | April 02,2022 | | PRC | | 51% | | Sale of medical consumables |
Hangzhou Woli Medical Treatment Technology Co., Ltd (“Hangzhou Woli”) | | July 22,2022 | | PRC | | 100% | | Sale of medical consumables |
Shanghai Chuqiang Medical Equipment Co., Ltd. (“Shanghai Chuqiang”) | | March 12, 2018 | | PRC | | 100% owned by Hangzhou Shanyou | | Sale of medical consumables |
Hangzhou Youshunhe Technology Co., Ltd. (“Hangzhou Youshunhe”) | | February 27, 2023 | | PRC | | 51% owned by Hangzhou Shanyou | | Sale of medical consumables |
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
(a) Basis of presentation and principles of consolidation
The accompanying unaudited condensed consolidated financial statements are prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”) and pursuant to the rules and regulations of the Securities and Exchange Commission (the “SEC”). The accompanying unaudited condensed consolidated financial statements include the financial statements of the Company and its subsidiaries. All inter-company balances and transactions are eliminated upon consolidation. For consolidated subsidiaries where the Group’s ownership in the subsidiary is less than 100%, the equity interest not held by the Group is shown as noncontrolling interests.
(b) Accounts receivable, net
Accounts receivable are stated at the original amount less an allowance for doubtful receivable.
The Group reviews the accounts receivable on a periodic basis and makes general and specific allowances when there is doubt as to the collectability of individual balances. The Group considers factors in assessing the collectability of its receivables, such as historical bad debts, changes in customers’ payment patterns,
F-36
Table of Contents
WORK MEDICAL TECHNOLOGY GROUP LTD
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(In U.S. dollars, except share and per share data)
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.)
credit-worthiness and financial condition of the customers, current economic trends and other specific circumstances related to the accounts. An allowance for doubtful accounts is recorded in the period in which a loss is determined to be probable. Accounts receivable balances are written off after all collection efforts have been exhausted.
(c) Advance to suppliers
Advances to suppliers refer to advances for the purchase of materials or provision of services, which are applied against accounts payable when the materials or services are received. The Group reviews a supplier’s credit history and background information before advancing a payment. If the financial condition of its suppliers were to deteriorate, resulting in an impairment of their ability to deliver goods or provide services, the Group would provide allowance for such amount in the period when it is considered impaired. Advances to suppliers as of March 31, 2024 and September 30, 2023 consisted of prepayments to suppliers relating to masks (including the supplies to produce masks), medical devices other than masks (including the supplies to produce medical devices other than masks), medical consumables, and other services.
(d) Revenue recognition
On October 1, 2019, the Group adopted Accounting Standards Codification (“ASC”) 606 using the modified retrospective approach.
The core principle of the new revenue standard is that a company should recognize revenue to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which the company expects to be entitled in exchange for those goods or services. The following five steps are applied to achieve that core principle:
Step 1: Identify the contract with the customer
Step 2: Identify the performance obligations in the contract
Step 3: Determine the transaction price
Step 4: Allocate the transaction price to the performance obligations in the contract
Step 5: Recognize revenue when the company satisfies a performance obligation
These criteria as they relate to each of the following major revenue generating activities are described below. Revenue is presented net of value added taxes (“VAT”) and surcharges. VAT tax to be collected from customers, net of VAT paid for purchases, is recorded as a liability in the unaudited condensed consolidated balance sheets until it is paid to the tax authorities.
Revenue from sales of self-manufactured masks and medical devices other than masks
The Group sells products to different distributor customers and direct-end user customers, primarily of sales of self-manufactured masks and medical devices other than masks. The Group does not accept returns of products or offer refunds for its customers after the expiration of quality objection periods. The Group usually offers a seven-day quality objection period and any quality deficiencies are determined by the testing of a third-party institution.
The Group grants credit sales for customers and the credit period varies among different customers. The Group normally grants the customers 30 to 180 days to complete their payments after the credit sales, and the length of the credit period is dependent on the customer’s creditworthiness and its transaction experience with the Group. The Group makes its judgment taking all of the facts and circumstances into account, including the PRC subsidiaries’ customary business practices and the knowledge of the customer, in determining whether it is probable that the PRC subsidiaries will collect substantially all of the consideration to which they will be entitled in exchange for the goods or services that the PRC subsidiaries expect to transfer to the customer.
F-37
Table of Contents
WORK MEDICAL TECHNOLOGY GROUP LTD
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(In U.S. dollars, except share and per share data)
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.)
Control of a product is transferred to a distributor customer or direct-end user customer upon delivery of the product to the designated place. The revenue is recognized at a point in time when the Group satisfies the performance obligation by transferring the promised product to distributor customers or direct-end user customers upon acceptance by them. The Group presents the revenue generated from its sales of products on a gross basis as the Group acts as a principal.
Revenue from commodity trading
Revenue from commodity trading is recognized on a net basis or gross basis based on whether the Group arranges the provision of products through third parties and control the specified products provided by the third parties before that products are transferred to the customers. The revenue is recognized at a point in time when the Group satisfies performance obligations by arranging the transfer of a promised product to a customer. When the Group acted as an agent, the revenue is measured at fixed consideration which is determined as the difference between the sales price that the Group expects to receive in exchange for arranging promised products to the customer and the settlement price with the third-party suppliers. When the Group acted as a principal, the revenue is measured at fixed consideration which is the sales price that the Group expects to receive in exchange for arranging promised products.
Shipping and handling activities are considered to be fulfilment activities rather than promised services and are not, therefore, considered to be separate performance obligations. The Group’s sales terms provide no right of return outside of a standard quality policy and returns are generally not significant.
Geographic information
The following table disaggregates the Group’s revenue by geographic market for the six months ended March 31, 2024 and 2023:
| | For the six months ended
March 31, |
| | | 2024 | | 2023 |
China domestic market | | $ | 4,630,186 | | $ | 8,818,866 |
Overseas market | | | 678,909 | | | 192,538 |
Net revenue | | $ | 5,309,095 | | $ | 9,011,404 |
Revenue by product categories
The following table disaggregates the Group’s revenue product categories for the six months ended March 31, 2024 and 2023:
| | For the six months ended
March 31, |
| | | 2024 | | 2023 |
Masks | | $ | 566,549 | | $ | 4,752,892 |
Medical devices other than masks(1) | | | 4,564,468 | | | 3,940,257 |
Commodity trading(2) | | | 178,078 | | | 257,530 |
Others | | | — | | | 60,725 |
Net revenue | | $ | 5,309,095 | | $ | 9,011,404 |
Contract balances
Payment terms are established on the Group’s pre-established credit requirements based upon an evaluation of customers’ credit. Contract assets are recognized for in related accounts receivable.
F-38
Table of Contents
WORK MEDICAL TECHNOLOGY GROUP LTD
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(In U.S. dollars, except share and per share data)
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.)
The contract liabilities consist of deferred revenue, which represents the billings or cash received for services in advance of revenue recognition and is recognized as revenue when all the Group’s revenue recognition criteria are met. The Group’s deferred revenue was $703,209 and $776,210 as of March 31, 2024 and September 30, 2023, respectively.
Other than accounts receivable and deferred revenue, the Group had no other material contract assets, contract liabilities or deferred contract costs recorded on its unaudited condensed consolidated balance sheets as of March 31, 2024 and September 30, 2023.
Contract costs
For the years ended March 31, 2024 and 2023, the Group did not have any significant incremental costs of obtaining contracts with customers incurred and/or costs incurred in fulfilling contracts with customers within the scope of ASC Topic 606, that shall be recognized as an asset and amortized to expenses in a pattern that matches the timing of the revenue recognition of the related contract.
(e) Recent accounting pronouncements
The Company is an “emerging growth company” (“EGC”) as defined in the Jumpstart Our Business Startups Act of 2012 (the “JOBS Act”). Under the JOBS Act, EGC can delay adopting new or revised accounting standards issued subsequent to the enactment of the JOBS Act until such time as those standards apply to private companies.
Recent accounting standards that have been issued by FASB that do not require adoption until a future date are not expected to have a material impact on the consolidated financial statements upon adoption. The Group does not discuss recent standards that are not anticipated to have an impact on or are unrelated to its consolidated financial position, results of operations, cash flows or disclosures.
3. ACCOUNTS RECEIVABLE, NET
Accounts receivable, net consisted of the following:
| | As of
March 31,
2024 | | As of
September 30,
2023 |
Accounts receivable | | $ | 2,185,406 | | | $ | 4,343,923 | |
Allowance for doubtful accounts | | | (937,766 | ) | | | (1,011,601 | ) |
Accounts receivable, net | | $ | 1,247,640 | | | $ | 3,332,322 | |
Bad debt expense of $81,616 and $264,348 was recorded for the six months ended March 31, 2024 and 2023, respectively. The Group recorded reversal of bad debt expense of $166.220 and nil for the six months ended March 31, 2024 and 2023, respectively.
| | As of
March 31,
2024 | | As of
September 30,
2023 |
Balance at the beginning of the year | | $ | 1,011,601 | | | $ | 1,344,480 | |
Current period addition | | | 81,616 | | | | 136,228 | |
Write-off | | | — | | | | (309,656 | ) |
Reversal | | | (166,220 | ) | | | (134,846 | ) |
Foreign currency translation adjustment | | | 10,769 | | | | (24,605 | ) |
Balance at the end of the year | | $ | 937,766 | | | $ | 1,011,601 | |
F-39
Table of Contents
WORK MEDICAL TECHNOLOGY GROUP LTD
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(In U.S. dollars, except share and per share data)
4. INVENTORIES, NET
Inventories consisted of the following:
| | As of
March 31,
2024 | | As of
September 30,
2023 |
Raw materials | | $ | 637,814 | | $ | 741,988 |
Work in progress | | | 1,878,537 | | | 2,030,108 |
Finished goods | | | 1,912,857 | | | 2,089,383 |
Less: impairment | | | 89,925 | | | 241,288 |
Inventories, net | | $ | 4,339,283 | | $ | 4,620,191 |
Impairment provided for the inventories were nil for the six months ended March 31, 2024 and 2023.
| | As of
March 31,
2024 | | As of
September 30,
2023 |
Balance at the beginning of the year | | $ | 241,288 | | | $ | 229,239 | |
Current period addition | | | — | | | | 18,320 | |
Reduction | | | (154,188 | ) | | | — | |
Foreign currency translation adjustment | | | 2,825 | | | | (6,271 | ) |
Balance at the end of the year | | $ | 89,925 | | | $ | 241,288 | |
5. PREPAID EXPENSES AND OTHER CURRENT ASSETS
Prepaid expenses and other current assets consisted of the following:
| | As of
March 31,
2024 | | As of
September 30,
2023 |
Interest-bearing loans to a third party(1) | | $ | 2,581,686 | | | $ | 2,604,167 | |
Deferred offering costs | | | 1,960,143 | | | | 1,356,176 | |
Interest-free loans to third parties(2) | | | 282,988 | | | | 550,706 | |
Receivable from third party | | | 227,981 | | | | 613,580 | |
Prepaid expenses | | | 11,912 | | | | 24,152 | |
Others | | | 269,277 | | | | 59,535 | |
Allowance for doubtful accounts | | | (356,636 | ) | | | (352,935 | ) |
Total prepaid expenses and other current assets | | $ | 4,977,351 | | | $ | 4,855,381 | |
F-40
Table of Contents
WORK MEDICAL TECHNOLOGY GROUP LTD
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(In U.S. dollars, except share and per share data)
6. PROPERTY, PLANT AND EQUIPMENT, NET
Property, plant and equipment, net, consisted of the following:
| | As of
March 31,
2024 | | As of
September 30,
2023 |
Property and buildings | | $ | 5,464,133 | | $ | 5,397,008 |
Machinery and equipment | | | 12,220,786 | | | 12,084,239 |
Vehicle | | | 157,756 | | | 156,119 |
Office and electric equipment | | | 146,823 | | | 137,050 |
Subtotal | | | 17,989,498 | | | 17,774,416 |
Less: impairment | | | 2,676,718 | | | 2,648,945 |
Less: accumulated depreciation | | | 9,248,993 | | | 8,498,697 |
Property, plant and equipment, net | | $ | 6,063,787 | | $ | 6,626,774 |
Depreciation expenses were $676,818 and $780,505 for the six months ended March 31, 2024 and 2023, respectively.
The Group did not record any impairment charge for the six months ended March 31, 2024 and 2023.
Written-off of impairment due to disposal of related assets were nil and $270,583 for the six months ended March 31, 2024 and 2023, respectively.
7. INTANGIBLE ASSETS, NET
Intangible assets consisted of the following:
| | As of
March 31,
2024 | | As of
September 30,
2023 |
Land use rights | | $ | 988,799 | | $ | 978,539 |
Others | | | 280,941 | | | 277,778 |
Subtotal | | | 1,269,740 | | | 1,256,317 |
Less: accumulated amortization | | | 290,056 | | | 257,867 |
Intangible assets, net | | $ | 979,684 | | $ | 998,450 |
Amortization expenses were $31,481 and $27,225 for the six months ended March 31, 2024 and 2023, respectively.
The following table presents future amortization as of March 31, 2024:
| | Amount |
For the remaining of the year ended September 30, 2024 | | $ | 31,420 |
2025 | | | 62,840 |
2026 | | | 62,840 |
2027 | | | 48,361 |
2028 | | | 39,270 |
Thereafter | | | 734,953 |
| | | $ | 979,684 |
8. OTHER NON-CURRENT ASSET
On December 5, 2023, the Group entered into a contract to purchase a building located in Hangzhou from a third party for use as the Group’s future operating center, exhibition hall and warehouse. Pursuant to the contract, the total areas of the building are about 4 thousand square meter. The building is expected to be ready for use within December 31, 2024. The Group had prepaid RMB42 million (approximately USD5.8 million) and recorded as other non-current asset as of March 31, 2024.
F-41
Table of Contents
WORK MEDICAL TECHNOLOGY GROUP LTD
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(In U.S. dollars, except share and per share data)
9. ACCRUED EXPENSES AND OTHER LIABILITIES
Accrued expenses and other liabilities consisted of the following:
| | As of
March 31,
2024 | | As of
September 30,
2023 |
VAT payable | | $ | 1,504,445 | | $ | 1,388,754 |
Payroll payable | | | 531,240 | | | 625,802 |
Other payable | | | 706,690 | | | 2,384,040 |
Total accrued expenses and other liabilities | | $ | 2,742,375 | | $ | 4,398,596 |
10. LEASES
The Group has one operating lease for its office space.
Operating lease right-of-use assets and liabilities are recognized at commencement date based on the present value of lease payments over the lease term. The discount rate used to calculate present value is incremental borrowing rate or, if available, the rate which is implicit in the lease. The Group determines the incremental borrowing rate for each lease based primarily on its lease term in PRC, which is approximately 4.75%.
Operating lease expenses were $43,286 and $12,906 for the six months ended March 31, 2024 and 2023, respectively.
The undiscounted future minimum lease payment schedule as follows:
For the year ended September 30 | | Lease
Payment |
2025 | | 60,939 |
Total lease payments | | 60,939 |
Interest | | 1,662 |
Total | | 59,277 |
11. TAXATION
Cayman Islands
The Company is incorporated in the Cayman Islands. Under the current laws of the Cayman Islands, the Company is not subject to income or capital gains taxes. In addition, dividend payments are not subject to withholdings tax in the Cayman Islands.
Hong Kong
According to Tax (Amendment) (No. 3) Ordinance 2018 published by Hong Kong government, since September 1, 2018, under the two-tiered profits tax rate regime, the profits tax rate for the first HKD2 million of assessable profits has been lowered to 8.25% (half of the rate specified in Schedule 8 to the Inland Revenue Ordinance (IRO)) for corporations. Work HK was not subject to Hong Kong profit tax for any period presented as it did not have assessable profit during the periods presented.
PRC
The Group’s subsidiaries in mainland China are all subject to PRC Enterprise Income Tax (“EIT”) on the taxable income in accordance with the relevant PRC income tax laws and regulations. The EIT rate for the Group’s operation in mainland China was 25% with the following exceptions.
F-42
Table of Contents
WORK MEDICAL TECHNOLOGY GROUP LTD
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(In U.S. dollars, except share and per share data)
11. TAXATION (cont.)
Hangzhou Shanyou was qualified as a “high and new technology enterprise strongly supported by the State” in November 2017 and renewed in December 2023, and entitled to an EIT rate of 15%, expiring on December 8, 2026.
The income tax provision consisted of the following components:
| | For the six months ended
March 31, |
| | | 2024 | | 2023 |
Current income tax expenses | | $ | 47,834 | | | $ | 267,157 | |
Deferred income tax benefit | | | (27,784 | ) | | | (46,486 | ) |
Total income tax expense | | $ | 20,050 | | | $ | 220,671 | |
A reconciliation between the Group’s actual provision for income taxes and the provision at the PRC, mainland statutory rate is as follows:
| | For the six months ended
March 31, |
| | | 2024 | | 2023 |
Income before income taxes | | $ | 254,904 | | | $ | 1,654,036 | |
Income tax expense computed at statutory EIT rate of 25% | | | 63,725 | | | | 413,509 | |
Reconciling items: | | | | | | | | |
Effect of preferred tax rate | | | (30,817 | ) | | | (153,376 | ) |
Additional deduction for R&D expenses | | | (26,817 | ) | | | (55,841 | ) |
Change in valuation allowance | | | 3,357 | | | | 16,367 | |
Tax effect of non-deductible items | | | 10,602 | | | | 12 | |
Income tax expense | | $ | 20,050 | | | $ | 220,671 | |
Effective tax rates | | | 7.9 | % | | | 13.3 | % |
Deferred tax assets/liability
As of March 31, 2024 and September 30, 2023, the significant components of the deferred tax assets and deferred tax liabilities are summarized below:
| | As of
March 31,
2024 | | As of
September 30,
2023 |
Deferred tax assets: | | | | | | | | |
Allowance for doubtful accounts | | $ | 505,804 | | | $ | 510,542 | |
Inventories write-down | | | 13,489 | | | | 36,193 | |
Impairment of equipment | | | 34,123 | | | | 33,768 | |
Net operating loss carry forwards | | | 4,937 | | | | 52,359 | |
Deferred tax assets, gross | | $ | 558,353 | | | $ | 632,862 | |
Less: valuation allowance | | | (4,937 | ) | | | (59,879 | ) |
Deferred tax assets, net | | | 553,416 | | | | 572,983 | |
Deferred tax liability: | | | | | | | | |
Depreciation* | | | 458,112 | | | | 506,111 | |
Deferred tax liability, net | | | 458,112 | | | | 506,111 | |
Net deferred tax asset: | | $ | 95,304 | | | $ | 66,872 | |
F-43
Table of Contents
WORK MEDICAL TECHNOLOGY GROUP LTD
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(In U.S. dollars, except share and per share data)
11. TAXATION (cont.)
Uncertain tax positions
The Group evaluates each uncertain tax position (including the potential application of interest and penalties) based on the technical merits, and measure the unrecognized benefits associated with the tax positions. As of March 31, 2024 and September 30, 2023, the Group did not have any significant unrecognized uncertain tax positions.
12. BORROWINGS
As of March 31, 2024 and September 30, 2023, summary of the borrowings is as following:
| | Annual
Interest Rate | | Maturity
date | | March 31,
2024 | | September 30,
2023 |
Bank of Jiangsu | | 4.05% | | September 2024 | | $ | 415,495 | | $ | 411,184 |
Subtotal | | | | | | $ | 415,495 | | $ | 411,184 |
Xiaoshan Rural Commercial Bank | | 4.40% – 4.70% | | August 2024 | | $ | 3,808,706 | | $ | 4,454,496 |
| | | 4.35% – 4.50% | | August 2024 | | | 1,384,984 | | | 1,370,614 |
Subtotal | | | | | | $ | 5,193,690 | | $ | 5,825,110 |
Bank of Beijing | | 3.55% | | June 2024 | | $ | 1,384,984 | | $ | 1,370,614 |
Subtotal | | | | | | $ | 1,384,984 | | $ | 1,370,614 |
China Citic Bank | | 3.90% | | June 2024 | | $ | 1,246,486 | | $ | 1,233,553 |
| | | 4.20% | | December 2024 | | | 5,816,933 | | | — |
Subtotal | | | | | | $ | 7,063,419 | | $ | 1,233,553 |
Total | | | | | | $ | 14,057,588 | | $ | 8,840,461 |
Interest expenses were $253,691 and $141,440 for the six months ended March 31, 2024 and 2023, respectively.
The borrowings from Xiaoshan Rural Commercial Bank were secured by the Group’s buildings and guarantee by Huiyu Holdings Group Co., Ltd.
The borrowing from China Citic Bank was guaranteed by the Group’s CEO, Shuang Wu, and secured by the CEO’s properties.
13. RELATED PARTY TRANSACTIONS
The following is a list of related parties which the Group has transactions with:
No. | | Name of Related Parties | | Relationship with the Group |
a | | Baiming Yu | | COO, Director and a shareholder of the Company |
b | | Qijia Yu | | Immediate family member of Baiming Yu |
c | | Liwei Zhang | | Shareholder of the Company and the spouse of Baiming Yu |
d | | Huiyu Chuanggu (Hangzhou) Equity Investment Fund Co., Ltd. (“Huiyu Chuanggu”) | | Shuang Wu acted as the executive director and owned 30% equity interests of this related party |
e | | Shuang Wu | | Chief Executive Officer of the Company |
f | | Hangzhou Shuige Technology Co., Ltd (“Hangzhou Shuige”) | | 55% equity interests owned by Baiming Yu |
g | | Hangzhou Qingniu Medical Instrument Co., Ltd (“Hangzhou Qingniu”) | | Owned by immediate family member of Baiming Yu |
h | | Hangzhou Xiaoshan Ance Building Materials Firm (“Xiaoshan Ance”) | | Owned by Liwei Zhang |
i | | Hangzhou Yuanqi Biotech Co., Ltd. (“Hangzhou Yuanqi”) | | Baiming Yu acted as director |
j | | Ming Zhao | | Shareholder of Shanghai Saitumofei |
F-44
Table of Contents
WORK MEDICAL TECHNOLOGY GROUP LTD
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(In U.S. dollars, except share and per share data)
13. RELATED PARTY TRANSACTIONS (cont.)
Amounts due from related parties
Amounts due from related parties consisted of the following for the periods indicated:
| | As of
March 31,
2024 | | As of
September 30,
2023 |
Hangzhou Qingniu(1) | | $ | 234,290 | | $ | 1,294,296 |
Huiyu Chuanggu(2) | | | 214,282 | | | 1,453,583 |
Shuang Wu(3) | | | 18,376 | | | 795,243 |
Qijia Yu(3) | | | 1,315 | | | — |
Baiming Yu(4) | | | — | | | 683,376 |
Liwei Zhang(5) | | | — | | | 11,184 |
Total | | $ | 468,263 | | $ | 4,237,682 |
Amounts due to related parties
Amounts due to related parties consisted of the following for the periods indicated:
| | As of
March 31,
2024 | | As of
September 30,
2023 |
Xiaoshan Ance(1) | | $ | 138,498 | | $ | 137,061 |
Hangzhou Shuige(1) | | | 9,001 | | | 17,818 |
Baiming Yu(2) | | | 9,354 | | | — |
Ming Zhao(2) | | | 4,089 | | | 4,048 |
Hangzhou Yuanqi(1) | | | 693 | | | 685 |
Total | | $ | 161,635 | | $ | 159,612 |
F-45
Table of Contents
WORK MEDICAL TECHNOLOGY GROUP LTD
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(In U.S. dollars, except share and per share data)
13. RELATED PARTY TRANSACTIONS (cont.)
Related party transactions
Nature | | For the six months ended
March 31, |
2024 | | 2023 |
Loan to Hangzhou Shuige(1) | | $ | — | | $ | 430,040 |
Repayment from Hangzhou Shuige(1) | | | — | | | 430,040 |
Loan from Hangzhou Shuige(2) | | | 416,297 | | | — |
Repayment to Hangzhou Shuige(2) | | | 425,318 | | | — |
Loan to Liwei Zhang(3) | | | 277,531 | | | 458,709 |
Repayment from Liwei Zhang(3) | | | 288,854 | | | 458,709 |
Reimbursement from Baiming Yu(4) | | | — | | | 651,797 |
Repayment from Baiming Yu(4) | | | 691,872 | | | — |
Loan from Baiming Yu(2) | | | 9,372 | | | |
Selling to Hangzhou Qingniu(5) | | | 233,109 | | | 429,093 |
Loan to Hangzhou Qingniu(1) | | | 2,708,432 | | | 2,072,392 |
Repayment from Hangzhou Qingniu(1&5&6) | | | 4,023,255 | | | 1,727,326 |
Rent income from Hangzhou Qingniu(6) | | | — | | | 58,373 |
Purchase from Hangzhou Qingniu(7) | | | 24,236 | | | — |
Advance from Shuang Wu(8) | | | — | | | 1,018,337 |
Repayment to Shuang Wu(8) | | | — | | | 1,653,679 |
Advance to Shuang Wu(9) | | | — | | | 1,077,790 |
Repayment from Shuang Wu(9) | | | 769,039 | | | 97,046 |
Reimbursement from Shuang Wu(9) | | | 17,681 | | | — |
Advance to Huiyu Chuanggu(9) | | | 447,796 | | | — |
Reimbursement from Huiyu Chuanggu(9) | | | 1,704,757 | | | — |
Advance to Qijia Yu(4) | | | 1,388 | | | — |
Reimbursement from Qijia Yu(4) | | | 69 | | | — |
F-46
Table of Contents
WORK MEDICAL TECHNOLOGY GROUP LTD
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(In U.S. dollars, except share and per share data)
14. ORDINARY SHARES
Ordinary Shares
The Company was established as an exempted company under the laws of Cayman Islands on March 1, 2022. The authorized number of Ordinary Shares was 50,000 with par value of $1 per share. The Company issued 50,000 shares to the shareholders at par value $1 per share.
On April 6, 2023, the shareholders of the Company unanimously passed resolutions effecting the subdivision of the Company’s authorized and issued share capital and the adoption of the amended and restated memorandum of association, pursuant to which, (1) the Company effectuated a 1:2000 share subdivision, whereupon the Company’s authorized share capital was amended from US$50,000 divided into 50,000 shares of par value US$1.00 each to US$50,000 divided into 100,000,000 Ordinary Shares of a par value of $0.0005 each; and (2) immediately after the share subdivision, the shareholders voluntarily surrendered, on a pro rata basis, a total of 87,500,000 Ordinary Shares of a par value of $0.0005 each, after which, the Company had an aggregate of 12,500,000 Ordinary Shares issued and outstanding.
Subscription receivable
As of March 31, 2024 and September 30, 2023 subscription receivable on the unaudited condensed consolidated balance sheets represented the unrecovered consideration of the 12,500,000 ordinary shares issued by the Company.
15. STATUTORY SURPLUS RESERVES AND RESTRICTED NET ASSETS
A significant portion of the Group’s operations are conducted through its PRC (excluding Hong Kong) subsidiaries, the Company’s ability to pay dividends is primarily dependent on receiving distributions of funds from our subsidiaries. Relevant PRC statutory laws and regulations permit payments of dividends by our subsidiaries only out of their retained earnings, if any, as determined in accordance with PRC accounting standards and regulations, and after it has met the PRC requirements for appropriation to statutory reserves. The Group is required to make appropriations to certain reserve funds, comprising the statutory surplus reserve and the discretionary surplus reserve, based on after-tax net income determined in accordance with generally accepted accounting principles of the PRC (“PRC GAAP”). Appropriations to the statutory surplus reserve are required to be at least 10% of the after-tax net income determined in accordance with PRC GAAP until the reserve is equal to 50% of the entity’s registered capital. Appropriations to the surplus reserve are made at the discretion of the Board of Directors. Paid-in capital of our subsidiaries included in the Company’s consolidated net assets are also non-distributable for dividend purposes.
As a result of these PRC laws and regulations, the Company’s PRC subsidiaries are restricted in their ability to transfer a portion of their net assets to the Company. As of March 31, 2024 and September 30, 2023, net assets restricted in the aggregate, which include paid-in capital and statutory reserve funds of the Company’s subsidiaries, that are included in the consolidated net assets were both $972,494.
16. CONCENTRATION OF CREDIT RISK
Financial instruments that potentially expose the Group to concentrations of credit risk consist primarily of accounts receivable. The Group conducts credit evaluations of its customers, and generally does not require collateral or other security from them. The Group evaluates its collection experience and long outstanding balances to determine the need for an allowance for doubtful accounts. The Group conducts periodic reviews of the financial condition and payment practices of its customers to minimize collection risk on accounts receivable.
There is no single customer that accounted 10% or more of the Group’s total revenue for the six months ended March 31, 2024 and 2023.
F-47
Table of Contents
WORK MEDICAL TECHNOLOGY GROUP LTD
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(In U.S. dollars, except share and per share data)
16. CONCENTRATION OF CREDIT RISK (cont.)
The following table sets forth a summary of single customer who represented 10% or more of the Group’s total accounts receivable:
| | As of
March 31,
2024 | | As of
September 30,
2023 |
Percentage of the Group’s accounts receivable | | | | | | |
Customer A | | 20 | % | | 19 | % |
Customer B | | * | | | 14 | % |
Customer C | | * | | | 13 | % |
The following table sets forth a summary of single suppliers who represented 10% or more of the Group’s total purchases:
| | For the six months ended
March 31, |
| | | 2024 | | 2023 |
Percentage of the Group’s purchase | | | | | | |
Supplier A | | 15 | % | | — | |
Supplier B | | 11 | % | | — | |
Supplier C | | — | | | 11 | % |
The following table sets forth a summary of single suppliers who represented 10% or more of the Group’s total advance to suppliers:
| | As of
March 31,
2024 | | As of
September 30,
2023 |
Percentage of the Group’s advance to suppliers | | | | | | |
Supplier D | | 34 | % | | — | |
Supplier E | | 19 | % | | 40 | % |
Supplier F | | * | | | 20 | % |
Supplier G | | * | | | 10 | % |
17. COMMITMENTS AND CONTINGENCIES
Commitments
As of March 31, 2024, the Group has no material purchase commitments or significant leases.
Contingencies
In the ordinary course of business, the Group may be subject to legal proceedings regarding contractual and employment relationships and a variety of other matters. The Group records contingent liabilities resulting from such claims, when a loss is assessed to be probable, and the amount of the loss is reasonably estimable. In the opinion of management, there were no material pending or threatened claims and litigation as of the issuance date of these consolidated financial statements.
F-48
Table of Contents
WORK MEDICAL TECHNOLOGY GROUP LTD
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(In U.S. dollars, except share and per share data)
18. CONDENSED FINANCIAL INFORMATION OF THE PARENT COMPANY
The condensed financial information of the parent company, WORK Medical Technology Group LTD, has been prepared using the same accounting policies as set out in the Group’s consolidated financial statements except that the parent company has used equity method to account for its investment in its subsidiaries.
The Company and its subsidiaries are included in the consolidated financial statements where the inter-company balances and transactions are eliminated upon consolidation. For the purpose of the Company’s stand-alone financial statements, its investments in subsidiaries are reported using the equity method of accounting. The Company’s share of income and losses from its subsidiaries is reported as earnings from subsidiaries in the accompanying condensed financial information of parent company.
The Company did not have significant capital and other commitments, long-term obligations, or guarantees as of March 31, 2024 and September 30, 2023.
PARENT COMPANY BALANCE SHEETS
| | As of
March 31,
2024 | | As of
September 30,
2023 |
| | | (unaudited) | | |
ASSETS | | | | | | | | |
Current Assets | | | | | | | | |
Prepaid expenses and other current assets | | $ | — | | | $ | — | |
Total current assets | | | — | | | | — | |
| | | | | | | | | |
Non-current Assets | | | | | | | | |
Long term investment | | | 10,214,314 | | | | 9,876,251 | |
Total non-current assets | | | 10,214,314 | | | | 9,876,251 | |
Total assets | | | 10,214,314 | | | | 9,876,251 | |
| | | | | | | | | |
LIABILITIES AND SHAREHOLDERS’ EQUITY | | | | | | | | |
Liabilities | | | | | | | | |
Amounts due to related parties | | | — | | | | — | |
Current Liabilities | | | — | | | | — | |
Total liabilities | | | — | | | | — | |
| | | | | | | | | |
Shareholders’ equity | | | | | | | | |
Ordinary Shares | | | 6,250 | | | | 6,250 | |
Subscription receivable | | | (6,250 | ) | | | (6,250 | ) |
Additional paid-in capital | | | 85,012 | | | | 85,012 | |
Statutory reserve | | | 887,482 | | | | 887,482 | |
Retained earnings | | | 9,807,110 | | | | 9,580,585 | |
Accumulated other comprehensive loss | | | (565,263 | ) | | | (676,828 | ) |
Total shareholders’ equity | | | 10,214,314 | | | | 9,876,251 | |
Total liabilities and shareholders’ equity | | $ | 10,214,314 | | | $ | 9,876,251 | |
F-49
Table of Contents
WORK MEDICAL TECHNOLOGY GROUP LTD
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(In U.S. dollars, except share and per share data)
18. CONDENSED FINANCIAL INFORMATION OF THE PARENT COMPANY (cont.)
PARENT COMPANY STATEMENTS OF INCOME AND COMPREHENSIVE INCOME
| | For the six months ended
March 31, |
| | | 2024 | | 2023 |
Other income: | | | | | | |
Equity in earnings of subsidiaries | | $ | 226,525 | | $ | 1,475,734 |
Income before income tax expense | | | 226,525 | | | 1,475,734 |
Income tax expense | | | — | | | — |
Net income | | | 226,525 | | | 1,475,734 |
Other comprehensive income: | | | | | | |
Foreign currency translation gain, net of nil income taxes | | | 111,565 | | | 400,594 |
Total comprehensive income | | | 338,090 | | | 1,876,328 |
PARENT COMPANY STATEMENTS OF CASH FLOWS
| | For the six months ended
March 31, |
| | | 2024 | | 2023 |
Cash flows from operating activities | | $ | — | | $ | — |
Cash flows from investing activities | | | — | | | — |
Cash flows from financing activities | | | — | | | — |
Effect of exchange rate changes | | | — | | | — |
Net increase in cash, cash equivalents and restricted cash | | | — | | | — |
Cash, cash equivalents and restricted cash, at the beginning of the year | | | — | | | — |
Cash, cash equivalents and restricted cash, at the end of the year | | $ | — | | $ | — |
19. SUBSEQUENT EVENTS
On April 30, 2024, the Group established a new PRC limited liability company subsidiary, Huangshan Saitumofei Medical Technology Co., Ltd. (“Huangshan Saitumofei”), with registered capital of RMB1 million. Huangshan Saitumofei does not have any operations as of the date of this prospectus.
On August 26, 2024, the Company closed its IPO of 2,000,000 Ordinary Shares, par value $0.0005 per share. The Ordinary Shares were priced at $4.00 per share, and the offering was conducted on a firm commitment basis. The Ordinary Shares were approved for listing on the Nasdaq Capital Market and commenced trading under the ticker symbol “WOK” on August 23, 2024.
On August 28, 2024, Kingswood Capital Partners, LLC, as the representative of the underwriters of the initial public offering of the Company, exercised its over-allotment option, in part, to purchase an additional 91,942 Ordinary Shares of the Company at the public offering price of US$4.00 per share. The closing for the sale of the over-allotment shares took place on August 29, 2024. As a result, the net proceeds of the Company’s IPO, including the proceeds from the sale of the over-allotment shares, totaled $5,654,525, after deducting underwriting discounts and other related expenses.
The Group has evaluated subsequent events from March 31, 2024 and through the date of issuance of the condensed consolidated financial statements which is December 20, 2024, other than the matters noted above, the Group did not identify any subsequent events with material financial impact or that required adjustment of the Group’s condensed consolidated financial statements.
F-50
Table of Contents
2,500,000 Ordinary Units (each Ordinary Unit consisting of one Ordinary Share, one Series A Warrant to purchase one Ordinary Share, and one Series B Warrant to purchase one Ordinary Share)
Up to 2,500,000 Pre-Funded Ordinary Units (each Pre-Funded Unit consisting of one Pre-Funded Warrant, one Series A Warrant to purchase one Ordinary Share, and one Series B Warrant to purchase one Ordinary Share)
Up to 7,500,000 Ordinary Shares underlying the Series A Warrants, Series B Warrants and Pre-Funded Warrants

WORK MEDICAL TECHNOLOGY GROUP LTD
____________________
PROSPECTUS
____________________
Univest Securities, LLC
Prospectus dated , 2024
Until [•], 2024 (25 days after the date of this prospectus), all dealers that buy, sell or trade these securities, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to the dealers’ obligation to deliver a prospectus when acting as an underwriter and with respect to its unsold allotments or subscriptions.
Table of Contents
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
ITEM 6. INDEMNIFICATION OF DIRECTORS AND OFFICERS.
Cayman Islands law does not limit the extent to which a company’s articles of association may provide for indemnification of officers and directors, except to the extent any such provision may be held by the Cayman Islands courts to be contrary to public policy, such as to provide indemnification against civil fraud or the consequences of committing a crime. Our amended and restated articles of association, which will become effective upon or before completion of this offering, provide that, to the extent permitted by law, we shall indemnify each existing or former secretary, director (including alternate director), and any of our other officers (including an investment adviser or an administrator or liquidator) and their personal representatives against:
(a) all actions, proceedings, costs, charges, expenses, losses, damages or liabilities incurred or sustained by the existing or former director (including alternate director), secretary or officer in or about the conduct of our business or affairs or in the execution or discharge of the existing or former director (including alternate director), secretary’s or officer’s duties, powers, authorities or discretions; and
(b) without limitation to paragraph (a) above, all costs, expenses, losses or liabilities incurred by the existing or former director (including alternate director), secretary or officer in defending (whether successfully or otherwise) any civil, criminal, administrative or investigative proceedings (whether threatened, pending or completed) concerning us or our affairs in any court or tribunal, whether in the Cayman Islands or elsewhere.
No such existing or former director (including alternate director), secretary or officer, however, shall be indemnified in respect of any matter arising out of his own dishonesty.
To the extent permitted by law, we may make a payment, or agree to make a payment, whether by way of advance, loan or otherwise, for any legal costs incurred by an existing or former director (including alternate director), secretary or any of our officers in respect of any matter identified in above on condition that the director (including alternate director), secretary or officer must repay the amount paid by us to the extent that it is ultimately found not liable to indemnify the director (including alternate director), the secretary or that officer for those legal costs.
We have entered into indemnification agreements with each of our directors and executive officers. Under these agreements, we have agreed to indemnify our directors and executive officers against certain liabilities and expenses incurred by such persons in connection with claims made by reason of their being such a director or executive officer.
The form of underwriting agreement filed as Exhibit 1.1 to this registration statement will also provide for indemnification of us and our officers and directors.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers or persons controlling us under the foregoing provisions, we have been informed that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable.
ITEM 7. RECENT SALES OF UNREGISTERED SECURITIES.
During the past three years, we have issued and sold the securities described below without registering the securities under the Securities Act. We believe that each of the following issuances was exempt from registration under the Securities Act in reliance on Regulation D under the Securities Act or pursuant to Section 4(a)(2) of the Securities Act regarding transactions not involving a public offering or in reliance on Regulation S under the Securities Act regarding sales by an issuer in offshore transactions.
II-1
Table of Contents
None of these transactions involved any underwriters’ underwriting discounts or commissions, or any public offering.
Purchaser | | Number of
Ordinary
Shares | | Date of
Sale or
Issuance | | Consideration |
Tricor Services (Cayman Islands) Limited* | | 1 | | March 1, 2022 | | $ | 1 |
LWY GROUP LTD | | 24,999 | | March 1, 2022 | | $ | 24,999 |
HJZ GROUP LTD | | 5,740 | | March 1, 2022 | | $ | 5,740 |
SANYOU NO.1 GROUP LTD | | 3,890 | | March 1, 2022 | | $ | 3,890 |
JPY GROUP LTD | | 2,870 | | March 1, 2022 | | $ | 2,870 |
SANYOU NO.2 GROUP LTD | | 2,500 | | March 1, 2022 | | $ | 2,500 |
YQJ GROUP LTD | | 2,500 | | March 1, 2022 | | $ | 2,500 |
ZHF GROUP LTD | | 2,500 | | March 1, 2022 | | $ | 2,500 |
ZLW GROUP LTD | | 2,500 | | March 1, 2022 | | $ | 2,500 |
ZXH GROUP LTD | | 2,500 | | March 1, 2022 | | $ | 2,500 |
ITEM 8. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES.
a) Exhibits
See Exhibit Index beginning on page II-6 of this registration statement.
The agreements included as exhibits to this registration statement contain representations and warranties by each of the parties to the applicable agreement. These representations and warranties were made solely for the benefit of the other parties to the applicable agreement and (i) were not intended to be treated as categorical statements of fact, but rather as a way of allocating the risk to one of the parties if those statements prove to be inaccurate; (ii) may have been qualified in such agreement by disclosure that was made to the other party in connection with the negotiation of the applicable agreement; (iii) may apply contract standards of “materiality” that are different from “materiality” under the applicable securities laws; and (iv) were made only as of the date of the applicable agreement or such other date or dates as may be specified in the agreement.
We acknowledge that, notwithstanding the inclusion of the foregoing cautionary statements, we are responsible for considering whether additional specific disclosure of material information regarding material contractual provisions is required to make the statements in this registration statement not misleading.
b) Financial Statement Schedules
Schedules have been omitted because the information required to be set forth therein is not applicable or is shown in our consolidated financial statements or the notes thereto.
ITEM 9. UNDERTAKINGS.
The undersigned registrant hereby undertakes to provide to the underwriter at the closing specified in the underwriting agreements, certificates in such denominations and registered in such names as required by the underwriter to permit prompt delivery to each purchaser.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the registrant pursuant to the provisions described in Item 6, or otherwise, the registrant has been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
II-2
Table of Contents
The undersigned registrant hereby undertakes that:
(1) For purposes of determining any liability under the Securities Act, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective.
(2) For the purpose of determining any liability under the Securities Act, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(3) For the purpose of determining liability under the Securities Act to any purchaser, each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness; provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use.
(4) For the purpose of determining any liability of the registrant under the Securities Act to any purchaser in the initial distribution of the securities, the undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser:
(i) Any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424;
(ii) Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant;
(iii) The portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and
(iv) Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser.
II-3
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, as amended, the registrant certifies that it has reasonable grounds to believe that it meets all of the requirements for filing on Form F-1 and has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in Hangzhou, Zhejiang Province, China, on December 20, 2024.
| | WORK Medical Technology Group LTD |
| | | By: | | /s/ Shuang Wu |
| | | | | Name: | | Shuang Wu |
| | | | | Title: | | Chief Executive Officer |
Power of Attorney
Each person whose signature appears below constitutes and appoints Shuang Wu as attorney-in-fact with full power of substitution, for him in any and all capacities, to do any and all acts and all things and to execute any and all instruments which said attorney and agent may deem necessary or desirable to enable the registrant to comply with the Securities Act, and any rules, regulations, and requirements of the SEC thereunder, in connection with the registration under the Securities Act of securities of the registrant, including, without limitation, the power and authority to sign the name of each of the undersigned in the capacities indicated below to the registration statement on Form F-1 (the “Registration Statement”) to be filed with the SEC with respect to such securities, to any and all amendments or supplements to such Registration Statement, whether such amendments or supplements are filed before or after the effective date of such Registration Statement, to any related Registration Statement filed pursuant to Rule 462(b) under the Securities Act, and to any and all instruments or documents filed as part of or in connection with such Registration Statement or any and all amendments thereto, whether such amendments are filed before or after the effective date of such Registration Statement; and each of the undersigned hereby ratifies and confirms all that such attorney and agent shall do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act, this registration statement has been signed by the following persons in the capacities and on the dates indicated.
Signature | | Title | | Date |
/s/ Shuang Wu | | Chief Executive Officer | | December 20, 2024 |
Shuang Wu | | (Principal executive officer) | | |
/s/ Ningfang Liang | | Chief Financial Officer | | December 20, 2024 |
Ningfang Liang | | (Principal financial and accounting officer) | | |
/s/ Baiming Yu | | Director | | December 20, 2024 |
Baiming Yu | | | | |
/s/ Xiaoyang Li | | Director | | December 20, 2024 |
Xiaoyang Li | | | | |
/s/ Zhongxuan Li | | Director | | December 20, 2024 |
Zhongxuan Li | | | | |
/s/ Robert Johnson | | Director | | December 20, 2024 |
Robert Johnson | | | | |
II-4
Table of Contents
SIGNATURE OF AUTHORIZED REPRESENTATIVE IN THE UNITED STATES
Pursuant to the Securities Act of 1933, the undersigned, the duly authorized representative in the United States of WORK Medical Technology Group LTD, has signed this registration statement or amendment thereto in New York, NY on December 20, 2024.
| | Cogency Global Inc. |
| | | Authorized U.S. Representative |
| | | By: | | /s/ Colleen A. De Vries |
| | | | | Name: | | Colleen A. De Vries |
| | | | | Title: | | Sr. Vice President on behalf of
Cogency Global Inc. |
II-5
Table of Contents
WORK MEDICAL TECHNOLOGY GROUP LTD
EXHIBIT INDEX
Exhibit
Number | | Description of Document |
1.1* | | Form of Underwriting Agreement |
3.1* | | Amended and Restated Memorandum of Association of the Registrant |
3.2* | | Amended and Restated Articles of Association of the Registrant |
4.1 | | Registrant’s Specimen Certificate for Ordinary Shares (incorporated by reference to Exhibit 4.1 of our Registration Statement on Form F-1 (File No. 333-271474) initially filed with the Securities and Exchange Commission on April 27, 2023) |
4.2* | | Form of Pre-Funded Warrant |
4.3* | | Form of Series A Warrant |
4.4* | | Form of Series B Warrant |
5.1* | | Opinion of Ogier (Cayman) LLP regarding the validity of the Ordinary Shares being registered |
5.2* | | Opinion of Hunter Taubman Fischer & Li LLC, U.S. counsel to Company, as to the enforceability of the Warrants |
10.1 | | Form of Indemnification Agreement with the Registrant’s directors and officers (incorporated by reference to Exhibit 10.1 of our Registration Statement on Form F-1 (File No. 333-271474) initially filed with the Securities and Exchange Commission on April 27, 2023) |
10.2 | | Form of Director Offer Letter between the Registrant and its directors (incorporated by reference to Exhibit 10.2 of our Registration Statement on Form F-1 (File No. 333-271474) initially filed with the Securities and Exchange Commission on April 27, 2023) |
10.3 | | English Translation of Form of Procurement Agreement (incorporated by reference to Exhibit 10.3 of our Registration Statement on Form F-1 (File No. 333-271474) initially filed with the Securities and Exchange Commission on April 27, 2023) |
10.4 | | English Translation of Form of Distribution Agreement (incorporated by reference to Exhibit 10.4 of our Registration Statement on Form F-1 (File No. 333-271474) initially filed with the Securities and Exchange Commission on April 27, 2023) |
10.5 | | English Translation of Advance Agreement dated October 3, 2021, by and between Hangzhou Shanyou and Baiming Yu (incorporated by reference to Exhibit 10.5 of our Registration Statement on Form F-1 (File No. 333-271474) initially filed with the Securities and Exchange Commission on April 27, 2023) |
10.6 | | English Translation of Advance Agreement dated October 3, 2021, by and between Hangzhou Shanyou and Liwei Zhang (incorporated by reference to Exhibit 10.6 of our Registration Statement on Form F-1 (File No. 333-271474) initially filed with the Securities and Exchange Commission on April 27, 2023) |
10.7 | | English Translation of Loan Agreement dated October 9, 2021, by and between Hangzhou Shanyou and Hangzhou Shuige (incorporated by reference to Exhibit 10.7 of our Registration Statement on Form F-1 (File No. 333-271474) initially filed with the Securities and Exchange Commission on April 27, 2023) |
10.8 | | English Translation of Real Estate Purchasing Agreement dated July 13, 2020, by and between Hangzhou Shanyou and Qijia Yu (incorporated by reference to Exhibit 10.8 of our Registration Statement on Form F-1 (File No. 333-271474) initially filed with the Securities and Exchange Commission on April 27, 2023) |
10.9 | | English Translation of Medical Devices Purchasing Agreement dated September 30, 2021, by and between Hangzhou Shanyou and Hangzhou Qingniu (incorporated by reference to Exhibit 10.9 of our Registration Statement on Form F-1 (File No. 333-271474) initially filed with the Securities and Exchange Commission on April 27, 2023) |
10.10 | | English Translation of Comprehensive Credit Agreement dated September 29, 2020, by and between Hangzhou Shanyou and Bank of Beijing (incorporated by reference to Exhibit 10.10 of our Registration Statement on Form F-1 (File No. 333-271474) initially filed with the Securities and Exchange Commission on April 27, 2023) |
10.11 | | English Translation of Loan Agreement dated September 29, 2020, by and between Hangzhou Shanyou and Bank of Beijing (incorporated by reference to Exhibit 10.11 of our Registration Statement on Form F-1 (File No. 333-271474) initially filed with the Securities and Exchange Commission on April 27, 2023) |
10.12 | | English Translation of Loan Agreement dated January 26, 2021, by and between Hangzhou Shanyou and Bank of Jiangsu (incorporated by reference to Exhibit 10.12 of our Registration Statement on Form F-1 (File No. 333-271474) initially filed with the Securities and Exchange Commission on April 27, 2023) |
II-6
Table of Contents
Exhibit
Number | | Description of Document |
10.13 | | English Translation of Loan Agreement dated December 22, 2016, by and between Hangzhou Shanyou and Zhejiang Xiaoshan Rural Commercial Bank (incorporated by reference to Exhibit 10.13 of our Registration Statement on Form F-1 (File No. 333-271474) initially filed with the Securities and Exchange Commission on April 27, 2023) |
10.14 | | English Translation of Loan Agreement dated January 6, 2020, by and between Hangzhou Shanyou and Zhejiang Xiaoshan Rural Commercial Bank (incorporated by reference to Exhibit 10.14 of our Registration Statement on Form F-1 (File No. 333-271474) initially filed with the Securities and Exchange Commission on April 27, 2023) |
10.15 | | English Translation of Loan Agreement dated January 15, 2020, by and between Hangzhou Shanyou and Zhejiang Xiaoshan Rural Commercial Bank (incorporated by reference to Exhibit 10.15 of our Registration Statement on Form F-1 (File No. 333-271474) initially filed with the Securities and Exchange Commission on April 27, 2023) |
10.16 | | English Translation of Loan Agreement dated March 2, 2020, by and between Hangzhou Shanyou and Zhejiang Xiaoshan Rural Commercial Bank (incorporated by reference to Exhibit 10.16 of our Registration Statement on Form F-1 (File No. 333-271474) initially filed with the Securities and Exchange Commission on April 27, 2023) |
10.17 | | English Translation of Loan Agreement dated June 28, 2020, by and between Hangzhou Shanyou and Zhejiang Xiaoshan Rural Commercial Bank (incorporated by reference to Exhibit 10.17 of our Registration Statement on Form F-1 (File No. 333-271474) initially filed with the Securities and Exchange Commission on April 27, 2023) |
10.18 | | English Translation of Loan Agreement dated July 2, 2020, by and between Hangzhou Shanyou and Zhejiang Xiaoshan Rural Commercial Bank (incorporated by reference to Exhibit 10.18 of our Registration Statement on Form F-1 (File No. 333-271474) initially filed with the Securities and Exchange Commission on April 27, 2023) |
10.19 | | English Translation of Loan Agreement dated September 29, 2021, by and between Hangzhou Shanyou and Zhejiang Xiaoshan Rural Commercial Bank (incorporated by reference to Exhibit 10.19 of our Registration Statement on Form F-1 (File No. 333-271474) initially filed with the Securities and Exchange Commission on April 27, 2023) |
10.20 | | English Translation of Loan Agreement dated September 29, 2021, by and between Hangzhou Shanyou and Zhejiang Xiaoshan Rural Commercial Bank (incorporated by reference to Exhibit 10.20 of our Registration Statement on Form F-1 (File No. 333-271474) initially filed with the Securities and Exchange Commission on April 27, 2023) |
10.21 | | English Translation of Loan Agreement dated September 1, 2022, by and between Hangzhou Shanyou and Zhejiang Xiaoshan Rural Commercial Bank (incorporated by reference to Exhibit 10.21 of our Registration Statement on Form F-1 (File No. 333-271474) initially filed with the Securities and Exchange Commission on April 27, 2023) |
10.22 | | English Translation of Loan Agreement dated September 2, 2022, by and between Hangzhou Shanyou and Zhejiang Xiaoshan Rural Commercial Bank (incorporated by reference to Exhibit 10.22 of our Registration Statement on Form F-1 (File No. 333-271474) initially filed with the Securities and Exchange Commission on April 27, 2023) |
10.23 | | English Translation of Loan Agreement dated September 7, 2022, by and between Hangzhou Shanyou and Zhejiang Xiaoshan Rural Commercial Bank (incorporated by reference to Exhibit 10.23 of our Registration Statement on Form F-1 (File No. 333-271474) initially filed with the Securities and Exchange Commission on April 27, 2023) |
10.24 | | English Translation of Loan Agreement dated September 7, 2022, by and between Hangzhou Shanyou and Zhejiang Xiaoshan Rural Commercial Bank (incorporated by reference to Exhibit 10.24 of our Registration Statement on Form F-1 (File No. 333-271474) initially filed with the Securities and Exchange Commission on April 27, 2023) |
10.25 | | English Translation of Lease Agreement dated January 1, 2020, by and between Hangzhou Shanyou and Guancuncun Village Committee (incorporated by reference to Exhibit 10.25 of our Registration Statement on Form F-1 (File No. 333-271474) initially filed with the Securities and Exchange Commission on April 27, 2023) |
10.26 | | English Translation of Lease Agreement dated January 1, 2011, by and between Hangzhou Shanyou and Louta Town Village Committee (incorporated by reference to Exhibit 10.26 of our Registration Statement on Form F-1 (File No. 333-271474) initially filed with the Securities and Exchange Commission on April 27, 2023) |
II-7
Table of Contents
Exhibit
Number | | Description of Document |
10.27 | | English Translation of Lease Agreement dated November 30, 2023, by and between Hangzhou Shanyou ang Hangzhou Tianxia Weaving Co., Ltd. (incorporated by reference to Exhibit 10.27 of our Registration Statement on Form F-1 (File No. 333-271474) initially filed with the Securities and Exchange Commission on April 27, 2023) |
10.28 | | English Translation of Lease Agreement dated February 27, 2018, by and between Shanghai Chuqiang and Ailiu Real Estate Co., Ltd. (incorporated by reference to Exhibit 10.28 of our Registration Statement on Form F-1 (File No. 333-271474) initially filed with the Securities and Exchange Commission on April 27, 2023) |
10.29 | | English Translation of Loan Agreement dated March 31, 2023, by and between Hangzhou Shanyou and Bank of Jiangsu (incorporated by reference to Exhibit 10.29 of our Registration Statement on Form F-1 (File No. 333-271474) initially filed with the Securities and Exchange Commission on April 27, 2023) |
10.30 | | English Translation of Patent Transfer Agreement dated April 1, 2021, by and between Hangzhou Shanyou and The Second Hospital of Jiaxing City (incorporated by reference to Exhibit 10.30 of our Registration Statement on Form F-1 (File No. 333-271474) initially filed with the Securities and Exchange Commission on April 27, 2023) |
10.31 | | English Translation of Patent Transfer Agreement dated December 26, 2019, by and between Hangzhou Hanshi and Zhejiang University (incorporated by reference to Exhibit 10.31 of our Registration Statement on Form F-1 (File No. 333-271474) initially filed with the Securities and Exchange Commission on April 27, 2023) |
10.32 | | Employment Agreement by and between Shuang Wu and the Registrant dated June 1, 2022 (incorporated by reference to Exhibit 10.32 of our Registration Statement on Form F-1 (File No. 333-271474) initially filed with the Securities and Exchange Commission on April 27, 2023) |
10.33 | | Employment Agreement by and between Ningfang Liang and the Registrant dated June 1, 2022 (incorporated by reference to Exhibit 10.33 of our Registration Statement on Form F-1 (File No. 333-271474) initially filed with the Securities and Exchange Commission on April 27, 2023) |
10.34 | | Employment Agreement by and between Baiming Yu and the Registrant dated June 1, 2022 (incorporated by reference to Exhibit 10.34 of our Registration Statement on Form F-1 (File No. 333-271474) initially filed with the Securities and Exchange Commission on April 27, 2023) |
10.35 | | English Translation of Loan Agreement dated June 28, 2023, by and between Hangzhou Shanyou and China CITIC Bank (incorporated by reference to Exhibit 10.35 of our Registration Statement on Form F-1 (File No. 333-271474) initially filed with the Securities and Exchange Commission on April 27, 2023) |
10.36 | | English Translation of Loan Agreement dated December 20, 2023, by and between Hangzhou Woli and China CITIC Bank (incorporated by reference to Exhibit 10.36 of our Registration Statement on Form F-1 (File No. 333-271474) initially filed with the Securities and Exchange Commission on April 27, 2023) |
10.37 | | English Translation of Loan Agreement dated June 29, 2023, by and between Hangzhou Shanyou and Bank of Beijing (incorporated by reference to Exhibit 10.37 of our Registration Statement on Form F-1 (File No. 333-271474) initially filed with the Securities and Exchange Commission on April 27, 2023) |
10.38 | | Form of Indemnification Escrow Agreement (incorporated by reference to Exhibit 10.38 of our Registration Statement on Form F-1 (File No. 333-271474) initially filed with the Securities and Exchange Commission on April 27, 2023) |
10.39 | | English Translation of Loan Agreement dated March 4, 2024, by and between Hangzhou Shanyou and Bank of Jiangsu (incorporated by reference to Exhibit 10.39 of our Registration Statement on Form F-1 (File No. 333-271474) initially filed with the Securities and Exchange Commission on April 27, 2023) |
10.40* | | English Translation of Loan Agreement dated July 26, 2024, by and between Hangzhou Shanyou and China CITIC Bank (Hangzhou) |
10.41* | | English Translation of Loan Agreement dated July 11, 2024, by and between Hangzhou Shanyou and Zhejiang Xiaoshan Rural Commercial Bank |
10.42* | | English Translation of Loan Extension Agreement dated August 26, 2024, regarding a loan of RMB10 million, by and between Hangzhou Shanyou and Zhejiang Xiaoshan Rural Commerical Bank |
10.43* | | English Translation of Loan Extension Agreement dated August 29, 2023, regarding a loan of RMB8 million, by and between Hangzhou Shanyou and Zhejiang Xiaoshan Rural Commercial Bank |
10.44* | | English Translation of Loan Extension Agreement dated August 26, 2024, regarding a loan of RMB8 million, by and between Hangzhou Shanyou and Zhejiang Xiaoshan Rural Commercial Bank |
10.45* | | English Translation of Loan Extension Agreement dated August 26, 2024, regarding a loan of RMB5 million, by and between Hangzhou Shanyou and Zhejiang Xiaoshan Rural Commercial Bank |
II-8
Table of Contents
II-9

































