As filed with the Securities and Exchange Commission on November 22, 2024
Registration No. 333-
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, DC 20549
Form F-1
REGISTRATION STATEMENT UNDER THE SECURITIES ACT OF 1933
NEWGENIVF GROUP LIMITED
(Exact name of registrant as specified in its charter)
| British Virgin Islands | | 8090 | | Not Applicable |
(State or other jurisdiction of
incorporation or organization) | | (Primary Standard Industrial
Classification Code Number) | | (I.R.S. Employer
Identification Number) |
Wing Fung Alfred Siu
Chief Executive Officer
1/F, Pier 2, Central
Hong Kong, 999077
Tel: +1 (212) 537-4406
(Address, including zip code, and telephone number,
including area code, of registrant’s principal executive offices)
Cogency Global Inc.
122 East 42nd Street, 18th Floor
New York, NY 10168
(212) 947-7200
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Copies to:
Steve Lin
Han Kun Law Offices LLP
Rooms 4301-10, 43/F., Gloucester Tower
The Landmark
15, Queen’s Road Central
Hong Kong
+852 2820 5600
Approximate date of commencement of proposed sale to the public: As soon as practicable after the effective date hereof.
If any of the securities being registered on this form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act, check the following box. ☒
If this form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933.
Emerging growth company ☒
If an emerging growth company that prepares its financial statements in accordance with U.S. GAAP, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act. ☐
The registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act or until the Registration Statement shall become effective on such date as the Commission, acting pursuant to said Section 8(a), may determine.
The information in this prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and is not soliciting an offer to buy these securities in any state where the offer or sale is not permitted.
| PRELIMINARY PROSPECTUS | SUBJECT TO COMPLETION | DATED NOVEMBER 22, 2024 |
40,000,000 Class A Ordinary Shares

NewGenIvf Group Limited
This prospectus relates to the resale by the selling shareholder identified in this prospectus (“Selling Shareholder”) of up to 40,000,000 Class A Ordinary Shares, no par value per share (“Ordinary Shares”) issuable by the Company to White Lion Capital, LLC (“White Lion”) in connection with the Common Stock Purchase Agreement dated as of November 21, 2024, by and between the Company and White Lion (the “White Lion Purchase Agreement”) (the “Resale Shares”).
The Resale Shares will be resold from time to time by the Selling Shareholder listed in the section titled “Selling Shareholder” beginning on page 86.
The Resale Shares represent vastly more than the number of our outstanding Ordinary Shares, and the sales of the Resale Shares, or the perception that those sales might occur, could depress the market price of our Ordinary Shares and could impair our ability to raise capital through the sale of additional equity securities.
The Ordinary Shares to which this prospectus relates may be issued to White Lion pursuant to the White Lion Purchase Agreement, which establishes an equity line of credit. Such Ordinary Shares include (a) up to 39,300,000 Ordinary Shares that we may elect, in our sole discretion, to issue and sell to White Lion from time to time during the Commitment Period (as defined below) under the White Lion Purchase Agreement, (b) 700,000 Ordinary Shares (the “Commitment Shares”) issuable to White Lion as consideration for entering into the White Lion Purchase Agreement. See “Selling Shareholder - White Lion Transaction” below for a description of the White Lion Purchase Agreement and for additional information regarding White Lion.
The actual number of Ordinary Shares issuable to White Lion will vary depending on the then-current market price of Ordinary Shares sold or issuable to White Lion under the White Lion Purchase Agreement and are subject to the further limitations set forth in the White Lion Purchase Agreement.
We are not selling any securities under this prospectus and will not receive any of the proceeds from the sale of the Resale Shares by the Selling Shareholder. Additionally, we will not receive any proceeds from the issuance or sale of the Commitment Shares. However, we may receive gross proceeds of up to $500 million from the sale of our Ordinary Shares to White Lion pursuant to the White Lion Purchase Agreement after the date of this prospectus, subject to certain contingencies as described herein. The actual proceeds from White Lion may be less than this amount, depending on the number of Ordinary Shares sold and the price at which the Ordinary Shares are sold.
The Selling Shareholder, or its respective transferees, pledgees, donees or other successors-in-interest, may sell the Resale Shares through public or private transactions at prevailing market prices, at prices related to prevailing market prices or at privately negotiated prices. The Selling Shareholder may sell any, all or none of the securities offered by this prospectus, and we do not know when or in what amount the Selling Shareholder may sell its Resale Shares hereunder following the effective date of the registration statement of which this prospectus forms a part. We provide more information about how the Selling Shareholder may sell its Resale Shares in the section titled “Plan of Distribution” on page 89. White Lion is an “underwriter” within the meaning of Section 2(a)(11) of the Securities Act.
The Selling Shareholder will pay all brokerage fees and commissions and similar expenses attributable to the sales of the Resale Shares. We will pay the expenses (except brokerage fees and commissions and similar expenses) incurred in registering the Resale Shares, including legal and accounting fees. See “Plan of Distribution.”
Our Ordinary Shares currently trade on The Nasdaq Global Market under the symbol “NIVF.” The last reported closing price of our Ordinary Shares on November 21, 2024 was $0.543. The Company’s common stock may be delisted by Nasdaq on November 20, 2024 for failing to comply with the minimum market value of listed securities set forth in Nasdaq Listing Rule 5450(b)(2)(A). The Company’s common stock may also be delisted by Nasdaq on November 20, 2024 for failing to comply with the minimum market value of publicly held shares set forth in Nasdaq Listing Rule 5450(b)(2)(C). See “Prospectus Summary – Nasdaq Deficiency” and “Risk Factors” regarding this possibility of delisting.
We qualify as a “foreign private issuer,” as defined in Rule 405 under the U.S. Securities Act of 1933, as amended, or the Securities Act, and are eligible for reduced public company reporting requirements.
NewGenIvf Group Limited (“NewGenIvf,” “Company,” “our,” “we,” or “us”) is a British Virgin Islands holding company with our operations conducted through our subsidiaries in the Cayman Islands (our wholly-owned subsidiary, NewGenIvf Limited) and in Asia (Hong Kong, Thailand, Kyrgyzstan, and the Kingdom of Cambodia). Under this holding company structure, investors are purchasing equity interests in NewGenIvf, a British Virgin Islands holding company, and obtaining indirect ownership interests in our Cayman Islands and Asian operating subsidiaries. Substantially all of NewGenIvf’s operations and assets are based in Thailand, Cambodia and Kyrgyzstan. As a result, its businesses and operations are subject to the changing economic conditions prevailing from time to time in such countries.
Investing in our Ordinary Shares involves a high degree of risk, including the risk of losing your entire investment. See “Risk Factors” starting on page 21 to read about the factors you should consider before buying the Ordinary Shares.
Neither the Securities and Exchange Commission, or the SEC, nor any state or other foreign securities commission has approved nor disapproved these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The date of this prospectus is , 2024
TABLE OF CONTENTS
You should rely only on the information contained in this prospectus and any free writing prospectus prepared by or on behalf of us or to which we have referred you. Neither we nor the Selling Shareholder have authorized anyone to provide you with different information. Neither we nor the Selling Shareholder are making an offer of these securities in any jurisdiction where the offer is not permitted. You should not assume that the information in this prospectus or any applicable prospectus supplement is accurate as of any date other than the date of the applicable document. Since the date of this prospectus, our business, financial condition, results of operations and prospects may have changed.
For investors outside of the United States: Neither we nor the Selling Shareholder have done anything that would permit this offering or possession or distribution of this prospectus in any jurisdiction where action for that purpose is required, other than in the United States. You are required to inform yourselves about and to observe any restrictions relating to this offering and the distribution of this prospectus.
In this prospectus, “we,” “us,” “our” and the “Company” refer to NewGenIvf Group Limited and its wholly owned subsidiary, NewGenIvf Limited, a Cayman Islands company.
Our reporting currency is the U.S. dollar. Unless otherwise expressly stated or the context otherwise requires, references in this prospectus to “dollars” or “$” are to U.S. dollars.
This prospectus includes statistical, market and industry data and forecasts which we obtained from publicly available information and independent industry publications and reports that we believe to be reliable sources. These publicly available industry publications and reports generally state that they obtain their information from sources that they believe to be reliable, but they do not guarantee the accuracy or completeness of the information. Although we believe that these sources are reliable, we have not independently verified the information contained in such publications.
Our consolidated financial statements are prepared and presented in accordance with accounting principles generally accepted in the United States of America, or U.S. GAAP.
The number of Ordinary Shares currently issued and outstanding was 10,149,386 as of the date of this prospectus. No new shares are being issued by the Company pursuant to this offering.
ABOUT THIS PROSPECTUS
This prospectus describes the general manner in which the Selling Shareholder identified in this prospectus may offer from time to time up to 40,000,000 Ordinary Shares through any means described in the section entitled “Plan of Distribution.” You should rely only on the information contained in this prospectus and the related exhibits, any prospectus supplement or amendment thereto and the documents incorporated by reference, or to which we have referred you, before making your investment decision. Neither we nor the Selling Shareholder have authorized anyone to provide you with different information. If anyone provides you with different or inconsistent information, you should not rely on it. This prospectus, any prospectus supplement or amendments thereto do not constitute an offer to sell, or a solicitation of an offer to purchase, the Resale Shares offered by this prospectus, any prospectus supplement or amendments thereto in any jurisdiction to or from any person to whom or from whom it is unlawful to make such offer or solicitation of an offer in such jurisdiction. You should not assume that the information contained in this prospectus, any prospectus supplement or amendments thereto, as well as information we have previously filed with the U.S. Securities and Exchange Commission (the “SEC”), is accurate as of any date other than the date on the front cover of the applicable document. The information contained in this prospectus is current only as of the date on the front cover of the prospectus. Our business, financial condition, results of operations, and prospects may have changed since that date.
If necessary, the specific manner in which the Ordinary Shares may be offered and sold will be described in a supplement to this prospectus, which supplement may also add, update or change any of the information contained in this prospectus. To the extent there is a conflict between the information contained in this prospectus and the prospectus supplement, you should rely on the information in the prospectus supplement, provided that if any statement in one of these documents is inconsistent with a statement in another document having a later date—for example, any prospectus supplement—the statement in the document having the later date modifies or supersedes the earlier statement.
GLOSSARY OF DEFINED TERMS
In this prospectus, unless otherwise indicated or the context otherwise requires, references to:
“ASCA” means A SPAC I Acquisition Corp., a British Virgin Islands business company.
“A SPAC I Mini Acquisition Corp.” means A SPAC I Mini Acquisition Corp., a British Virgin Islands business company.
“Business Combination” means the transactions contemplated by the Merger Agreement, pursuant to which (i) ASCA reincorporated to the British Virgin Islands by merging with and into the Company; and (ii) Merger Sub merged with and into Legacy NewGenIvf, resulting in Legacy NewGenIvf being a wholly-owned subsidiary of the Company.
“BVI” means British Virgin Islands.
“BVI Act” means BVI Business Companies Act (As Revised).
“Class A Ordinary Share” means Class A ordinary shares of the Company, no par value per share.
“Class B Ordinary Share” means (x) the Company’s Class B ordinary shares with no par value per share, and (y) any shares into which such ordinary shares shall have been changed or any shares resulting from a reclassification of such ordinary shares.
“Closing” means the consummation of the Business Combination, which occurred on April 3, 2024.
“Company” means NewGenIvf Group Limited, a British Virgin Islands business company, the surviving entity of the Business Combination.
“Legacy NewGenIvf” means NewGenIvf Limited, a Cayman Islands exempted company, which became a wholly owned subsidiary of ASCA upon the Closing.
“Merger Agreement” means the Merger Agreement entered into on February 15, 2023, and as amended on June 12, 2023 and December 6, 2023, between ASCA, A SPAC I Mini Acquisition Corp., Merger Sub, Legacy NewGenIvf, and certain shareholders of Legacy NewGenIvf, pursuant to which the Reincorporation Merger and Acquisition Merger were consummated.
“Merger Sub” means A SPAC I Mini Sub Acquisition Corp., a Cayman Islands exempted company and former wholly-owned subsidiary of A SPAC I Mini Acquisition Corp.
“Memorandum and Articles of Association” means the Company’s Amended and Restated Memorandum and Articles of Association, as and restated on September 23, 2024.
“NewGenIvf” means NewGenIvf Group Limited, a British Virgin Islands business company, the surviving entity of the Business Combination, unless the context so requires.
“Ordinary Shares” means the Class A Ordinary Shares.
“Preferred Shares” means preferred shares of the Company, no par value per share.
“Reincorporation Merger” means the first step of the Business Combination which occurred pursuant to the Merger Agreement, in which ASCA reincorporated to the British Virgin Islands by merging with and into A SPAC I Mini Acquisition Corp.
PROSPECTUS SUMMARY
This summary highlights information contained elsewhere in this prospectus. This summary does not contain all of the information you should consider before investing in our securities. Before you decide to invest in our securities, you should read the entire prospectus carefully, including the “Risk Factors” section and the financial statements and related notes appearing at the end of this prospectus.
Unless the context otherwise requires, all references in this Prospectus Summary to “NewGenIvf,” “we,” “our,” and “us” refer to Legacy NewGenIvf and its subsidiaries as they existed prior to the Closing if described in relation to a date prior to April 3, 2024. Any references to “NewGenIvf,” “we,” “our,” and “us” with respect to the present time, a future time, or a date after April 3, 2024 refers to NewGenIvf, a British Virgin Islands company, and its subsidiaries, whose existence continued after the Closing.
Overview
We are an assisted reproductive services (“ARS”) provider in Asia-Pacific. Since the opening of our first clinic in Thailand in 2014, we have established ourself as a long-standing ARS provider in this region. Our strategic presence in Thailand, Cambodia, and Kyrgyzstan positions us to take advantage of opportunities across Asia-Pacific. According to China Insights Consultancy (“CIC”), from 2014 to 2022, there was a rising number of women in the key ARS-targeted age group (ages 15 to 49) in Asia Pacific and a growing trend towards later maternal age. The number of married women of reproductive age in Asia Pacific has risen from 816.4 million in 2014 to 833.2 million in 2022. Additionally, according to CIC, there was increasing social acceptance of ARS use in Asia Pacific countries such as China, India, and Thailand during the same period. For example, the number of ARS users in China has risen from 136.8 thousand in 2017 to 184.9 thousand in 2022 approximately and that in Japan has risen from 98.0 thousand in 2017 to 128.5 thousand in 2022.
According to CIC, the prevalence of infertility in Asia-Pacific developing countries is substantial. For example, the infertility rate in Thailand, India and China was about 15.4%, 13.8% and 17.8%, respectively, in 2022. In India, the infertility rate in 2020 was approximately 13.1%, representing an annual growth of 2.6%. The infertility rate in China was around 17.6% in 2020, representing an annual growth of 0.6%. Infertility is increasingly gaining society’s attention as individuals are more openly discussing their struggles. Despite the prevalence of infertility, access to treatment is often limited in the Asia Pacific region. According to CIC, financial challenges, costs of treatment, and limited availability or capacity of fertility medical care are some of the main challenges in the fertility marketplace in Asia-Pacific region. Religious, social and cultural roadblocks can also prevent hopeful couples from realizing their dream to have children. We believe that we can help address some of these key challenges of Asia-Pacific fertility industry.
History and Development of the Company
Prior to the Business Combination, on April 29, 2021, A SPAC I Acquisition Corp. (“ASCA”), was incorporated as a British Virgin Islands business company, specifically a blank check company formed for the purpose of effecting a merger, share exchange, asset acquisition, share purchase, recapitalization, reorganization or similar business combination with one or more target businesses.
The Business Combination
On February 15, 2023, ASCA entered into the Merger Agreement (as amended on June 12, 2023 and December 6, 2023, the “Merger Agreement,” and the transactions contemplated thereunder, the “Business Combination”) with A SPAC I Mini Acquisition Corp., Merger Sub, NewGenIvf Limited, a Cayman Islands exempted company (“Legacy NewGenIvf”) and certain shareholders of Legacy NewGenIvf. Pursuant to the Merger Agreement, the Business Combination was effected in two steps: (i) ASCA was reincorporated to the British Virgin Islands by merging with and into A SPAC I Mini Acquisition Corp. (such transaction, the “Reincorporation Merger”); and (ii) Merger Sub merged with and into Legacy NewGenIvf, resulting in Legacy NewGenIvf being a wholly-owned subsidiary of the Company (such second step in isolation, the “Acquisition Merger”). The surviving entity of the Business Combination, together with its subsidiaries is referred to in this prospectus as “NewGenIvf,” the “Company,” “we,” “our,” or “us,” unless the context otherwise requires.
On June 12, 2023, the parties to the Merger Agreement entered into the First Amendment to Merger Agreement (the “First Amendment”), pursuant to which Legacy NewGenIvf agreed to provide non-interest bearing loans in an aggregate principal amount of up to $560,000 (the “Loan”) to ASCA to fund any amount that would be required in order to further extend the period of time available for ASCA to consummate a business combination and for ASCA’s working capital, payment of professional, administrative and operational fees and expenses, and other purposes as mutually agreed by ASCA and Legacy NewGenIvf. Such loans were to become repayable upon the closing of the Acquisition Merger. In addition, pursuant to the First Amendment, subject to receipt of at least $140,000 as part of the Loan from NewGenIvf, ASCA agreed to waive its termination rights and the right to receive any break-up fee due to Legacy NewGenIvf’s failure to deliver audited financial statements by no later than February 28, 2023.
On December 6, 2023, the parties to the Merger Agreement entered into the Second Amendment to the Merger Agreement (the “Second Amendment”) which amended and modified the Merger Agreement to, among other things, (i) reduce the size of NewGenIvf’s board of directors following the consummation of the Business Combination to five (5) directors, two (2) of whom would be executive directors designated by NewGenIvf and three (3) of whom will be designated by NewGenIvf to serve as independent directors in accordance with Nasdaq requirements, (ii) provide for the conversion of NewGenIvf shares issued by NewGenIvf following the original date of the Merger Agreement into Class A Ordinary Shares in connection with the Acquisition Merger, and (iii) remove the condition that ASCA have in excess of $5,000,000 in net tangible assets immediately after the consummation of the Business Combination.
On April 3, 2024, the Business Combination was consummated with the Company as the surviving entity.
NewGenIvf’s Business
With a focus on providing fertility treatments to fulfil the dreams of building families, NewGenIvf mainly offers two services, namely: (i) in vitro fertilization (“IVF”) treatment service, comprising traditional IVF and egg donation; and (ii) surrogacy and ancillary caring services. Currently, we have three clinics: one clinic in Thailand, one clinic in Cambodia, and one clinic in Kyrgyzstan.
| | ● | IVF treatment service: For the years ended December 31, 2023 and 2022, we generated approximately 78.3% and 47.4%, of its revenue from IVF treatments services. We primarily provide our clients with conventional IVF/intracytoplasmic sperm injection (“ICSI”) and embryo transfer services. As technology has progressively advanced, we have been able to, through technologies and facilities provided by MicroSort technology, help fulfill the family-balancing dreams of its clients and avoiding certain gender-related hereditary diseases. IVF treatment involves the performance of a series of medical treatment and procedures that are not separately distinct and only brings benefits to clients when embryo is successfully implanted, therefore revenue from IVF treatment is recognized at a point in time when it is completed in clinic. The completion of this treatment is evidenced by a written IVF report indicating successful embryo implantation. |
| | ● | Surrogacy and ancillary caring services: We also generate revenue from surrogacy services and related ancillary caring services in Kyrgyzstan. For the years ended December 31, 2023 and 2022, we generated approximately 21.7% and 52.6%, of our revenue from surrogacy and ancillary caring services. For surrogacy services, NewGenIvf conducts implantation of embryos from biological parents in surrogate mothers. In addition, NewGenIvf provides a “success guarantee” program for egg donation services in Cambodia and surrogacy services in Kyrgyzstan. Under this optional program, patients pay additional fees of approximately 40% of the original price and can have repeated attempts of IVF cycles, egg donation services and/or surrogacy services until the procedures are successful. The additional costs to NewGenIvf are generally limited and amount to approximately 30% of the original costs because NewGenIvf’s clinics, together with the patients, can choose suitable egg donors and surrogate mothers to limit the additional costs. During the pregnancy period, NewGenIvf provides ancillary caring services including regular body check and provision of vitamins, supplements and medicines to surrogate mothers. Revenue from surrogacy and ancillary caring services is recognized at a point in time when the surrogate mother gives birth. Surrogacy services provide infertile couples with an alternative method of having children. |
For the years ended December 31, 2023 and 2022, NewGenIvf’s revenue was US$5,136,153 and US$5,944,190, and its net income was US$108,418 and US$135,847, respectively.
Market Opportunity
According to CIC, NewGenIvf’s core market for fertility services is substantial and growing rapidly, driven by, among other things, societal and cultural shifts, such as people starting families later in life and other health-related challenges which could impact couples’ and individuals’ ability to have children. In addition, NewGenIvf believes that continued overall de-stigmatization of infertility will help drive better access to, and stronger demand for, fertility treatment services, thereby further enabling the expansion of NewGenIvf’s addressable market. According to CIC, the market size of fertility treatments in Asia Pacific was increasing steadily and the potential size of the Asia fertility market is expected to reach US$37.4 billion by 2030. NewGenIvf believes its market opportunity is substantial and is continuing to grow as a result of the rising demand for fertility services, the lack of adequate offerings in the market and the increasing awareness of the challenges of infertility.
Competitive Strengths
NewGenIvf believes that the following competitive strengths have positioned it to meet growing opportunities in the fertility market across Asia-Pacific, and have differentiated it from its competitors:
Broad-range ARS Provider Offering Comprehensive Fertility Treatment Services
With almost a decade of experience in the fertility market, NewGenIvf has built a reputation in the IVF industry in Asia-Pacific. NewGenIvf has reinforced its long-standing position through expanding its service offerings and locations to address the evolving clients’ needs or requests.
NewGenIvf’s comprehensive fertility treatment offerings in Thailand, Cambodia, and Kyrgyzstan, primarily including IVF, egg donation (in Cambodia) and surrogacy services (in Kyrgyzstan), make it convenient for clients in Asia-Pacific market to have access to various fertility services but with a relatively low cost, as compared with the US market. According to CIC, the average cost per IVF cycle in the US is around US$12,000 (excluding medication), which is 65% higher than that of Asia-Pacific market. Meanwhile, the average cost per IVF cycle by NewGenIvf is around US$7,000 (excluding medication). Each of NewGenIvf’s clinics in Thailand, Cambodia, and Kyrgyzstan has its own specialty, and together, NewGenIvf is able to provide more flexibility and options to its patients. For example, NewGenIvf’s Thailand clinic focus on IVF and related ancillary services including HIV sperm washing, egg freezing, and chromosome screening. The clinic in Cambodia specializes in providing both IVF services and egg donation services. NewGenIvf opened the clinic in Kyrgyzstan in 2019, which broadened NewGenIvf’s services by being legally qualified/received approval letter from The Ministry of Health of Kyrgyzstan to offer surrogacy services. As of December 31, 2023, NewGenIvf was the one of the few ARS providers in Kyrgyzstan and one of the few companies in Kyrgyzstan that is licensed to offer surrogacy services in Kyrgyzstan.
NewGenIvf attributes its track record of success to its experienced physicians and its ability to provide comprehensive ARS services, allowing it to meet patients’ increasing demand for advanced, high-end, and sophisticated ARS, a higher standard and a wider range of advanced services.
NewGenIvf has extensive experience serving Asia-Pacific patients and a deep understanding of their general profiles. In particular, NewGenIvf has personnel speaking multiple languages, including nurses, facilitators, and translators, who are familiar with the health condition and culture of Asia-Pacific patients from different countries in the region. NewGenIvf believes that it is therefore well-positioned to benefit from market growth driven by Asia-Pacific patients travelling to its clinics for treatment.
Attractive Market with Significant Demand and Fast Growth
NewGenIvf operates in the ARS market in Asia Pacific, positioning it to leverage on an attractive market with compelling underlying growth potential. According to CIC, during the years ended December 31, 2021 and 2022, the ARS market in Asia Pacific has experienced growth underpinned by long-term demographic and social trends. These trends include a rising demand for fertility services, the lack of adequate offerings in the market and the increasing awareness of the challenges of infertility, according to CIC.
According to CIC, the Asia Pacific ARS market is a large, multi-billion dollar industry growing at a strong pace of approximately 15% in 2022 as increased awareness and acceptance of IVF and surrogacy services continue to drive demand. Additionally, according to CIC, the market is underserved as a substantial percentage of patients in need of ARS treatments go untreated. The industry also remains constrained in capacity, thereby creating challenges in providing access to ARS to the volume of patients in need. According to CIC, as of December 31, 2022, there were more than 213 million infertile couples in Asia Pacific. While there have been substantial increases in the use of ARS, according to CIC, only approximately 1.47 million ARS cycles, including IVF, and other fertility treatments, were performed in Asia Pacific in 2022. This amounts to less than 1.1% of the infertile couples in Asia Pacific being treated and only 0.7% having a child though ARS in 2022, indicating significant unmet demand for ARS.
Asia-Pacific fertility markets, in particular India and China, present a vast opportunity for ARS providers in the region. China’s ARS market has been driven by an increasing rate of infertility, the implementation of the Three-Child Policy in May 2021, a decreasing number of couples at childbearing age and increasing affordability and awareness of ARS, according to CIC. China’s ARS market size in 2021 and 2022 was US$2,105 million and US$2,069 million, respectively, and is expected to further grow to US$2.3 billion in 2023, according to CIC. India’s ARS market size increased from US$1.2 billion in 2021 to US$1.5 billion in 2022, and is expected to grow further to US$1.6 billion in 2023, according to CIC. NewGenIvf believes that its existing market presence and reputation in Thailand, Cambodia, and Kyrgyzstan well positions it to capitalize on the fast-growing Asia-Pacific fertility market.
According to CIC, the significant entry barriers in Asia-Pacific ARS industry are expected to continue to constrain supply in the industry. The industry is heavily regulated and a significant number of stringent requirements must be satisfied in order to obtain relevant licenses to conduct IVF, egg donation and surrogacy procedures in the relevant countries. NewGenIvf believes that such barriers to entry can help it maintain its market position in Asia Pacific as the fertility market in the region continues to expand.
Built on years of experience, NewGenIvf has established a strong reputation in its industry, which in turn attracted potential business partners to approach NewGenIvf to negotiate cooperations and referrals. Over the years, NewGenIvf sends representatives to medical expos mostly held in the PRC to approach potential business partners and establish new partnerships by entering into agency agreements with each agent. NewGenIvf has become a significant partner with approximately 90 fertility service agents in China as well as in India. Normally, each agency agreement has a maximum term of one year, which is renewable upon mutual agreement. Agents typically market and promote NewGenIvf’s services by word-to-mouth referrals and other measures and NewGenIvf pays the agents commission at a range of 10% to 25% of the treatment fees upon the completion of client’s treatment. Normally, agents provide potential clients’ contact information to the sales team of NewGenIvf, who then approach potential clients and provide consultation on services. Overall, approximately 50% of NewGenIvf’s patients are referrals from agents, among which approximately 80% are referrals from China and the remaining 20% from India, whereas the remaining 50% of NewGenIvf’s patients are patients who contact NewGenIvf directly through its websites from social media promotions. With its partnerships in various countries, NewGenIvf believes it is able to better benefit from the growing market opportunities.
Exclusively Licensed Technology for Family Planning and Access to Mature Fertility Technologies
NewGenIvf believes that its licenses and/or access to mature technologies contribute to its ability to identify and tailor ARS services to individual patient’s needs. These technologies include:
| | ● | MicroSort Technology: NewGenIvf holds an exclusive license granted by a division of the Genetics and IVF Institute, to use MicroSort technology in Thailand and Cambodia, which is a form of pre-conception gender selection technology for humans. MicroSort technology aims to separate male sperm cells based on which gender chromosome they contain, which results in separated semen samples that contain a higher percentage of sperm cells that carry the same gender chromosome. The technology ultimately helps couples choose the gender of their future child by choosing semen samples that predominately contain sperm with the X chromosome for a female or Y chromosome for a male. Traditionally and naturally, gender selection occurs after conception, meaning after the eggs are fertilized. As a result, some fertilized eggs will go unused. However, with MicroSort technology, NewGenIvf is able to increase the ratio of male or female embryos, based on the patient’s preference. Eggs are more likely to be fertilized according to the preferences of the parents. Other improvements that MicroSort treatment could help achieve include prevention of certain gender-related hereditary diseases. As of December 31, 2023, NewGenIvf was one of the only seven exclusive license holders of MicroSort technology world-wide. |
| | ● | Preimplantation Genetic Screening (“PGS”): PGS is used in parallel with an IVF treatment cycle. PGS is the practice of determining the presence of aneuploidy (either too many or too few chromosomes) in a developing embryo. PGS improves success rates of in vitro fertilization by ensuring the transfer of euploid embryos that have a higher chance of implantation and resulting in a live birth. PGS has improved clinical outcomes for NewGenIvf by achieving a higher implantation rate of 70.9% and reducing miscarriage rates by 26.6%. |
| | ● | Next-Generation Sequencing (“NGS”): NGS is a high-throughput technology for determining the sequence of deoxyribonucleic acid (“DNA”) or ribonucleic acid (“RNA”) to study genetic variation associated with diseases or other biological phenomena. NGS determines the sequence of a sample all at once by using parallel sequencing. Traditional Sanger sequencing determines the sequence of a sample one section at a time. Sequencing thousands of gene fragments simultaneously with NGS reduces time and cost associated with sequencing and increases the coverage quality and data output. |
| | ● | Preimplantation Genetic Diagnosis (“PGD”): Similar to PGS, PGD is also used in parallel with an IVF treatment cycle. But PGD is a process more enhanced than PGS since it scans for individual genes. PGD is the practice of evaluating embryos for specific genetic abnormalities, such as sickle cell disease or cystic fibrosis, where carrier status has been documented in each of the parents. By using this technique, physicians are able to check the genes or chromosomes for a specific genetic condition. PGD can decrease the risk of miscarriage and this technology can help women better achieve a healthy pregnancy. Individuals who suspect or know they carry genes for serious medical conditions may opt to screen for healthy embryos ahead of time. |
Well Established Brand with Reliable Reputation
The founders of NewGenIvf entered the fertility market as agents in 2011 by introducing patients in need to a Thailand clinic for fertility treatments. The founders of NewGenIvf started to operate their own clinic in Thailand in 2014 and subsequently added clinics in Cambodia and Kyrgyzstan. Since then, NewGenIvf has attracted clients from countries throughout Asia-Pacific, including Mainland China, Hong Kong, India, Thailand, Australia and Taiwan.
NewGenIvf benefits from the favourable geographic locations of its clinics, especially its clinic in Thailand. Located in central Bangkok and situated in one of the biggest shopping malls of the city, the clinic is located in close proximity to various transportation facilities and popular tourist attractions, such as the Erawan Shrine. In this regard, NewGenIvf believes that its business has benefited from, and will continue to benefit from, the convenience of its locations.
NewGenIvf has developed a relatively replicable and scalable operating model that supports high productivity at its assisted reproductive medical facilities in Asia. Under this model, NewGenIvf’s medical facilities have established standardized operating procedures to select the treatment process according to each patient’s profile. NewGenIvf’s medical and operational personnel are organized into specialized teams according to the different stages of the treatment process and different patient profiles. When patients are initially admitted or would like to seek additional medical services later on, they are assigned to one of the optimal medical teams, which NewGenIvf believes is better suited after taking into account the patient’s diagnosis and preferences. NewGenIvf believes that this model allows each team to improve its efficiency and arrange suitable physicians for patients.
The physicians of NewGenIvf have also developed and employed an operating model that seeks to increase the effectiveness of physicians by utilizing standardized workflows and operating procedures with teams of supporting nurses and medical assistants. This helps to increase the number of IVF treatment cycles that physicians can perform while providing treatment customized based on patient conditions.
With its established client service history, accumulated experience as well as its continuous upgrades and development of treatment models, NewGenIvf believes that it will be able to better monetize its brands through its business.
Experienced Management Team
The NewGenIvf management team has considerable experience in the ARS market and the broader healthcare industry. A considerable number of NewGenIvf’s management are physicians or laboratory technicians who possess extensive experience in the ARS industry and are experts in their respective fields. NewGenIvf’s Chief Executive Officer, Mr. Alfred Siu, has more than 13 years of experience in the fertility service market. Dr. Wiphawee Luangtangvarodom had over 8 years of experience as an obstetrician and gynecologist. NewGenIvf’s two lab supervisors, Ms. Anussara Phinyong, and Ms. Araya Boonchaisitthipong, each had over eight years of experience in the embryologist field. These individuals have extensive experience in managing assisted reproductive medical facilities. NewGenIvf is also led by other members of the professional management team, who are intimately involved in the operational and financial management of NewGenIvf’s Group. Leveraging their experience, NewGenIvf believes that it is well positioned to expand its network and aims to become a leader in the Asia Pacific ARS market.
Strategies
NewGenIvf’s vision is to provide tailored ARS solutions to fulfil patients’ dreams of becoming a parent. To realize this vision, NewGenIvf plans to adopt the following strategies:
Offer Broad Fertility Services for Fertility Tourists across Asia Pacific
NewGenIvf intends to provide broad fertility services for fertility tourists seeking high quality, cost effective and comprehensive fertility solutions. According to CIC, the demand for fertility tourism is driven by a variety of factors including the prevalence of infertility, the introduction of the Three-Child policy in China, the improved understanding of assisted reproductive technology and increased affordability of ARS. To address these needs, NewGenIvf plans to offer its customers a “hassle-free”, seamless and integrated ARS and hospitality arrangement experience. To complement its fertility services, NewGenIvf intends to integrate its offerings with additional services for traveling patients, most of whom are first-time fertility tourists, such as translation service, hotel arrangement and airport pickup services. NewGenIvf plans to enhance its customers’ experience by entering into exclusive cooperation arrangements with local premium hospitality providers.
Furthermore, NewGenIvf expects the easing of COVID-19 travel restrictions to contribute to an increase in tourists seeking fertility services. According to CIC, the COVID-19 pandemic led to a delay in many patients’ plans for fertility treatments, with travel restrictions and border closures impacting their ability to access care. On May 5, 2023, the WHO Director-General Dr. Tedros Adhanom Ghebreyesus announced that COVID-19 no longer constituted a public health emergency of international concern. The pent-up demand for these services is expected to be released with the lifting of the travel restrictions, leading to a surge in patients seeking fertility treatment. NewGenIvf’s believes that its strategy of offering a comprehensive approach to fertility treatments will help it capture a share of the growing market for fertility tourism in Asia Pacific.
Continue to Invest in Laboratories and Facilities
NewGenIvf believes laboratories and treatment facilities are critical to supporting its future research, development and clients experience. NewGenIvf currently operates two laboratories that offer IVF services, one in Thailand and one in Cambodia, and plans to continue to scale up its existing laboratories. NewGenIvf plans to continue to invest in upgrading its laboratories and facilities to complement its growth and expansion, which it believes will help NewGenIvf maintain an edge over its competitors with regard to technology, operational efficiency, scalability, and client experience.
NewGenIvf intends to develop advanced facilities for its existing laboratories, which will be conducting research on ARS related basic science and experiments relating to emerging technologies to improve ARS success rates and lower costs. NewGenIvf also plans to correlate its data on patient treatment protocols to the embryo physiologic data and the pregnancy success rate-related data to identify better treatment protocols to increase ARS success rates. NewGenIvf intends to continue to actively promote technological cooperation with tertiary institutions to discover ways to improve its IVF success rates. Furthermore, NewGenIvf seeks to actively deploy the technology that it possesses to expand the services it provides.
NewGenIvf has accumulated experience in treating patients over 40 years old with premature ovarian failure and patients who have had recurrent ARS implementation failure, by, for the example, injecting platelet rich plasma into the ovaries to stimulate and support growth of the follicles. NewGenIvf is also implementing certain technological advancements relevant to the ARS industry, including microfluidics, automated sperm analysers, time lapsed incubators, non-invasive preimplantation genetic testing (“PGT”) of cell-free DNA in spent media, automated systems for oocyte/embryo vitrification to reduce reagent consumption and decrease labor intensity, mitochondria replacement therapy to reconstruct oocytes by nuclear transfer of polar body genome from an MII oocyte into an enucleated donor MII cytoplasm, to increase the number of oocytes available for the treatment of infertile women, preimplantation methylome screening. There are also breakthrough developments in science including organ culture systems, induced pluripotent stem cells, embryonic stem cells, spermatogonial stem cells for creation of functional gametes, but these techniques are not yet ready for human clinical trials.
NewGenIvf also intends to develop clinically customised interior design concepts for its medical facilities, including improved service rooms, consultation rooms, reception areas, nutrition food areas, and traditional Chinese medicine (such as acupuncture) facilities.
Increase Brand Awareness and Market Share
NewGenIvf intends to maintain and strengthen its brand awareness and market share in Asia Pacific. In order to expand its reach and increase patient numbers, NewGenIvf plans to collaborate with local hospitals, companies, premium hospitality providers and other key players in the ARS industry in Asia Pacific. Additionally, NewGenIvf intends to increase brand awareness through social media promotions and marketing initiatives, and establishing its business development team with the goal of attracting new patients and partners across Asia Pacific. Meanwhile, NewGenIvf intends to provide innovative treatment services to attract more clients. For example, NewGenIvf plans to introduce IVF mental health services, which allows clients who fail in IVF treatments to access online consultation for further treatment plans such as egg donation and surrogacy. These new treatments services aim to enable NewGenIvf to attract potential clients. By adopting a comprehensive strategy to expand its market share, NewGenIvf aims to strengthen its reputation as a long-standing ARS provider and capture additional market share of the growingly ARS market in Asia-Pacific.
Expand Service Reach Through Acquisitions and Partnerships
Leveraging its reputation and footprint in its current markets, NewGenIvf intends to expand its reach, services offering and client base through strategic acquisitions and/or partnerships in Asia Pacific. Acquisitions of or by companies offering similar services could not only allow NewGenIvf to diversify its client base, but also allow it to benefit from potential economies of scale and increasing efficiency through consolidation. NewGenIvf could also leverage the acquired or acquiring company’s customer base, reputation and expertise to further improve its offerings and operations. NewGenIvf intends to focus on ARS providers in Asia Pacific which possess all conventional licenses and locally recognized brands. For the global market beyond Asia Pacific, NewGenIvf intends to expand its footprint through partnerships with other IVF clinics.
In addition, NewGenIvf plans to explore expanding its client base by offering its fertility services as part of corporate benefit programs in Asia. NewGenIvf believes that there is potential in Asia in offering fertility treatments as a benefit for employees, particularly in companies with a large number of female employees of childbearing age. By partnering with corporate clients to provide fertility benefits, NewGenIvf can increase its market reach, enhance its brand reputation, and drive client growth. NewGenIvf’s broad range of fertility services, including IVF and egg freezing, can help corporate partners differentiate their employee benefits in the competitive employment landscape, which could make them more attractive to potential employees. Additionally, by offering these services, companies can help address the growing concern of delayed childbearing, which is becoming more common among women according to CIC. NewGenIvf plans to collaborate with potential corporate clients to develop customized fertility benefit programs that cater to their specific needs, and to provide comprehensive support and counselling throughout the process.
Meanwhile, NewGenIvf also intends to attract more clients by establishing its “home country gynecologist partnership program”. Under the program, NewGenIvf may, subject to its discretion and screening process, offer treatment services to clients with reduced time requirements to be spent overseas. Depending on local laws, the potential clients may be able to complete their treatments with gynecologists NewGenIvf partners with, in their home countries.
NewGenIvf had entered into a non-binding term sheet dated June 3, 2024 (the “Term Sheet”) with COVIRIX Medical Pty Ltd (“COVIRIX”) for a proposed reverse merger (the “Proposed Transaction”). However, on September 21, 2024, COVIRIX withdrew from the Proposed Transaction, as such the Proposed Transaction was terminated with no cost to the Company.
White Lion Transaction
On November 21, 2024, the Company entered into a Common Shares Purchase Agreement (the “White Lion Purchase Agreement”) with White Lion Capital, LLC (“White Lion”) and a related Registration Rights Agreement (the “RRA”). Pursuant to the White Lion Purchase Agreement, the Company has the right, but not the obligation, to require White Lion to purchase, from time to time, up to One Hundred Million Dollars ($100,000,000) in aggregate gross purchase price of newly issued Ordinary Shares, with an automatic increase to Three Hundred Million Dollars ($300,000,000) upon any substantial M&A or Material Transaction (as defined in the White Lion Purchase Agreement) and a further option to increase to Five Hundred Million Dollars ($500,000,000) after Two Hundred and Fifty Million Dollars ($250,000,000) has been issued and sold to White Lion under the White Lion Purchase Agreement, subject to certain limitations and conditions set forth in the White Lion Purchase Agreement.
Subject to the satisfaction of certain customary conditions including, without limitation, the effectiveness of the registration statement of which this prospectus forms a part (the “Registration Statement”) registering the resale of the shares issuable pursuant to the White Lion Purchase Agreement, the Company’s right to sell shares to White Lion commenced on the date of the execution of White Lion Purchase Agreement and extends until (i) 36 months from the date of execution of the White Lion Purchase Agreement, or (ii) at the Company’s option, until 65 months from the date of the execution of the White Lion Purchase Agreement in the event that $100,000,000 of purchases under the White Lion Purchase Agreement have been completed prior to the 36 month anniversary of the Execution Date (the “Commitment Period”).
During the Commitment Period, subject to the terms and conditions of the White Lion Purchase Agreement, the Company may exercise its right to sell its Ordinary Shares. The Company may deliver a Regular Purchase Notice (as such term is defined in the White Lion Purchase Agreement), pursuant to which the Company can require White Lion to purchase up to a number of Ordinary Shares equal to the lesser of (i) $3,000,000 divided by the highest closing price of the Ordinary Shares over the most recent five (5) Business Days immediately preceding the Purchase Notice, or (ii) 40% of Average Daily Trading Volume (as such term is defined in the White Lion Purchase Agreement), subject to a maximum Investment Limit of $3,000,000.
The Company may also deliver a Rapid Purchase Notice (as such term is defined in the White Lion Purchase Agreement), pursuant to which the Company may require White Lion to purchase up to a number of Ordinary Shares equal to $3,000,000 divided by the highest closing price of the Ordinary Shares over the most recent five business days immediately prior to the receipt of the notice. White Lion may waive such limits under any notice at its discretion and purchase additional shares.
The price to be paid by White Lion for any shares that the Company requires White Lion to purchase will depend on the type of purchase notice that the Company delivers. For shares being issued pursuant to a Regular Purchase Notice, the purchase price per share will be the lower of (i) the closing price of Ordinary Shares prior to the receipt of the applicable Purchase Notice, or (ii) the product of (a) the lowest daily VWAP of the Ordinary Shares during the Regular Purchase Valuation Period (as defined in the White Lion Purchase Agreement), and (b) 98%.
For shares being issued pursuant to a Rapid Purchase Notice, the Company may opt for the purchase price per share to be (i) equal to the lowest traded price of the Ordinary Shares on the date that the notice is delivered, or (ii) 97% of the lowest traded price of the Ordinary Shares two hours following White Lion’s written confirmation of the acceptance of the Rapid Purchase Notice.
No purchase notice shall result in White Lion beneficially owning (as calculated pursuant to Section 13(d) of the Securities Exchange Act of 1934, as amended, and Rule 13d-3 thereunder) more than 4.99% (subject to increase, in the sole discretion of White Lion, to 9.99%) of the number of Ordinary Shares outstanding immediately prior to the issuance of Ordinary Shares issuable pursuant to a purchase notice.
The Company has the right to terminate the White Lion Purchase Agreement in the event of a material breach of the White Lion Purchase Agreement by White Lion. The White Lion Purchase Agreement also automatically terminates upon the earlier of (i) the end of the Commitment Period and (ii) the date that the Company commences a voluntary bankruptcy proceeding, a custodian is appointed for the Company or for all or substantially all of its property, or the Company makes a general assignment for the benefit of its creditors.
In consideration for the commitments of White Lion, as described above, the Company has agreed that it will issue to White Lion 700,000 Common Shares (“Commitment Shares”). In addition, the Company has agreed that (i) if the Company fails to sign a binding term sheet for a Material Transaction within 90 days of the execution of the White Lion Purchase Agreement, it will issue to White Lion an additional 100,000 Common Shares; (ii) upon the completion of a Material Transaction (as defined in the White Lion Purchase Agreement), it will issue an additional amount of Ordinary Shares equal to $500,000 divided by the closing price of the Ordinary Shares on the date of the public filing announcing the closing of the Material Transaction; and (iii) in the event the gross investment by White Lion reaches $250,000,000, the Company shall issue an additional amount of Ordinary Shares equal to $250,000 divided by the closing price of the Ordinary Shares on the Closing Date the gross investment reaches $250,000,000. The Commitment Shares will be fully earned by White Lion regardless of termination of the White Lion Purchase Agreement.
Concurrently with the White Lion Purchase Agreement, the Company entered into the RRA with White Lion, pursuant to which the Company agreed to file, within 10 business days following the execution of the White Lion Purchase Agreement, the Registration Statement with the SEC covering the resale by White Lion of the number of shares determined appropriate by the Company and permitted to be included therein in accordance with applicable SEC rules, regulations and interpretations and the Commitment Shares. The RRA also contains usual and customary damages provisions for failure to file and failure to have the Registration Statement declared effective by the SEC within the time periods specified therein.
The White Lion Purchase Agreement and the RRA contain customary representations, warranties, conditions and indemnification obligations of the parties. The representations, warranties and covenants contained in such agreements were made only for purposes of such agreements and as of specific dates, were solely for the benefit of the parties to such agreements and may be subject to limitations agreed upon by the contracting parties.
Business Model
With a focus on providing fertility treatments to fulfil couples and individuals’ dreams of raising children, NewGenIvf offers mainly two services, namely: (i) IVF treatment service, comprising traditional IVF and egg donation; and (ii) surrogacy and ancillary caring services. The following table sets forth NewGenIvf’s revenue by service offerings and as a percentage of total revenue for the periods indicated:
| | | For the Year ended December 31, | |
| | | 2023 | | | 2022 | |
| | | US$ | | | % | | | US$ | | | % | |
| IVF Treatment Service | | | 4,021,696 | | | | 78.3 | | | | 2,819,163 | | | | 47.4 | |
| Surrogacy and Ancillary Caring Services | | | 1,114,457 | | | | 21.7 | | | | 3,125,027 | | | | 52.6 | |
| Total Revenue | | | 5,136,153 | | | | 100.0 | | | | 5,944,190 | | | | 100.0 | |
IVF Treatment Service
NewGenIvf primarily provides its clients with conventional IVF/ICSI and embryo transfer services. NewGenIvf is also able to, through MicroSort technology, help fulfill the family-balancing dreams of its clients and avoiding certain gender-related hereditary diseases.
IVF treatments that NewGenIvf provides address tubal factor, ovulatory dysfunction, diminished ovarian reserve, endometriosis, uterine factor, male factor, unexplained infertility and other causes. IVF bypasses the function of the fallopian tube by achieving fertilization within a laboratory environment. Ovarian hyper-stimulation is common with IVF treatments to recruit numerous follicles to increase the chances for success. Follicles are retrieved trans-vaginally using a vaginal probe and ultrasound guidance. Anaesthesia is frequently used due to the number of follicles retrieved and the resulting discomfort experienced by the patient. The eggs are identified in the follicular fluid and combined with sperm and culture medium in culture dishes, which are placed in an incubator with a temperature and gas environment designed to mimic the condition of the fallopian tubes. Once the embryos develop, typically over a 3-to-5-day period, they are transferred to the uterine cavity. According to CIC, the average clinical pregnancy success rates, using 5-day incubation, averaged approximately 64.6% (with no PGT) for IVF, with live birth rate at approximately 28.7%.
As a long-standing IVF treatments provider in Asia-Pacific, NewGenIvf had completed over 4,000 cycles of IVF treatments from 2014 to 2023. For the years ended December 31, 2023 and 2022, the revenue from NewGenIvf’s IVF treatments was US$4,021,696 and US$2,819,163, respectively, representing 78.3% and 47.4% of its total revenue in the corresponding periods.
IVF Treatments Process
A typical IVF treatment process mainly includes two stages, the pre-IVF treatment stage and the IVF treatment stage. During the IVF treatment process, NewGenIvf also provides support services such as nutrition guidance and psychological counselling. The flow chart below shows the stages involved in a typical IVF treatment process:
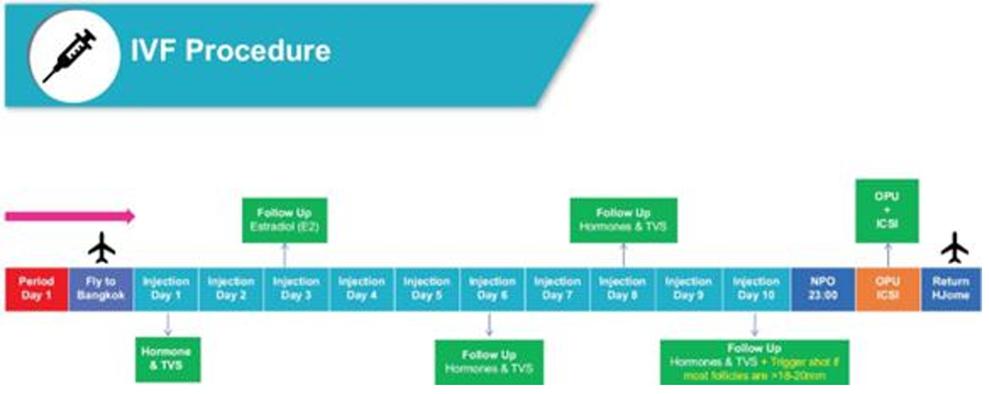
At the pre-IVF treatment stage, clients attend an initial consultation, undergo pre-IVF tests, and undergo treatment for gynaecological and andrological diseases, if needed. At the initial consultation, a physician reviews the clients’ detailed medical history to collect more information relating to the potential cause of their infertility. The client then undergoes various pre-IVF tests, which may include, among other things, blood pressure, hormone level, ultrasound, infectious disease screening, uterine evaluation and male fertility test. The physician will then design treatment plans based on the client’s medical history and results of the tests. If the client is satisfied with treatment plan and the test results are acceptable to the physician, the physician will prescribe medications and start stimulation treatment.
The first step of the cycle is to boost egg production through injecting synthetic hormones. Over about one week of ovarian stimulation, clients are monitored on a regular basis with blood test and transvaginal ultrasound. If follicles have reached at least 10 mm in size, an additional antagonist drug will be added into the daily injection schedule. This is used to prevent ovulation before ovum pickup time. After another few days of ovarian simulation, if follicle growth is consistent and majority of follicles are around 16 mm to 17 mm, the final injection of a human chorionic gonadotropin will be administered. The trigger injection is the final step of the stimulation process and is for the maturation of the eggs in the follicles before they are collected. The next major step is to retrieve the eggs with a minor surgical procedure called Trans Vaginal Follicle Aspiration conducted under anaesthesia. At the same time the male partner collects the sperms for fertilizing the eggs in the laboratory by a process known as intracytoplasmic sperm injection. The fertilized embryos are cultured in the laboratory for two to six days. Embryos that grow well are biopsied and tested by PGT to detect potential genetic diseases.
The final step is to transfer the embryos into the uterus using a catheter. Within eight days after the embryo transfer, a blood test can be conducted to detect whether the implantation was successful.
MicroSort Technology
MicroSort technology is a preconception process developed by the Genetics and IVF Institute, Inc. that aims to improve the chances that the baby to be conceived will be of the desired gender and prevents certain gender-related hereditary diseases.
Semen samples usually contain equal amounts of sperm carrying the Y chromosome (which will produce a boy), and sperm carrying the X chromosome (which will produce a girl). During the MicroSort process, the sperm sample is washed to remove seminal liquid and nonmotile cells. After the washing, the sample is stained with a special fluorescent material that attaches to the DNA contained in the sperm. The stained sperm cells are analyzed one by one by a flow cytometer, in which cells pass through a laser to make the stain attach to the DNA fluoresce. The sperm containing the X chromosome (which have more DNA and therefore more stain) will shine brighter than the sperm containing the Y chromosome. The flow cytometer uses a special software to identify X and Y chromosome sperm based on their fluorescence signature. The sperm carrying the chromosome that will produce the desired gender are separated from the rest of the sample -resulting in an enriched sperm sample ready for use.
NewGenIvf holds an exclusive license granted by a division of the Genetics and IVF Institute, MicroSort International, to use the MicroSort technology in Thailand and Cambodia. MicroSort licenses for NewGenIvf’s operation in Thailand and Cambodia are each provided under a lease and service agreement. In April 2019, First Fertility PGS entered into a Lease and Services Agreement with MicroSort International to use MicroSort equipment in Thailand and in March 2019, Phnom Penh Center entered into a Lease and Services Agreement with MicroSort International to use MicroSort equipment in Cambodia (together, the “Lease and Services Agreements”). Pursuant to the Lease and Services Agreements, First Fertility PGS and Phnom Penh Center each has the exclusive right to utilize the MicroSort equipment and to market and sell MicroSort sperm sorting services in Thailand and Cambodia, respectively. MicroSort International is responsible for the maintenance of MicroSort equipment and technical and engineering support. The term of each Lease and Service Agreements is initially from 2019 to 2024, which shall be automatically renewed for one year unless a written notice of at least 180 days prior to the intended termination date is provided. The consideration under each of the Lease and Services Agreements is US$9,000 per month after six months from the effective date of the agreements. MicroSort International was entitled to a down payment of US$15,000 per agreement and the aggregated amounts received by it under the agreements was US$328,500. During the term of each lease and service agreement, MicroSort grants NewGenIvf the exclusive right in that country to utilize the MicroSort equipment and market MicroSort services. The term of each lease and service agreement is initially from 2019 to 2024, which shall be automatically renewed for one year unless a written notice at least 180 days prior to the intended termination date is provided. The flow chart below shows the process involved in MicroSort:

Preimplantation Genetic Screening
PGS is used in parallel with an IVF treatment cycle. PGS is the practice of determining the presence of aneuploidy (either too many or too few chromosomes) in a developing embryo. PGS improves success rates of in vitro fertilization by ensuring the transfer of euploid embryos that have a higher chance of implantation and resulting in a live birth. PGS has improved clinical outcomes for NewGenIvf by achieving a higher implantation rate of 70.9% and reducing miscarriage rates by 26.6%.
Next-Generation Sequencing
NGS is a high-throughput technology for determining the sequence of deoxyribonucleic acid DNA or RNA to study genetic variation associated with diseases or other biological phenomena. NGS determines the sequence of a sample all at once by using parallel sequencing. Traditional Sanger sequencing determines the sequence of a sample one section at a time. Sequencing thousands of gene fragments simultaneously with NGS reduces time and cost associated with sequencing and increases the coverage quality and data output.
Preimplantation Genetic Diagnosis
Similar to PGS, PGD is also used in parallel with an IVF treatment cycle. But PGD is a more enhanced process than PGS since it scans for individual genes. PGD is the practice of evaluating embryos for specific genetic abnormalities, such as sickle cell disease or cystic fibrosis, where carrier status has been documented in each of the parents. By using this technique, physicians are able to check the genes or chromosomes for a specific genetic condition. PGD can decrease the risk of miscarriage and this technology can help women achieve a healthy pregnancy. Individuals who suspect or know they carry genes for serious medical conditions may opt to screen for healthy embryos ahead of time.
Surrogacy and Ancillary Caring Services
NewGenIvf also generated revenue from surrogacy services and related ancillary caring services in Kyrgyzstan. NewGenIvf conducts implantation of embryos from biological parents in surrogate mothers. During the pregnancy period, NewGenIvf provides ancillary caring services including regular body check and provision of vitamins, supplements and medicines to surrogate mothers. Revenue from surrogacy and ancillary caring services is recognized when the surrogate mother gives birth. Surrogacy services provide infertile couples with an alternative method of having children. In general, NewGenIvf provides certain discount to clients if they wish to pursue additional services such as egg donation and surrogacy, after several cycles of IVF treatments failures due to medical reasons including, but not limited to, the poor egg quality of aged female clients.
As compared to other countries, Kyrgyzstan has the following features that allow NewGenIvf to operates its surrogacy services: (i) surrogacy is legal and regulated, which means that there are less restrictions on either intended parents or surrogate mothers, and a parent-child relationship can be requested before the child’s birth; and (ii) the costs of operation and surrogate mother is favourable, given the cost of living in Kyrgyzstan is relatively low.
In addition to the regular surrogacy services, NewGenIvf is also able to assist the clients with birth certificate applications and facilitate the application of infants’ passports and visas as supplemental services.
For the years ended December 31, 2023 and 2022, the revenue from NewGenIvf’s surrogacy and ancillary caring services was US$1,114,457 and US$3,125,027, respectively, representing 21.7% and 52.6% of its total revenue in the corresponding periods.
The flow chart below shows the stages involved in a typical surrogacy process:

In Kyrgyzstan, NewGenIvf also provides ancillary fertility services when carrying out surrogacy services. These ancillary fertility services include: (i) maternity caring service, and (ii) documentation service.
Network of Facilities
As of December 31, 2023, NewGenIvf had one marketing and sales support office located in Hong Kong and three clinics located in Thailand, in Cambodia, and in Kyrgyzstan, respectively. The integration of the medical facilities in Thailand help NewGenIvf provide a more seamless one-stop experience to its clients. Set out below is an illustration of the locations of NewGenIvf’s clinics and marketing and sales office:

The following table sets forth the approximate aggregate average gross floor area (“G.F.A.”) of each of NewGenIvf’s clinics that were under lease and actively used for client service as of December 31, 2023:
| | | As of
December 31,
2023 | |
| | | (Square Feet) | |
| Thailand | | | |
First Fertility PGS Center Co., Ltd.
(“First Fertility PGS Center”) | | | 14,750 | |
| | | | | |
| Cambodia | | | | |
First Fertility Phnom Penh Center
(“Phnom Penh Center”) | | | 18,567 | |
| | | | | |
| Kyrgyzstan | | | | |
First Fertility Bishkek Limited Liability Company
(“First Fertility Bishkek”) | | | 2,368 | |
| | | | | |
| Aggregate G.F.A | | | 35,685 | |
To increase the scale of NewGenIvf’s operations, NewGenIvf expanded its Thailand fertility services by leasing a new property for its second clinic Erawan Consultation Clinic in May 2023. Consisting of approximately 2,500 sq. ft., Erawan Consultation Clinic is expected to open in 2024.
Currently, IVF treatments are performed in its Thailand and Cambodia clinics, egg donation services are provided in its Cambodia clinic, and surrogacy services are provided in its Kyrgyzstan clinic. The following table summarises the services available at NewGenIvf’s clinics:
| | | IVF
Treatments | | Surrogacy
Services |
| Thailand | | | | |
| First Fertility PGS Center | | √ | | × |
| | | | | |
| Cambodia | | | | |
| Phnom Penh Center | | √ | | × |
| | | | | |
| Kyrgyzstan | | | | |
| First Fertility Bishkek | | × | | √ |
The following table sets forth a breakdown of revenue from services performed at NewGenIvf’s medical centers for the periods indicated:
| | | For the Year ended December 31, | |
| | | 2023 | | | 2022 | |
| | | US$ | | | % | | | US$ | | | % | |
| HK SAR | | | 34,038 | | | | 0.7 | | | | — | | | | — | |
| Thailand | | | 1,356,903 | | | | 26.4 | | | | 505,609 | | | | 8.5 | |
| Cambodia | | | 621,619 | | | | 12.1 | | | | 377,608 | | | | 6.4 | |
| Kyrgyzstan | | | 3,123,593 | | | | 60.8 | | | | 5,060,973 | | | | 85.1 | |
| Total Revenue | | | 5,136,153 | | | | 100.0 | | | | 5,944,190 | | | | 100.0 | |
Thailand Clinic
As of December 31, 2023, NewGenIvf had one clinic in Thailand. At the clinic in Thailand, NewGenIvf offers its clients customized fertility treatment solutions including IVF/ICSI, embryo culture, hormonal blood tests, infectious diseases tests, chromosome screening by PGT, hysteroscopy, sperm analysis, sorting, washing and freezing, and egg freezing. Its medical and operational personnel are organized into specialized teams according to the different stages of the IVF treatment process and different patient profiles. When clients are admitted, they are assigned to a team which NewGenIvf believes is better suited the clients after taking into account the clients’ diagnosis and preferences. Furthermore, NewGenIvf also provides related value-added services such as nutrition guidance, psychological counselling, acupuncture, and translation interpreters to supplement the IVF treatment. NewGenIvf prides itself on providing quality and customized treatment to its clients on a day-to-day basis.
As of December 31, 2023, the clinic in Thailand had six nurses, 8 full time lab physicians and embryologists, 14 administrative staff, totaling 28 staff members.
Cambodia Clinic
NewGenIvf has one clinic, Phnom Penh Center, in Cambodia. Phnom Penh Center is staffed with one Cambodian physician, three embryologists, five nurses and twelve other staff, and offers similar IVF treatments as in Thailand and egg donation services. Phnom Penh Center operates under a license issued by Cambodia MOH for the Cambodian physician, who has entered into an agreement with Phnom Penh Center for the exclusive use of such license.
After eight years of development since its opening in 2015, Phnom Penh Center has become one of the long-standing ARS providers in Cambodia. According to CIC, it was the first to use conventional IVF technology which led to a successful birth in 2016 in Cambodia. Since its establishment, Phnom Penh Center achieved more than 1,600 IVF treatment cycles as of December 31, 2023. As of December 31, 2023, Phnom Penh Center’s IVF philosophy concentrates on three key points in the treatment process: the mother’s wellbeing, the technology used to assist mothers deliver a strong and healthy baby and the medical science used to ensure every chance of success for women in various age spectrums.
Clinic in Kyrgyzstan
NewGenIvf established First Fertility Bishkek in October 2019 in Kyrgyzstan for its surrogacy services, as Kyrgyzstan has supply of surrogate candidates at a relatively low cost and a more friendly legal environment for surrogacy services. In 2020, First Fertility Bishkek obtained the license to provide ARS and surrogacy services, becoming one of the few facilities licensed to offer ARS and one of the facilities licensed to offer surrogacy services in Kyrgyzstan as of December 31, 2023, according to CIC. In addition, NewGenIvf also provide related ancillary fertility services when carrying out surrogacy services. These ancillary fertility services include: (i) maternity caring service, and (ii) documentation service.
Physicians at First Fertility Bishkek have expertise in sourcing surrogate mothers, techniques of embryo transfers, prenatal care, baby delivery, and postnatal care. First Fertility Bishkek also collaborates closely with Phnom Penh Center in arranging shipment of frozen embryos. NewGenIvf hires local physicians and local staff. NewGenIvf also provides training for newly admitted Kyrgyzstan physicians and embryologists in Thailand. Some personnel who had relevant experience in Kyrgyzstan had also been sent from Cambodia to Kyrgyzstan to help manage such operations from time to time.
As of December 31, 2023, First Fertility Bishkek had one full-time physician, one embryologist, two nurses, and ten other staff.
Professionals
Licensed Physicians
As of December 31, 2023, NewGenIvf contracted with five licensed physicians, among which one was based in Cambodia and the other four were based in Thailand. Most of NewGenIvf’s physicians had over 10 years of experience or above. The following table summarises the number and types of such licensed physicians as of December 31, 2023.
| Country | | Licensed physician | | Licenses and
Approvals | | Effective Period | | Issuing
Authority |
| Cambodia | | Mr. Keut Serey | | Decision on permission for beauty | | December 14, 2022 – | | The Ministry of Health of |
| | | | | treatment operation | | December 14, 2026 | | Cambodia |
| Thailand | | Dr Patsama Vichinsartvichai | | Medical Facility Operating License | | August 12, 2022 – December 31, | | The Ministry of Health of |
| | | | | number 288006 | | 2023 | | Thailand |
| | | | | Number 30920 Medical | | April 1, 2004 – Indefinite | | The Ministry of Health of |
| | | | | Practitioner License | | | | Thailand |
| | | | | Number 26443/2556 Reproductive | | July 1, 2013 – Indefinite | | Medical Council of |
| | | | | Medicine Diploma | | | | Thailand |
| | | | | Certificate number obscured OB- | | October 13, 2010 – Indefinite | | Medical Council of |
| | | | | Gyn License | | | | Thailand |
| Thailand | | Dr Keatthisak Boonsimma | | Number 31801 Medical | | April 1, 2005 – Indefinite | | Royal Thai College of |
| | | | | Practitioner License | | | | Obstetricians and |
| | | | | | | | | Gynaecologists of Thailand |
| | | | | Number 22624/2554 OB-Gyn | | July 1, 2014 – Indefinite | | Medical Council of |
| | | | | License | | | | Thailand |
| | | | | Number 40962/2563 Reproductive | | July 1, 2020 – Indefinite | | Medical Council of |
| | | | | Medicine Diploma | | | | Thailand |
| Country | | Licensed physician | | Licenses and
Approvals | | Effective Period | | Issuing
Authority |
| Thailand | | Dr Seree Teerapong | | Number 15231/2564 Reproductive | | July 1, 2021 – Indefinite | | Medical Council of |
| | | | | Medicine License | | | | Thailand |
| | | | | Number 4576/2533 OB-Gyn | | July 12, 1990 – Indefinite | | Medical Council of |
| | | | | License | | | | Thailand |
| | | | | Number 11544 (replacement) | | April 12, 1984 – Indefinite | | Medical Council of |
| | | | | Medical Practitioner License | | | | Thailand |
| Thailand | | Dr Wiphawee Luangtangvarodom | | Number 38347/2562 OB-Gyn | | August 1, 2019 – Indefinite | | Medical Council of |
| | | | | License | | | | Thailand |
| | | | | Number 43217/2564 Reproductive | | July 1, 2021 – Indefinite | | Medical Council of |
| | | | | Medicine License | | | | Thailand |
| | | | | Number 48510 Medical | | April 1, 2014 – Indefinite | | Medical Council of |
| | | | | Practitioner License | | | | Thailand |
Agreements with Physicians
NewGenIvf enters into independent physician agreements or employment contracts with its physicians. The terms and conditions and the format of the agreements NewGenIvf enters into with each of its physicians vary, depending on the physician’s seniority and practise nature.
Customers
For the years ended December 31, 2023 and 2022, the majority of NewGenIvf’s clients were from China (including mainland China and Hong Kong). The number of Thai and Cambodian local patients generally increased in 2022 and 2023 compared with earlier years due to the impact of COVID-19 on international travel. NewGenIvf enters into a service agreement with each of its customers that outline, among other things, the scope of services, service fees, payment terms and rights, responsibilities and obligations of each party. Customers are not entitled to enjoy the relevant services until outstanding amounts have been settled pursuant to the relevant contract. Sales to individual consumers did not vary significantly and none of the customers contribute more than 10% of NewGenIvf’s revenue for the years ended December 31, 2023 and 2022.
The following table sets forth a breakdown of NewGenIvf’s total customers by major countries (determined by the passports they provided to NewGenIvf for registration) and as a percentage of the total customers for the periods indicated(1):
| | | For the Year ended December 31, | |
| | | 2023 | | | 2022 | |
| | | First
Fertility
PGS
Center | | | Phnom
Penh
Center | | | Total | | | % | | | First
Fertility
PGS
Center | | | Phnom
Penh
Center | | | Total | | | % | |
| China(2) | | | 34 | | | | 87 | | | | 121 | | | | 42 | | | | 66 | | | | 117 | | | | 183 | | | | 72 | |
| India | | | 16 | | | | — | | | | 16 | | | | 6 | | | | 16 | | | | — | | | | 16 | | | | 6 | |
| Thailand | | | 103 | | | | — | | | | 103 | | | | 36 | | | | 25 | | | | 3 | | | | 28 | | | | 11 | |
| Cambodia | | | — | | | | 7 | | | | 7 | | | | 2 | | | | — | | | | 22 | | | | 22 | | | | 9 | |
| Others(3) | | | 31 | | | | 9 | | | | 40 | | | | 14 | | | | — | | | | 5 | | | | 5 | | | | 2 | |
| Total | | | 184 | | | | 103 | | | | 287 | | | | 100 | | | | 107 | | | | 147 | | | | 254 | | | | 100 | |
| (1) | Customers of First Fertility Bishkek are the same customers of Phnom Penh Center. |
| (2) | Include customers from mainland China and Hong Kong. |
| (3) | Include customers from Philippines, Singapore, USA, Korea, Nigeria and UK. |
In addition to significant customers using NewGenIvf’s IVF treatment services and surrogacy and ancillary caring services, NewGenIvf also has customers who only use its relatively insignificant services, such as check-ups services, blood test services and other minor services (the latter category of customers are referred to as “consultation customers”).
Sales and Marketing
For the years ended December 31, 2023 and 2022, NewGenIvf promoted brand awareness through its sales teams and, in many cases, through cooperating with third-party agencies and partners.
NewGenIvf’s sales teams have broad experience in fertility services and are responsible for identifying potential clients and managing the overall sales process. NewGenIvf’s sales team primarily relies on social media marketing, word-of-mouth referrals, recognition of its brand, printed advertisements and marketing events. NewGenIvf spends marketing expenses on placing advertisements through popular social media platforms, maintaining the official website of NewGenIvf and sending information through its official accounts on social media platforms.
Supply and Procurement
NewGenIvf’s procurement is mainly for medications, laboratory media and reagents, laboratory consumables, and blood test reagents. As of December 31, 2023 and 2022, one and four suppliers individually contributed more than 10% of the Group’s trade payable, in aggregate accounting for 30.6% and 69.8% of the Group’s trade payables, respectively. For the year ended December 31, 2023 and 2022, nil and two vendors contributed more than 10% of total purchases of the Group, in aggregate accounting for nil and 55.3% of the Group’s total purchases, respectively. NewGenIvf’s procurement team is experienced in selecting cost-effective supplies as well as selecting reliable suppliers. NewGenIvf’s major suppliers are pharmaceutical companies.
Competition
NewGenIvf believes that it is a long-standing provider of ARS in Asia Pacific that competes primarily based on the following competitive factors:
| ● | the value and comprehensiveness of the solutions; |
| ● | treatment that is effective and achieves desired outcomes; |
| ● | clients’ experience, including dedicated patient education, clinical guidance and emotional support; and |
| ● | access to a network of high-quality fertility specialists. |
NewGenIvf competes primarily with other regional fertility service providers. While NewGenIvf does not believe any single competitor offers a comparably robust and integrated fertility solution package as NewGenIvf in the regions that it operates, NewGenIvf’s competitors may compete in a variety of ways, including by providing better services, having established local connections, fulfilling evolving client needs, as well as conducting brand promotions and other marketing activities.
As NewGenIvf may introduce new ancillary services and other companies may introduce similar fertility services as NewGenIvf’s, NewGenIvf may become subject to additional competition.
Facilities
As of December 31, 2023, in addition to its clinics, NewGenIvf leased one property in Hong Kong with an aggregate square footage of approximately 8,000 for its administration support offices. NewGenIvf also operates its medical facilities as described above in “— Network of Facilities” above. NewGenIvf believes that its existing facilities are suitable and adequate to meet its current needs.
C. Organizational Structure
The following is a list of our principal subsidiaries and consolidated affiliated entities as of the date of this prospectus:
| Name | | Place of Formation | | Relationship |
| | | | | |
| Legacy NewGenIvf | | Cayman Islands | | Wholly-owned subsidiary |
| | | | | |
| FFPGS (HK) Ltd | | Hong Kong | | Indirect subsidiary, wholly owned by Legacy NewGenIvf |
| | | | | |
| First Fertility Bishkek LLC | | Kyrgyzstan | | Indirect subsidiary, wholly owned by Legacy NewGenIvf |
| | | | | |
| First Fertility PGS Center Limited | | Thailand | | Indirect subsidiary, wholly owned by Well Image Limited HK |
| | | | | |
| First Fertility Phnom Penh Ltd | | Kingdom of Cambodia | | Indirect subsidiary, wholly owned by Legacy NewGenIvf |
| | | | | |
| Med Holdings Limited | | Thailand | | Indirect subsidiary, wholly owned by Well Image Limited HK |
| | | | | |
| Well Image Limited HK | | Hong Kong | | Indirect subsidiary, wholly owned by Legacy NewGenIvf |
D. Property, Plants and Equipment
The Company leases the premises for its principal executive office located at 36/39-36/40, 13th Floor, PS Tower, Sukhumvit 21 Road (Asoke) Khlong Toei Nuea Sub-district, Watthana District, Bangkok 10110, Thailand. This property contains approximately 14,750 square feet. The Company leases one property in Hong Kong with an aggregate square footage of approximately 8,000 for its administration support offices.
The Company also leases several premises to operate its clinics in various countries. In Kyrgyzstan, the Company operates the First Fertility Bishkek Limited Liability Company, which premises have an aggregate area of 2,368 square feet. In Cambodia, the Company operates the First Fertility Phnom Penh Center, which premises have an aggregate area of 18,567 square feet. In Thailand, the Company operates a clinic named First Fertility PGS Center Co., Ltd., which premises have an aggregate area of 14,750 square feet.
The Company also leases premises located in Thailand for its anticipated Erawan Consultation Clinic clinic, with an aggregate area of approximately 2,500 square feet. This property is used as the Company’s second clinic in Thailand, which is expected to open in 2024.
Nasdaq Deficiency
On October 8, 2024, the Company received a deficiency letter (“Bid Price Deficiency Letter”) from the Listing Qualifications Department (the “Staff”) of Nasdaq notifying the Company that it is currently not in compliance with the closing bid price requirement under Nasdaq Listing Rule 5450(a)(1) (the “Minimum Bid Price Rule”). The Bid Price Deficiency Letter stated that, for the preceding 30 consecutive business days, the Company’s Class A Ordinary Shares did not meet the minimum closing bid price of $1 per share pursuant to the Minimum Bid Price Rule. The Company has an initial compliance period of 180 calendar days, or until April 7, 2025 to regain compliance with the Minimum Bid Price Rule. The Deficiency letter stated that if at any time the closing bid price of the Company’s Class A Ordinary Shares is at least $1 for a minimum of ten consecutive business days, Nasdaq will provide the Company written confirmation of compliance with this requirement, as applicable. If the Company does not regain compliance with the above requirements by April 7, 2025, the Company will receive written notification that its securities are subject to delisting. The Company may be eligible for additional time to regain compliance, which will require the Company to submit to Nasdaq written notice of its intent to cure the deficiency ahead of April 7, 2025. The Company intends to monitor the minimum bid price of its Class A Ordinary Shares and may, if appropriate, consider available options to regain compliance with the Nasdaq requirements.
On May 24, 2024, the Company received a deficiency letter (“MVLS Deficiency Letter”) from the Staff of Nasdaq notifying the Company that, for the preceding 35 consecutive business days, the Class A Shares did not meet the minimum $50,000,000 Market Value of Listed Securities requirement (“MVLS Requirement”) for continued listing on Nasdaq pursuant to Nasdaq Listing Rules 5450(b)(2)(A) (the “MVLS Requirement,” and the Company’s non-compliance with this requirement, the “MVLS Deficiency”). In accordance with Nasdaq Rule 5810(c)(3)(C), the Company has been provided an initial period of 180 calendar days, or until November 20, 2024 (the “Compliance Date”), to regain compliance with the MVLS Requirement. If, at any time before the Compliance Date, the MVLS for the Class A Shares is at least $50,000,000 for a minimum of ten consecutive business days, the Staff will provide the Company written confirmation of compliance with the MVLS Requirement. In the event the Company does not regain compliance with the above requirement prior to the expiration of the compliance period, it will receive written notification that its securities are subject to delisting.
On May 24, 2024, the Company received a deficiency letter (“MVPHS Deficiency Letter”) from the Staff of Nasdaq notifying the Company that, for the preceding 35 consecutive business days, the Company’s Class A Ordinary Shares did not meet the minimum $15,000,000 Market Value of Publicly Held Shares (“MVPHS”) requirement for continued listing on Nasdaq pursuant to Nasdaq Listing Rules 5450(b)(2)(C) (the “MVPHS Requirement,” and the Company’s non-compliance with this requirement, the “MVPHS Deficiency”). In accordance with Nasdaq Rule 5810(c)(3)(D), the Company has until the Compliance Date to regain compliance with the MVPHS Requirement. If, at any time before the Compliance Date, the MVPHS for the Class A Shares is at least $15,000,000 for a minimum of ten consecutive business days, the Staff will provide the Company written confirmation of compliance with the MVPHS Requirement. In the event the Company does not regain compliance with the above requirement prior to the expiration of the compliance period, it will receive written notification that its securities are subject to delisting. Alternatively, the Company may apply to transfer the Company’s securities to The Nasdaq Capital Market.
On November 21, 2024, the Company received a notice from the Staff of Nasdaq notifying the Company that its securities are subject to delisting due to the MVPHS Deficiency and MLVS Deficiency. The Company will formally request a hearing to appeal the delisting determination and intends to leverage several strategic options to ensure its securities remain publicly traded. In addition, the Company will apply to transfer its securities from the Nasdaq Global Market to the Nasdaq Capital Market.
Capitalization and Indebtedness
Below is the Company’s capitalization and indebtedness as of September 30, 2024:
| | | US$ | |
| | | | |
| Cash and Cash equivalents | | | 169,661 | |
| EQUITY AND LIABILITIES | | | | |
| Share capital | | | 0 | |
| Additional paid-in capital | | | 1,415,000 | |
| Accumulated deficit | | | (7,999,906 | ) |
| Total capitalization | | | (6,415,245 | ) |
Statements of indebtedness as of September 30, 2024
| | | US$ | |
| Current liabilities | | | |
| Legal fee payable | | | 2,074,114 | |
| Other payables, accruals and advance receipt | | | 398,208 | |
| Contract liabilities | | | 8,400 | |
| Amount with related companies/parties | | | 309,795 | |
| Lease liabilities, operating leases | | | 213,546 | |
| Income tax payable | | | 486,705 | |
| | | | | |
| Total current liabilities | | | 3,490,768 | |
| | | | | |
| Non-current liabilities | | | | |
| Lease liabilities, operating leases | | | 122,981 | |
| Lease liabilities, finance lease | | | 1,453,861 | |
| Convertible Promissory Note | | | 4,300,000 | |
| Discount on convertible note | | | (819,762 | ) |
| Total non-current liabilities | | | 5,057,080 | |
| | | | | |
| Total Indebtedness | | | 8,547,848 | |
Dilution
The following information is presented as of September 30, 2024:
| | | Existing shareholder | |
| | | 30/9/2024 | |
| | | | |
| Total comprehensive income attributable to the shareholders of the company | | $ | (405,742 | ) |
| Earning per share – basic | | | (0.04 | ) |
| - diluted | | | (0.03 | ) |
| Weighted average shares outstanding – Basic | | | 10,149,386 | |
| - Diluted | | | 14,770,914 | |
Implications of being a “Foreign Private Issuer”
We are subject to the information reporting requirements of the Exchange Act that are applicable to “foreign private issuers,” and under those requirements, we file reports with the SEC. As a foreign private issuer, we are not subject to the same requirements that are imposed upon U.S. domestic issuers by the SEC. Under the Exchange Act, we are subject to reporting obligations that, in certain respects, are less detailed and less frequent than those of U.S. domestic reporting companies. For example, we are not required to issue quarterly reports, proxy statements that comply with the requirements applicable to U.S. domestic reporting companies or individual executive compensation information that is as detailed as that required of U.S. domestic reporting companies. We also have four months after the end of each fiscal year to file our annual report with the SEC and are not required to file current reports as frequently or promptly as U.S. domestic reporting companies. Our officers, directors and principal shareholders are exempt from the requirements to report transactions in our equity securities and from the short-swing profit liability provisions contained in Section 16 of the Exchange Act. As a foreign private issuer, we are not subject to the requirements of Regulation FD (Fair Disclosure) promulgated under the Exchange Act. In addition, as a foreign private issuer, we are permitted to follow certain home country corporate governance practices instead of those otherwise required under the rules of Nasdaq for domestic U.S. issuers and are not required to be compliant with all Nasdaq rules as of the date of our initial listing on Nasdaq as would domestic U.S. issuers. These exemptions and leniencies will reduce the frequency and scope of information and protections available to you in comparison to those applicable to a U.S. domestic reporting company. We intend to take advantage of the exemptions available to us as a foreign private issuer.
Summary of Risk Factors
Investing in our Ordinary Shares involves significant risks. You should carefully consider all of the information in this prospectus before making an investment in our shares. Below please find a summary of the principal risks we face, organized under relevant headings. These risks are discussed more fully in the section titled “Risk Factors” and in Part I, Item 3, D. Risk Factors in our most recent Annual Report on Form 20-F.
Risks Related to NewGenIvf’s Business and Industry
| | ● | We may not be able to continue operating as a going concern. |
| | | |
| | ● | The fertility market in which NewGenIvf participates is competitive, and if NewGenIvf does not continue to compete effectively, its results of operations could be materially and adversely affected. |
| | | |
| | ● | NewGenIvf has a limited operating history with its current platform of solutions, which makes it difficult to predict its future prospects, financial performance and results of operations. |
| | | |
| | ● | NewGenIvf’s marketing efforts depend significantly on its ability to receive positive references from its existing clients. |
| | | |
| | ● | If NewGenIvf is unable to attract new clients, its business, financial condition and results of operations would be adversely affected. |
| | | |
| | ● | NewGenIvf’s business depends on its ability to maintain its existing client demographics. Any failure to do so would harm its business, financial condition and results of operations. |
| | ● | If NewGenIvf fails to offer high-quality support, its reputation could suffer. |
| | | |
| | ● | NewGenIvf’s failure to effectively develop and expand its marketing and sales capabilities could harm its ability to increase its client base and achieve broader market acceptance of solutions NewGenIvf provides. |
| | | |
| | ● | NewGenIvf may experience net losses and may not sustain profitability in the future. |
| | | |
| | ● | NewGenIvf’s future revenue may not grow at the rates it historically has, or at all. |
| | | |
| | ● | NewGenIvf’s quarterly and annual results may fluctuate significantly and may not fully reflect the underlying performance of NewGenIvf’s business. |
| | | |
| | ● | If the estimates and assumptions NewGenIvf uses to determine the size of the target markets for its services are inaccurate, its future growth rate may be impacted and its business would be harmed. |
| | | |
| | ● | NewGenIvf may not be able to successfully manage its growth, and if NewGenIvf is not able to grow efficiently, its business, financial condition and results of operations could be harmed. |
| | | |
| | ● | If NewGenIvf’s new solutions and services are not adopted by its clients, or if it fails to innovate and develop new offerings that are adopted by its clients, its revenue and results of operations may be adversely affected. |
| | | |
| | ● | If NewGenIvf fails to adapt and respond effectively to the changing medical landscape, changing regulations, changing client needs, requirements or preferences, its offerings may become less competitive. |
| | | |
| | ● | If NewGenIvf fails to maintain and enhance its brand, its ability to expand its client base will be impaired and its business, financial condition and results of operations may suffer. |
| | | |
| | ● | If NewGenIvf fails to retain and motivate members of its management team or other key employees, or fails to attract additional qualified personnel to support its operations, its business and future growth prospects could be harmed. |
| | | |
| | ● | To successfully market and sell its services and products in Asia-Pacific markets, NewGenIvf must address many international business risks with which NewGenIvf has limited experience. |
| | | |
| | ● | Ethical, legal and social concerns related to the use of assisted reproductive technology could reduce demand for the fertility services provided by the medical facilities in NewGenIvf’s network, and thus may adversely affect the business, financial conditions and results of operations of the medical facilities in its network. |
| | | |
| | ● | NewGenIvf is reliant on revenue from international clients. |
| | | |
| | ● | Fluctuations in exchange rates could have a material and adverse effect on NewGenIvf’s results of operations and the value of your investment. |
| | | |
| | ● | Governmental control of currency conversion may limit NewGenIvf’s ability to utilize NewGenIvf’s net revenue effectively and affect the value of your investment. |
| | | |
| | ● | Substantially all of NewGenIvf’s assets and operations are located in Thailand, Cambodia and Kyrgyzstan and they are subject to economic, legal and regulatory uncertainties in such countries. |
| | ● | Failure to comply with the terms of future financing arrangements could result in default, which could have an adverse effect on NewGenIvf’s cash flow and liquidity. |
| | | |
| | ● | NewGenIvf requires a significant amount of capital to fund its operations and growth. If NewGenIvf cannot obtain sufficient capital on acceptable terms, its business, financial condition, and prospects may be materially and adversely affected. |
| | ● | The defects in certain leased property interests and failure to register certain lease agreements may materially and adversely affect NewGenIvf’s business, financial condition, results of operations, and prospects. |
| | | |
| | ● | NewGenIvf currently has no insurance coverage for its operations. |
| | | |
| | ● | NewGenIvf may not be successful in adapting to technological developments, which may affect its business and results of operations. |
| | | |
| | ● | If its computer systems, or those of its providers, specialty pharmacies or other downstream vendors lag, fail or suffer security breaches, NewGenIvf may incur a material disruption of its services, which could materially impact its business and the results of operations. |
| | | |
| | ● | We may not be able to comply with the filing deadlines for reports that we file pursuant to the Exchange Act,, and our failure to timely file such reports may have material adverse consequences on our business. |
| | | |
| | ● | If we are unable to continue to meet the listing requirements of Nasdaq, our Class A Ordinary Shares will be delisted |
Risks Related to NewGenIvf’s Relationships with Third Parties
| | ● | NewGenIvf’s business depends on its ability to maintain its network of high-quality fertility specialists and other healthcare providers. If NewGenIvf is unable to do so, its future growth would be limited and its business, financial condition and results of operations would be harmed. |
| | | |
| | ● | The medical facilities and professionals in NewGenIvf’s network could become the subject of litigation, allegations and other claims, and NewGenIvf is not insured against these liabilities. |
| | | |
| | ● | The assisted reproductive medical facilities in NewGenIvf’s network have limited control over the quality of the pharmaceuticals, medical equipment, medical consumables and other supplies used in its operations, and cannot guarantee that the products in use are not defective or counterfeit. NewGenIvf also has no control over independent sub-contractors and cannot guarantee the services thereof. |
| | | |
| | ● | If NewGenIvf loses its relationship with one or more key pharmaceutical manufacturers, its business and results of operations could be adversely affected. |
| | | |
| | ● | NewGenIvf has engaged in transactions with related parties, and such transactions present potential conflicts of interest that could have an adverse effect on its business and results of operations. |
| | | |
| | ● | NewGenIvf may be subject to claims and allegations relating to intellectual property and other causes. |
| | | |
| | ● | Certain data and information in this prospectus relied on by NewGenIvf were obtained from third-party data and polls. These metrics were not independently verified by NewGenIvf and may not be accurate. |
Risks Related to Government Regulation
| | ● | NewGenIvf operates in a highly regulated industry and must comply with a significant number of complex and evolving requirements. Any lack of requisite approvals, licenses, or permits applicable to NewGenIvf’s business may have a material and adverse impact on NewGenIvf’s business, financial condition, and results of operations. |
| | | |
| | ● | Changes in NewGenIvf’s effective tax rate or tax liability may have an adverse effect on its results of operations. |
| | | |
| | ● | NewGenIvf’s reported financial results may be adversely affected by changes in accounting principles generally accepted in relevant jurisdictions. |
| | | |
| | ● | NewGenIvf’s reported financial results may be adversely affected by changes in accounting principles generally accepted in relevant jurisdictions. |
| | | |
| | ● | If NewGenIvf’s estimates or judgments relating to its critical accounting policies prove to be incorrect, its results of operations could be adversely affected. |
| | | |
| | ● | NewGenIvf is subject to anti-corruption, anti-bribery, anti-money laundering, and similar laws, and non-compliance with such laws can subject it to criminal or civil liability and harm its business, financial condition and results of operations. |
THE OFFERING
This prospectus relates to the resale by the Selling Shareholder identified in this prospectus of up to 40,000,000 Ordinary Shares. All of the Ordinary Shares, when sold, will be sold by this Selling Shareholder. The Selling Shareholder may sell its Ordinary Shares from time to time at prevailing market prices. We will not receive any proceeds from the sale of the Ordinary Shares by the Selling Shareholder.
| Ordinary Shares currently issued and outstanding | | 10,149,386 Class A Ordinary Shares |
| | | |
| Ordinary Shares offered by the Selling Shareholder | | Up to 40,000,000 Class A Ordinary Shares, consisting of (i) up to 39,300,000 Ordinary Shares that may be issued by us to White Lion pursuant to the White Lion Purchase Agreement, and (ii) 700,000 Commitment Shares issuable to White Lion as consideration for entering into the White Lion Purchase Agreement. |
| | | |
| Ordinary Shares after this offering | | Up to 50,149,386 Class A Ordinary Shares |
| | | |
| Use of proceeds | | We will not receive any of the proceeds from the resale of the Resale Shares. However, we may receive up to $500,000,000 in gross proceeds under the White Lion Purchase Agreement from sales of Ordinary Shares that we may elect to make to White Lion pursuant to the White Lion Purchase Agreement after the date of this prospectus subject to certain contingencies as described herein, if any, from time to time in our sole discretion, during the Commitment Period. However, we will not receive any proceeds from the issuance of the Commitment Shares. The proceeds from White Lion that we receive under the White Lion Purchase Agreement, if any, are currently expected to be used for general corporate purposes, including working capital. Accordingly, we retain broad discretion over the use of the net proceeds from the sale of our Ordinary Shares under the White Lion Purchase Agreement. The precise amount and timing of the application of such proceeds will depend upon our liquidity needs and the availability and cost of other capital over which we have little or no control. As of the date hereof, we cannot specify with certainty the particular uses for the net proceeds from the sales of our Ordinary Shares, if any, to White Lion under the White Lion Purchase Agreement. See “Use of Proceeds.” |
| | | |
| Risk factors | | You should read the “Risk Factors” section starting on page 21 of this prospectus for a discussion of factors to consider carefully before deciding to invest in our securities. |
| | | |
| Nasdaq symbol | | “NIVF” (Class A Ordinary Shares); “NIVFW” (Warrants to purchase Class A Ordinary Shares). |
The number of Class A Ordinary Shares issued and outstanding is 10,149,386 as of November 21, 2024. No new Class A Ordinary Shares will be issued by us under this offering.
RISK FACTORS
Investing in our Class A Ordinary Shares involves a high degree of risk. You should carefully consider the risks in this prospectus, the risk factors described under the caption “Risk Factors” in any applicable prospectus supplement and any risk factors set forth in our other filings with the SEC pursuant to Sections 13(a), 13(c), 14 or 15(d) of the Securities Exchange Act of 1934, as amended, or the Exchange Act, before making a decision about investing in our Ordinary Shares. The risks and uncertainties we have described are not the only ones we face. Additional risks and uncertainties not presently known to us or that we currently deem immaterial may also affect our operations. If any risks actually occur, our business, financial condition and results of operations may be materially and adversely affected. In such an event, the trading price of our Ordinary Shares could decline and you could lose part or all of your investment.
Additionally, we are also subject to the following risk factors.
Risks Related to NewGenIvf’s Business and Industry
We may not be able to continue operating as a going concern.
As of December 31, 2023, the Company had bank balance of $54,104 and may have challenge to settle its obligations when payment become due. The Company is always closely monitoring the market opportunities and is currently in the process of exercising various fundraising projects with various potential investors to improve the Company’s cash flow position for its operation and short-term payables.
One fundraising project was completed on April 3, 2024. As of April 4, 2024, the Company settled $2 million to any payment with respect to accounts payable, but not, directly or indirectly, for (i) except for expenses relating to the Business Combination, the satisfaction of any indebtedness of the Company or any of its Subsidiaries, (ii) the redemption or repurchase of any securities of the Company or any of its Subsidiaries, or (iii) the settlement of any outstanding litigation as at December 31, 2023. The Company secured funding subsequent to year-end with total of $2 million, and that the Company received $2 million funding to date.
The Company can make no assurance that required financings will be available for the amounts needed, or on terms commercially acceptable to the Company, if at all. If one or all of these events does not occur or subsequent capital raises are insufficient to bridge financial and liquidity shortfall, there would likely be a material adverse effect on the Company and its financial statements.
The consolidated financial statements do not reflect adjustments that would be necessary if the going concern basis was not appropriate. If the going concern basis was not appropriate for these consolidated financial statements, then adjustments would be necessary in the carrying value of the assets and liabilities, the reported revenues and expenses, and the balance sheet classifications used. These adjustments could be material.
The fertility market in which NewGenIvf participates is competitive, and if NewGenIvf does not continue to compete effectively, its results of operations could be materially and adversely affected.
The market for NewGenIvf’s solutions is competitive and is likely to attract increased competition, which could make it hard for it to succeed. NewGenIvf faces significant competition from other fertility companies and other players in the fertility market. Some of NewGenIvf’s competitors are more established, have a longer operating history and a larger client base, benefit from greater brand recognition and have substantially greater financial, technical and marketing resources than NewGenIvf does. NewGenIvf’s competitors may compete with NewGenIvf in a variety of ways, including seeking to develop or integrating solutions and services that may become more efficient or appealing to NewGenIvf’s existing and potential clients, achieving superior clinical outcomes, having access to a network of more high-quality fertility specialists, establishing more comprehensive data reporting and sharing systems, conducting brand promotions and other marketing activities, and making investments in and acquisitions of NewGenIvf’s business partners. While NewGenIvf believes that one of its key competitive advantages is its ability to provide a broad range of services, and NewGenIvf does not believe any competitors have developed a similar broad range services in Asia Pacific at this time, current or future competitors may be successful in doing so in the future. If current or future competitors are successful at developing a similar broad range of services, NewGenIvf’s financial performance may be negatively impacted.
In addition, NewGenIvf believes that there is growing awareness of the demand for fertility services. As the fertility services field gains more attention, more competitors may be drawn into the market. NewGenIvf also could be adversely affected if NewGenIvf fails to identify or effectively respond to changes in market dynamics. As a result of any of these factors, NewGenIvf may not be able to continue to compete successfully against its current or future competitors, and this competition could result in the decrease in its clients base and market share and the failure of its platform to continue to maintain market acceptance, which would materially and adversely affect its business, financial condition and results of operations.
NewGenIvf has a limited operating history with its current platform of solutions, which makes it difficult to predict its future prospects, financial performance and results of operations.
The predecessor entity of the Company prior to the Business Combination in April of 2024, NewGenIvf Limited, a Cayman Islands exempted company, was established in 2019, and although it launched its fertility services in 2014, has a limited operating history. As a result of its limited operating history with its current platform of solutions, as well as a limited amount of time serving a majority of its client base, its ability to accurately forecast its future results of operations, key operating data, net revenue, cash flows, and operating margins is limited and subject to a number of uncertainties, including its ability to plan for and model future growth. NewGenIvf’s historical revenue growth should not be considered indicative of its future performance. Further, in future periods, its revenue growth could slow or decline for a number of reasons, including risks, challenges and uncertainties that NewGenIvf has encountered and may continue to encounter that are frequently experienced by companies at an early stage, slowing demand for its solutions and fertility services in general, changes in utilization trends by its clients, general economic slowdown, an increase in unemployment, an increase in competition, changes to health care trends and regulations, changes to science relating to the fertility market, a decrease in the growth of the fertility market, or its failure, for any reason, to continue to take advantage of growth opportunities. If NewGenIvf’s assumptions regarding these risks and uncertainties and its future revenue growth are incorrect or change, or if it does not address these risks successfully, its operating and financial results could differ materially from its expectations, and its business could suffer.
NewGenIvf’s marketing efforts depend significantly on its ability to receive positive references from its existing clients.
NewGenIvf’s marketing efforts depend significantly on its ability to call on its current clients to provide positive references to new, potential clients. Given its limited number of long-term clients, the loss or dissatisfaction of any client could substantially harm its brand and reputation, inhibit the market adoption of its offering and impair its ability to attract new clients and maintain existing clients. Any of these consequences could have an adverse effect on its business, financial condition and results of operations.
As a public reporting company, we are subject to filing deadlines for reports that we file pursuant to the Exchange Act, and our failure to timely file such reports may have material adverse consequences on our business.
In the past, we have not been able to, and may continue to be unable to produce timely financial statements, and file these financial statements as part of a periodic report in a timely manner with the SEC. For example, we failed to timely file with the SEC the requisite Form 20-F for the year ended December 31, 2023. Consequently, we were not compliant with the periodic reporting requirements under the Exchange Act at such time. We cannot guarantee that in the future our reporting will always be timely. Our failure to timely file future periodic reports with the SEC could subject us to enforcement action by the SEC and shareholder lawsuits and could eventually result in the delisting of our Class A Ordinary Shares from Nasdaq, regulatory sanctions from the SEC, and/or the breach of covenants in our credit facilities or of any preferred equity or debt securities we may issue in the future, any of which could have a material adverse impact on our operations and your investment in our Class A Ordinary Shares, and our ability to register with the SEC public offerings of our securities for our benefit or the benefit of our security holders. Additionally, our failure to file our past periodic reports and future periodic reports has resulted in and could result in investors not receiving adequate information regarding us with which to make investment decisions. As a result, investors may not have access to current or timely financial information about our business.
If we are unable to continue to meet the listing requirements of Nasdaq, our Class A Ordinary Shares will be delisted.
On October 8, 2024, the Company received a deficiency letter (“Bid Price Deficiency Letter”) from the Listing Qualifications Department (the “Staff”) of Nasdaq notifying the Company that it is currently not in compliance with the closing bid price requirement under Nasdaq Listing Rule 5450(a)(1) (the “Minimum Bid Price Rule”). The Bid Price Deficiency Letter stated that, for the preceding 30 consecutive business days, the Company’s Class A Ordinary Shares did not meet the minimum closing bid price of $1 per share pursuant to the Minimum Bid Price Rule. The Company has an initial compliance period of 180 calendar days, or until April 7, 2025 to regain compliance with the Minimum Bid Price Rule. The Deficiency letter stated that if at any time the closing bid price of the Company’s Class A Ordinary Shares is at least $1 for a minimum of ten consecutive business days, Nasdaq will provide the Company written confirmation of compliance with this requirement, as applicable. If the Company does not regain compliance with the above requirements by April 7, 2025, the Company will receive written notification that its securities are subject to delisting. The Company may be eligible for additional time to regain compliance, which will require the Company to submit to Nasdaq written notice of its intent to cure the deficiency ahead of April 7, 2025. The Company intends to monitor the minimum bid price of its Class A Ordinary Shares and may, if appropriate, consider available options to regain compliance with the Nasdaq requirements.
On May 24, 2024, the Company received a MVLS Deficiency Letter from the Listing Qualifications Department (the “Staff”) of Nasdaq notifying the Company that, for the preceding 35 consecutive business days, the Class A Shares did not meet the minimum MVLS Requirement for continued listing on Nasdaq pursuant to Nasdaq Listing Rules 5450(b)(2)(A). In accordance with Nasdaq Rule 5810(c)(3)(C), the Company has been provided an initial period of 180 calendar days, or until November 20, 2024, the Compliance Date, to regain compliance with the MVLS Requirement. If, at any time before the Compliance Date, the MVLS for the Class A Shares is at least $50,000,000 for a minimum of ten consecutive business days, the Staff will provide the Company written confirmation of compliance with the MVLS Requirement. In the event the Company does not regain compliance with the above requirement prior to the expiration of the compliance period, it will receive written notification that its securities are subject to delisting.
On May 24, 2024, the Company received a MVPHS Deficiency Letter from the Staff of Nasdaq notifying the Company that, for the preceding 35 consecutive business days, the Company’s Class A Ordinary Shares did not meet the minimum $15,000,000 MVPHS Requirement for continued listing on Nasdaq pursuant to Nasdaq Listing Rules 5450(b)(2)(C) . In accordance with Nasdaq Rule 5810(c)(3)(D), the Company has until the Compliance Date to regain compliance with the MVPHS Requirement. If, at any time before the Compliance Date, the MVPHS for the Class A Shares is at least $15,000,000 for a minimum of ten consecutive business days, the Staff will provide the Company written confirmation of compliance with the MVPHS Requirement. In the event the Company does not regain compliance with the above requirement prior to the expiration of the compliance period, it will receive written notification that its securities are subject to delisting. Alternatively, the Company may apply to transfer the Company’s securities to The Nasdaq Capital Market.
On November 21, 2024, the Company received a notice from the Staff of Nasdaq notifying the Company that its securities are subject to delisting due to the MVPHS Deficiency and MLVS Deficiency. The Company will formally request a hearing to appeal the delisting determination and intends to leverage several strategic options to ensure its securities remain publicly traded. In addition, the Company will apply to transfer its securities from the Nasdaq Global Market to the Nasdaq Capital Market.
If we are unable to achieve and maintain compliance with such listing standards or other Nasdaq listing requirements in the future, our Class A Ordinary Shares could be delisted from Nasdaq. A delisting of our Class A Ordinary Shares and our inability to list on another national securities market could negatively impact us by: (i) reducing the liquidity and market price of our Class A Ordinary Shares; (ii) reducing the number of investors willing to hold or acquire our Class A Ordinary Shares, which could negatively impact our ability to raise equity financing; (iii) limiting our ability to use certain registration statements to offer and sell freely tradable securities, thereby limiting our ability to access the public capital markets; and (iv) impairing our ability to provide equity incentives to our employees.
If NewGenIvf is unable to attract new clients, its business, financial condition and results of operations would be adversely affected.
To increase its revenue, NewGenIvf must continue to attract new clients. NewGenIvf’s ability to do so depends in large part on the success of its sales and marketing efforts, and the success of references through existing clients. Potential clients may seek out other options; therefore, NewGenIvf must demonstrate that its solutions are valuable and superior to alternatives. If NewGenIvf fails to provide high-quality solutions and convince clients of the benefits of its model and value proposition, NewGenIvf may not be able to attract new clients. If the markets for NewGenIvf’s solutions decline or grow more slowly than it expects, or if the number of clients that contract with it for its solutions declines or fails to increase as it expects, its financial results could be harmed. As the markets in which NewGenIvf participate mature, fertility solutions and services evolve and competitors begin to enter into the market and introduce differentiated solutions or services that are perceived to compete with its solutions, particularly if such competing solutions are adopted by its competitors, its ability to sell its solutions could be impaired. As a result of these and other factors, NewGenIvf may be unable to attract new clients, which would have an adverse effect on its business, financial condition and results of operations.
NewGenIvf’s business depends on its ability to maintain its existing client demographics. Any failure to do so would harm its business, financial condition and results of operations.
As part of its growth strategy, NewGenIvf is focused on maintaining its services within its existing client demographics. NewGenIvf mainly competes with mid-level private clinics and hospitals, which have improved and developed their services and equipment over the years. In addition to private clinics and hospitals already existing, foreign medical companies may also enter the markets where NewGenIvf operates. Such foreign medical companies may be well-placed to compete with NewGenIvf due to their larger network size, reputation as global players and access to more advanced technology and financial resources. The expansion of existing competitors in the industry may erode NewGenIvf’s existing market share or decrease its traditional client pool. There can be no assurance that NewGenIvf will be able to compete effectively and therefore its future business growth may suffer.
A significant reduction in the utilization of NewGenIvf’s solutions could have an adverse effect on its business, financial condition and results of operations.
A significant reduction in the number of clients using NewGenIvf’s solutions could adversely affect its business, financial condition and results of operations. Factors that could contribute to a reduction in the use of its solutions include: general economic downturn that results in adverse financial conditions; regulatory changes; failure to adapt and respond effectively to changing medical landscape, changing regulations, changing client needs, requirements or preferences; negative publicity, through social media or otherwise and news coverage.
If NewGenIvf fails to offer high-quality support, its reputation could suffer.
NewGenIvf relies on its client account management personnel and the patient navigators (the “PNs”) to resolve client issues and help clients realize the full benefits that its solutions and services provide. High-quality support is also important for the renewal and expansion of its services to existing clients. The importance of its support functions will increase as NewGenIvf expands its business and pursue new clients. If NewGenIvf does not help its clients quickly resolve issues and provide effective ongoing supports, its ability to maintain and expand its offerings to existing and new clients could suffer, and its reputation with existing or potential clients could suffer. Further, to the extent that NewGenIvf is unsuccessful in hiring, training and retaining adequate PNs and client account management personnel, its ability to provide adequate and timely support to its clients would be negatively impacted, and its clients’ satisfaction with its solutions and services would be adversely affected.
NewGenIvf’s failure to effectively develop and expand its marketing and sales capabilities could harm its ability to increase its client base and achieve broader market acceptance of solutions NewGenIvf provides.
NewGenIvf’s ability to increase its client base and achieve broader market acceptance of solutions it provides will depend to a significant extent on its ability to expand its marketing and sales capabilities. NewGenIvf plans to continue expanding its direct sales force and to dedicate significant resources to sales and marketing programs, including direct sales, inside sales, targeted direct marketing, advertising, digital marketing, e-newsletter and conference sponsorships. All of these efforts will require it to invest significant financial and other resources. Its business and results of operations could be harmed if its sales and marketing efforts do not generate significant increases in revenue. NewGenIvf may not achieve anticipated revenue growth from expanding its sales and marketing efforts if it is unable to hire, develop, integrate and retain talented and effective sales personnel, if its new and existing sales personnel, on the whole, are unable to achieve desired productivity levels in a reasonable period of time, or if its sales and marketing programs are not effective.
NewGenIvf may experience net losses and may not sustain profitability in the future.
NewGenIvf experienced significant revenue decrease from 2019 to 2020, due to the impact of COVID-19. NewGenIvf is not certain whether it will obtain sufficient levels of sales to sustain its growth or maintain profitability in the future. NewGenIvf also expects its costs and expenses to increase in future periods, which could negatively affect its future results of operations if its revenue does not increase accordingly. In particular, NewGenIvf intends to continue to incrementally expand its sales and client account management teams to educate potential clients and drive new client adoption. NewGenIvf also expects to incur additional costs as it introduces new solutions and services to enhance its comprehensive fertility offering. NewGenIvf will also face increased compliance costs associated with growth, the expansion of its client base and being a public company. NewGenIvf’s efforts to grow its business may be costlier than it expects, and NewGenIvf may not be able to increase its revenue enough to offset its increased operating expenses. NewGenIvf may incur significant losses in the future for a number of reasons, including the other risks described herein, and unforeseen expenses, difficulties, complications and delays, and other unknown events. If NewGenIvf is unable to sustain profitability, the value of its business and common stock may significantly decrease.
NewGenIvf’s future revenue may not grow at the rates it historically has, or at all.
NewGenIvf has experienced growth since its business operations started in 2014. Revenue and NewGenIvf’s client base may not grow at the same rates they historically have, or they may decline in the future. NewGenIvf’s future growth will depend, in part, on its ability to:
| ● | continue to attract new clients and/or maintain existing clients; |
| ● | price its solutions and services effectively so that it is able to attract new clients, expand sales to its existing clients and maintain profitability; |
| ● | provide its clients with client support that meets their needs, including through dedicated PNs; |
| | ● | maintain successful collection of applicable receivable balances; |
| | ● | retain and maintain relationships with high-quality and respected fertility specialists; |
| | ● | attract and retain highly qualified personnel to support all clients; and |
| | ● | increase awareness of its brand and successfully compete with other competitors. |
NewGenIvf may not successfully accomplish all or any of these objectives, which may affect its future revenue, and which makes it difficult for it to forecast its future results of operations. In addition, if the assumptions that NewGenIvf uses to plan its business are incorrect or change in reaction to changes in its market, it may be difficult for it to maintain profitability. NewGenIvf’s shareholders should not rely on its revenue for any prior quarterly or annual periods as any indication of its future revenue or revenue growth.
In addition, NewGenIvf expects to continue to expend substantial financial and other resources on:
| | ● | technology infrastructure, including systems architecture, scalability, availability, performance and security; and |
| | ● | general administration, including increased legal and accounting expenses associated with being a public company. |
These investments may not result in increased revenue growth in its business. If NewGenIvf is unable to increase its revenue at a rate sufficient to offset the expected increase in its costs, its business, financial position, and results of operations will be harmed, and NewGenIvf may not be able to maintain profitability over the long term. Additionally, NewGenIvf may encounter unforeseen operating expenses, difficulties, complications, delays and other unknown factors that may result in losses in future periods.
If its revenue growth does not meet its expectations in future periods, NewGenIvf may not maintain profitability in the future, its business, financial position and results of operations may be harmed.
NewGenIvf’s quarterly and annual results may fluctuate significantly and may not fully reflect the underlying performance of NewGenIvf’s business.
NewGenIvf’s quarterly and annual results of operations, including the levels of NewGenIvf’s revenues, expenses, net (loss)/income and other key metrics, may vary significantly in the future due to a variety of factors, some of which are outside of NewGenIvf’s control, and period-to-period comparisons of NewGenIvf’s operating results may not be meaningful, especially given NewGenIvf’s limited operating history. Accordingly, the results for any one fiscal quarter or any one fiscal year are not necessarily an indication of future performance. Fluctuations in quarterly and/or annual financial results may adversely affect the price of NewGenIvf’s ordinary shares. Factors that may cause fluctuations in NewGenIvf’s quarterly and annual financial results include:
| ● | NewGenIvf’s ability to attract new customers and maintain relationships with existing customers; |
| ● | changes in NewGenIvf’s products and services offered and introduction of new services and products; |
| ● | the amount and timing of operating expenses related to marketing and the maintenance and expansion of NewGenIvf’s business, operations and infrastructure; |
| ● | general economic, industry and market conditions; and |
| ● | the timing of expenses related to the development or acquisition of technologies or businesses. |
If the estimates and assumptions NewGenIvf uses to determine the size of the target markets for its services are inaccurate, its future growth rate may be impacted and its business would be harmed.
Market opportunity estimates and growth forecasts are subject to significant uncertainty and are based on assumptions and estimates that may not prove to be accurate. Market opportunity estimates and growth forecasts included in this prospectus, including those NewGenIvf has generated itself, are subject to significant uncertainty and are based on assumptions and estimates that may not prove to be accurate, including the risks described in this prospectus. Even if the markets in which NewGenIvf competes achieve the forecasted growth, its business could fail to grow at similar rates, if at all.
NewGenIvf’s estimates of the market opportunity for its services are based on the assumption that the purpose-built, data-driven and disruptive fertility services platform with the plan design NewGenIvf offers will be attractive to clients. Clients may pursue alternatives or may not see the value in providing enhanced fertility-related services. In addition, NewGenIvf believes that it is expanding the size of the fertility market as NewGenIvf enhances demand and increase awareness for fertility services. If these assumptions prove inaccurate, or if the increase in awareness of fertility services attracts potential competitors to the market and results in greater competition, NewGenIvf’s business, financial condition and results of operations could be adversely affected.
It is difficult to predict the demand for NewGenIvf’s solutions, the entry of competitive solutions or the future growth rate and size of the fertility market. The expansion of the fertility market depends on a number of factors, including, but not limited to: the continued trend of individuals starting families later in life, increase in the number of single mothers by choice, adoption of non-traditional paths to parenthood and continued de-stigmatization of infertility.
If there is a reduction in demand caused by a lack of client acceptance, weakening economic conditions, data security or privacy concerns, governmental regulation, competing offerings or otherwise, the market for its solutions and services might not continue to develop or might develop more slowly than NewGenIvf expects, which would adversely affect its business, financial condition and results of operations.
NewGenIvf may not be able to successfully manage its growth, and if NewGenIvf is not able to grow efficiently, its business, financial condition and results of operations could be harmed.
As usage of its solutions grows, NewGenIvf will need to devote additional resources to improving and maintaining its infrastructure. In addition, NewGenIvf will need to appropriately scale its internal business systems and its client account management and services personnel to serve its growing client base. Any failure of or delay in these efforts could result in reduced client satisfaction, resulting in decreased sales to new clients and lower renewal and utilization rates by existing clients, which could hurt its revenue growth and its reputation. Even if NewGenIvf is successful in these efforts, they will require the dedication of management time and attention. NewGenIvf could also face inefficiencies or service disruptions as a result of its efforts to scale its internal infrastructure. NewGenIvf cannot be sure that the expansion and improvements to its internal infrastructure will be effectively implemented on a timely basis, and such failures could harm its business, financial condition and results of operations.
If NewGenIvf’s new solutions and services are not adopted by its clients, or if it fails to innovate and develop new offerings that are adopted by its clients, its revenue and results of operations may be adversely affected.
To date, NewGenIvf has derived a substantial majority of its revenue from sales of its fertility services. As NewGenIvf operates in an evolving industry, its long-term results of operations and continued growth will depend on its ability to successfully develop and market new successful solutions and services to its clients. If its existing clients do not value and/or are not willing to make additional payments for such new solutions or services, it could adversely affect its business, financial condition and results of operations. If NewGenIvf is unable to predict clients’ preferences, if the markets in which NewGenIvf participates change, including in response to government regulation, or if NewGenIvf is unable to modify its solutions and services on a timely basis, NewGenIvf may lose clients. Its results of operations would also suffer if its innovations were not responsive to the needs of the clients, appropriately timed with market opportunity or effectively brought to market.
If NewGenIvf fails to adapt and respond effectively to the changing medical landscape, changing regulations, changing client needs, requirements or preferences, its offerings may become less competitive.
The market in which NewGenIvf competes is subject to a changing medical landscape and changing regulations, as well as changing client needs, requirements and preferences. The success of its business will depend, in part, on its ability to adapt and respond effectively to these changes on a timely basis. NewGenIvf’s business strategy may not effectively respond to these changes, and NewGenIvf may fail to recognize and position itself to capitalize upon market opportunities. NewGenIvf may not have sufficient advance notice and resources to develop and effectively implement an alternative strategy. There may be scientific or clinical changes that require it to change its solutions or that make its solutions less competitive in the marketplace. If there are sensitivities to its model or its existing competitors and new entrants create new disruptive business models and/or develop new solutions that clients prefer to its solutions, NewGenIvf may lose clients, and its results of operations, cash flows and/or prospects may be adversely affected. The future performance of NewGenIvf’s business will depend in large part on its ability to design and implement market appropriate strategic initiatives, some of which will occur over several years in a dynamic industry. If these initiatives of NewGenIvf do not result in met objectives, NewGenIvf’s results of operations could be adversely affected.
If NewGenIvf fails to maintain and enhance its brand, its ability to expand its client base will be impaired and its business, financial condition and results of operations may suffer.
The growth of NewGenIvf’s business partially depends on the recognition of NewGenIvf’s brand and reputation. NewGenIvf believes that maintaining and enhancing its brand is important to support the marketing and sale of its existing and future solutions to new clients and expand sales of its solutions to existing clients. NewGenIvf also believes that the importance of brand recognition will increase as competition in its market increases. Successfully maintaining and enhancing its brand will depend largely on the effectiveness of its marketing efforts, its ability to provide reliable services that continue to meet the needs of its clients at competitive prices, its ability to maintain its clients’ trust, its ability to continue to develop new solutions, and its ability to successfully differentiate its platform from competitive solutions and services. NewGenIvf’s brand promotion activities may not generate client awareness or yield increased revenue, and even if they do, any increased revenue may not offset the expenses NewGenIvf incurs in building its brand. If NewGenIvf fails to successfully promote and maintain its brand, its business, financial condition and results of operations may suffer.
If NewGenIvf fails to retain and motivate members of its management team or other key employees, or fails to attract additional qualified personnel to support its operations, its business and future growth prospects could be harmed.
NewGenIvf’s success and future growth depend largely upon the continued services of its management team and its other key employees. From time to time, there may be changes in its executive management team or other key employees resulting from the hiring or departure of these personnel. Its executive officers and other key employees are employed on an at-will basis, which means that these personnel could terminate their employment with it at any time. The loss of one or more of its executive officers, or the failure by its executive team to effectively work with its employees and lead its company, could harm its business.
In addition, to execute its growth plan, NewGenIvf must attract and retain highly qualified personnel. Competition for these personnel is intense, especially for experienced medical officers and scientific staffs and sales and client account management personnel. There is no guarantee NewGenIvf will be able to attract such personnel or that competition among potential employers will not result in increased salaries or other benefits. From time to time, NewGenIvf has experienced, and NewGenIvf expects to continue to experience, difficulty in hiring and retaining employees with appropriate qualifications. Many of the companies with which NewGenIvf competes for experienced personnel have greater resources than NewGenIvf has. If NewGenIvf hires employees from competitors or other companies, their former employers may attempt to assert that these employees or NewGenIvf has breached their legal obligations, resulting in a diversion of its time and resources. In addition, prospective and existing employees often consider the value of the equity awards they receive in connection with their contribution to the company. If the perceived value of its equity awards declines, experiences significant volatility, or increases such that prospective employees believe there is limited upside to the value of its equity awards, it may adversely affect its ability to recruit and retain key employees. If NewGenIvf fails to attract new personnel or fails to retain and motivate its current personnel, its business and future growth prospects could be harmed.
Furthermore, in order to attract and retain key personnel and employees, the compensation amounts for NewGenIvf’s executive officers may change significantly after consummation of the Business Combination, although there are currently no agreements in place relating to any such post Business Combination compensation arrangements. As a result, NewGenIvf’s expenses associated with the compensation may increase, which may also have an adverse effect on its results of operations.
To successfully market and sell its services and products in Asia-Pacific markets, NewGenIvf must address many international business risks with which NewGenIvf has limited experience.
NewGenIvf’s business is subject to risks in connection with changes in international, national and local economic and market conditions, including the effects of global financial crises, effects of terrorist acts and war and global pandemics. Such economic changes could negatively impact infertile couples’ abilities to pay for fertility treatments around the world.
NewGenIvf’s strategy is to increase its international presence in Asia-Pacific countries and its international sales are subject to a number of risks, including:
| | ● | increased competition as a result of more products and procedures receiving regulatory approval or otherwise free to market in international markets; |
| | ● | longer accounts receivable payment cycles and difficulties in collecting accounts receivable; |
| | ● | reduced or varied protection for intellectual property rights in some countries; |
| | ● | export restrictions, trade regulations, and foreign tax laws; |
| | ● | fluctuations in currency exchange rates; |
| | ● | foreign certification and regulatory clearance or approval requirements; |
| | ● | customs clearance and shipping delays; |
| | ● | political, social, and economic instability abroad, terrorist attacks, and security concerns in general; |
| | ● | preference for locally provided services; |
| | ● | potentially adverse tax consequences, including the complexities of foreign value-added tax systems; |
| | ● | the burdens of complying with a wide variety of foreign laws and different legal standards; and |
| | ● | increased financial accounting and reporting burdens and complexities. |
If one or more of these risks are realized, its business, financial condition and results of operations could be adversely affected.
Ethical, legal and social concerns related to the use of assisted reproductive technology could reduce demand for the fertility services provided by the medical facilities in NewGenIvf’s network, and thus may adversely affect the business, financial conditions and results of operations of the medical facilities in its network.
Patient sentiment and distrust of the use of assisted reproductive technology may lead to less demand for fertility services. Assisted reproductive technologies, including genetic testing, technologies used for surrogacy and egg donation and gender selection, have raised ethical, legal and social issues regarding privacy and the appropriate uses of the resulting information. Government authorities could, for social or other purposes, limit or regulate the use of assisted reproductive technology to certain conditions. Similarly, these concerns may lead patients to refuse to use, or physicians to be reluctant to order, assisted reproductive services even if permissible. These and other ethical, legal and social concerns may limit market acceptance of fertility services or reduce patient demand for such services, either of which could have a material adverse effect on the business, financial condition and results of operations of the medical facilities in NewGenIvf’s network, and NewGenIvf itself.
NewGenIvf is reliant on revenue from international clients.
Fertility services revenue from international clients are an important part of NewGenIvf’s revenue, though NewGenIvf is expanding rapidly into the local markets. The number of international clients travelling to Thailand, Cambodia and Kyrgyzstan to seek fertility services may, however, be affected by a number of factors, including the economic status of the foreign client’s country of origin, the relative exchange rate of the client’s home currency to the relevant authorities, which may affect the cost of treatment, natural disasters, pandemics like COVID-19, and political tension or acts of terrorism in such countries and the region. For example, the COVID-19 has had resulted in a number of countries declaring a state of emergency and a number of countries, including the countries in Asian Pacific, imposing extensive travel restrictions, which in turn caused a decrease in the numbers of internal clients traveling to Thailand, Cambodia or Kyrgyzstan for treatments.
These events could cause a postponement or a reduction in the number of clients traveling to Thailand, Cambodia or Kyrgyzstan, and could in turn affect revenues from international clients, which is the significant contributor in terms of volume. A decline in the medical tourism industry may have a material adverse effect on NewGenIvf’s financial condition and results of operations.
Fluctuations in exchange rates could have a material and adverse effect on NewGenIvf’s results of operations and the value of your investment.
NewGenIvf’s reporting currency is U.S. dollars. The functional currency of NewGenIvf and its subsidiaries include Hong Kong dollar (“HK$”), Thai baht (“THB”), Cambodian riel (“KHR”) and United States dollar (“USD”). Accordingly, fluctuations in the value of HK$, THB and KHR relative to the USD could affect its results of operations due to translational remeasurements. As its international operations expand, an increasing portion of its revenue and operating expenses may be denominated in non- HK$, THB or KHR currencies. Accordingly, NewGenIvf’s revenue and operating expenses will become increasingly subject to fluctuations due to changes in foreign currency exchange rates. If NewGenIvf is not able to successfully hedge against the risks associated with currency fluctuations, NewGenIvf’s business, financial condition and results of operations could be materially adversely affected.
Governmental control of currency conversion may limit NewGenIvf’s ability to utilize NewGenIvf’s net revenue effectively and affect the value of your investment.
NewGenIvf’s revenue and expenses for its businesses are substantially denominated in THB, which are currently not freely convertible currencies. A portion of such revenue must be converted into other currencies in order to meet its foreign currency obligations. For example, NewGenIvf’s subsidiaries will need to obtain foreign currency to make payments of declared dividends, if any, on its shares.
Under the existing foreign exchange regulations in Thailand, NewGenIvf will be able to make current account foreign exchange transactions. However, in the future, governments may take measures, at its discretion, to restrict access to foreign currencies for capital account and current account transactions under certain circumstances. If such measures are implemented, NewGenIvf may not be able to pay dividends in foreign currencies to holders of its shares. Foreign exchange transactions under its capital account are subject to significant foreign exchange controls and require certain approvals. These limitations could affect our ability to obtain foreign exchange through offshore financing.
The value of the THB against the U.S. dollar and other currencies fluctuates, and is subject to changes resulting from policies of the Thailand and other governments, and depends to a large extent on domestic and international economic and political developments as well as supply and demand in the local market. For example, the Bank of Thailand, which is the central bank of Thailand, is responsible for formulating and implementing monetary policies in the country to maintain the price stability and promote economic stability and sustainable growth. The Bank of Thailand imposes (four) measures in preventing THB fluctuation. Those are measures to limit THB liquidity, to curb capital inflows, to limit the flows on Non-resident Bank Account and Non-resident Baht for Securities, and to limit the flows on Non-Deliverable Forward transactions. With an increased floating range of the THB’s value against foreign currencies and a more market-oriented mechanism for determining the mid-point exchange rates, the THB may further appreciate or depreciate significantly in value against the U.S. dollar or other foreign currencies in the long-term, depending on the fluctuation of the basket of currencies against which it is currently valued, or it may be permitted to enter into a full float, which may also result in a significant appreciation or depreciation of the THB against the U.S. dollar or other foreign currencies. It cannot be assured that THB will not experience significant appreciation or depreciation against the U.S. dollar or other foreign currencies in the future.
Furthermore, NewGenIvf is also currently required to obtain approvals before converting significant sums of foreign currencies into THB. All of these factors could materially and adversely affect its business, results of operations, financial condition and prospects, and could reduce the value of, and dividends payable on, its shares in foreign currency terms.
Sales of a substantial number of our securities in the public market by the Selling Shareholder and/or by our existing securityholders could cause the price of our Ordinary Shares to decrease significantly.
The Selling Shareholder can resell, under this prospectus, up to 4,000,000 Ordinary Shares. The securities being offered in this prospectus represent a substantial percentage of our issued and outstanding Ordinary Shares, and the sale of such securities in the public market by the Selling Shareholder, or the perception that those sales might occur, could depress the market price of our Ordinary Shares, and could impair our ability to raise capital through the sale of additional equity securities. We are unable to predict the effect that such sales may have on the prevailing market price of our Ordinary Shares.
Substantially all of NewGenIvf’s assets and operations are located in Thailand, Cambodia and Kyrgyzstan and they are subject to economic, legal and regulatory uncertainties in such countries.
Substantially all of NewGenIvf’s operations and assets are based in Thailand, Cambodia and Kyrgyzstan. As a result, its businesses and operations are subject to the changing economic conditions prevailing from time to time in such countries. Since 2020, Thailand’s economy has been experiencing a slowdown. According to the National Economic and Social Development Board of Thailand (the “NESDB”) the GDP growth rate of Thailand declined to minus 6.1% in 2020 and slightly recovered to 1.6% in 2021 and 2.6% in 2022. Under such conditions, the NESDB projected that Thailand’s economy will only grow by 3.0% to 4.0% in 2023, lower than the previously growth in historical years. Meanwhile, Cambodia’s post-pandemic economic recovery has gained momentum, but remains uneven. Traditional growth drivers, especially manufacturing and agricultural commodities exports, have fully recovered. However, while travel and tourism have improved, the sector remains well below pre-COVID-19 levels. The subsequent impact also caused the vendors and customers preference change, lower the willingness travelling to Kyrgyzstan for surrogacy services. The economy is projected to grow, underpinned by merchandise exports and domestic economic activity. Foreign direct investment, while diversified, remains affected by China’s related COVID-19 policies.
NewGenIvf also derives a substantial portion of its revenue from Chinese clients and as such, its maintenance of PRC-sourced revenues and access to new and existing clients from the PRC are also subject to the economic conditions of China. However, the near-term growth prospects of the PRC economy are unclear due to the uncertain effects of ongoing economic stress caused by policies to contain the COVID-19 pandemic, trade and national security policies, and the elevated levels of private and public indebtedness, among others. According to the National Statistics Bureau of the PRC, growth rate of China’s GDP for the year 2022 slowed down to 3.0% on a year-on-year basis compared to the growth rate of approximately 8.4% for the year 2021. In the second quarter of 2023, China’s GDP grew only 0.8% on a quarter basis, a significant slowdown from the 2.2% quarter growth registered in the first quarter of 2023. A prolonged downturn in the PRC economy generally could materially and adversely affect NewGenIvf’s results of operations.
Factors that may adversely affect the economy and conditions in such countries include:
| | ● | political instability (e.g., Thailand’s national election in May 2023); |
| | ● | global economic conditions; |
| | ● | exchange rate fluctuations and the exchange control policy of the banks; |
| | ● | a prolonged period of inflation or increase in regional interest rates; |
| | ● | changes in government policies affecting import and export volumes; |
| | ● | natural disasters, including tsunamis, earthquakes, fires, floods, drought and similar events; |
| | ● | a potential recurrence or outbreak of avian influenza, severe acute respiratory syndrome or other infectious or contagious diseases like COVID-19 in Asian countries, and governmental policies to address such outbreak; |
| | ● | scarcity of credit or other financing, resulting in lower demand for products and services provided by companies in the region; |
| | ● | increases in oil prices and other commodity prices; |
| | ● | decreased consumer confidence; |
| | ● | other external recessions or potential economic downturns in the United States, Asia or other parts of the world; and |
| | ● | other regulatory, political or economic developments in or affecting the countries. |
The economic conditions in Thailand, Cambodia, Kyrgyzstan and China are also affected by global economic conditions. The global credit markets have experienced, and may continue to experience, volatility and liquidity disruptions, which have resulted in the consolidation, failure or near failure of a number of institutions in the banking and insurance industries. There remains a concern that a return of the debt crisis in Europe, the political unrest in the Middle East and Eastern Europe as well as rumors or threats or actual terrorist attacks or conflicts in the Middle East, Southeast Asia, Eastern Europe or other regions will impinge upon the health of the global financial system. These or other such events could adversely affect NewGenIvf’s business, financial condition, results of operations and prospects.
There is no assurance that the economies and social conditions of Thailand, Cambodia, Kyrgyzstan and China will meet current projections or improve in the future. Any instability or economic downturn could have a material adverse effect on NewGenIvf’s business, financial condition, results of operations and prospects.
Failure to comply with the terms of future financing arrangements could result in default, which could have an adverse effect on NewGenIvf’s cash flow and liquidity.
NewGenIvf may from time to time enter into credit facilities and debt financing arrangements containing financial and other covenants that could, among other things, restrict NewGenIvf’s business and operations. If NewGenIvf breaches any of these covenants, including the failure to maintain certain financial ratios, NewGenIvf’s lenders may be entitled to accelerate NewGenIvf’s debt obligations. Any default under the credit facility could result in the repayment of these loans prior to maturity as well as the inability to obtain additional financing, which in turn may have a material adverse effect on NewGenIvf’s cash flow and liquidity.
NewGenIvf requires a significant amount of capital to fund its operations and growth. If NewGenIvf cannot obtain sufficient capital on acceptable terms, its business, financial condition, and prospects may be materially and adversely affected.
NewGenIvf requires a significant amount of capital and resources for its operations and continued growth. NewGenIvf expects to make significant investments to fund operations, laboratory upgrades, among other things, which may significantly increase NewGenIvf’s net cash used in operating activities. In addition, NewGenIvf will continue to invest in laboratory and facilities which are fundamental to NewGenIvf’s business operation and future growth. However, NewGenIvf cannot assure you that these investments will generate the optimal returns, if at all. To date, NewGenIvf has historically funded its cash requirements primarily through operational, capital contributions from its shareholders and short-term or long-term borrowings. If these resources are insufficient to satisfy NewGenIvf’s cash requirements, NewGenIvf may seek to raise funds through additional equity offering or debt financing or additional bank facilities. NewGenIvf’s ability to obtain additional capital in the future, however, is subject to a number of uncertainties, including those relating to its future business development, financial condition, and results of operations, general market conditions for financing activities by companies in its industry, and macro-economic and other conditions in Thailand, Cambodia, Kyrgyzstan and globally. If NewGenIvf cannot obtain sufficient capital on acceptable terms to meet its capital needs, NewGenIvf may not be able to execute its growth strategies, and NewGenIvf’s business, financial condition, and prospects may be materially and adversely affected.
The defects in certain leased property interests and failure to register certain lease agreements may materially and adversely affect NewGenIvf’s business, financial condition, results of operations, and prospects.
NewGenIvf leases premises in Thailand, Cambodia and Kyrgyzstan in various locations. With respect to property leased by First Fertility PGS Center in Thailand, the lessors did not have or provide NewGenIvf with property ownership certificates or other documents evidencing their rights to lease such premises to First Fertility PGS Center. Therefore, NewGenIvf cannot assure that it will not be subject to any challenges, lawsuits, or other actions taken against First Fertility PGS Center with respect to its leased premises for which the relevant lessors do not have valid title or right to lease. If First Fertility PGS Center’s lessors’ right to lease premises is successfully challenged by any third party, First Fertility PGS Center’s lease agreements may not be enforceable and NewGenIvf may be forced to vacate the premises and relocate to a different location. Under such circumstances, NewGenIvf expects to incur relocation costs of up to THB3 million and expects that there would not be material business interruption costs, if any.
In addition, the failure of the lessor to provide sufficient legal evidence of its right to lease the premises has prevented First Fertility PGS Center from registering the clinic with the Bangkok Metropolitan Authority (“BMA”) as required under the Public Health Act B.E. 2535 (1992) (the “PHA”). Under Section 71 of the PHA, First Fertility PGS Center and its directors are subject to imprisonment of up to 6 (six) months and a fine of up to THB50,000, or both. The BMA could also order First Fertility PGS Center to stop operating the clinic which would require relocation of the clinic if First Fertility PGS Center could not make the necessary registration. Under such circumstances, First Fertility PGS Center expects to incur relocation costs of up to THB3 million and expects that there would not be material business interruption costs, if any.
Only one of NewGenIvf’s directors or officers, namely Ms. Fong, Hei Yue Tina, is also a director of First Fertility PGS Center. NewGenIvf believes that if First Fertility PGS Center’s directors, including Ms. Fong, are found guilty of the above offence and subject to imprisonment, the resulting impact on NewGenIvf’s business, results of operations and financial conditions would be limited, as Ms. Fong has limited involvement in the day-to-day management of First Fertility PGS Center’s operations and Mr. Siu, Wing Fung Alfred and the other directors and officers of NewGenIvf and its subsidiaries would be able to keep operating the group’s and First Fertility PGS Center’s activities with limited disruptions.
In addition, NewGenIvf has not registered the lease agreements of First Fertility Bishkek in Kyrgyzstan with the relevant government authorities. The enforceability of the lease of property may therefore be subject to restrictions under relevant laws and regulations and NewGenIvf may be forced to vacate the premises and relocate to a different premise. Under such circumstances, NewGenIvf expects to incur relocation costs of up to USD150,000 and expects that there would not be material business interruption costs, if any. Meanwhile, First Fertility Bishkek may be required to pay a penalty for the late registration of the lease agreement with a lease term of 3 or more years, the maximum amount of which is KGS3060 ($35).
NewGenIvf currently has no insurance coverage for its operations.
The assisted reproductive medical facilities in NewGenIvf’s network are exposed to potential liabilities that are inherent to the provision of services. Medical and other liabilities may not be fully covered by insurance and the medical facilities may face claims in excess of the insurance coverage or claims which are not covered by insurance due to other policy limitations or exclusions or where the medical facilities in NewGenIvf’s network have failed to comply with the terms of the policy. Any uninsured risks may result in substantial costs and the diversion of resources, which could adversely affect its results of operations and financial condition.
The insurance industries in Thailand, Cambodia and Kyrgyzstan are still at early stages of development, and insurance companies in Thailand, Cambodia and Kyrgyzstan currently offer limited business-related insurance products. NewGenIvf does not currently maintain insurance. NewGenIvf cannot assure you that the medical facilities in its network will be able to obtain and/or maintain medical liability insurance on acceptable terms or without substantial premium increases or at all in the future.
In addition, as NewGenIvf’s business expands, the cost for each medical facility in its network and NewGenIvf to maintain an adequate level of insurance may become increasingly high. NewGenIvf cannot ensure that the medical facilities in its network will be able to locate or purchase appropriate insurance to cover the expanding operations in time, on commercially reasonable terms or at all. Any significant uninsured loss could have material and adverse effects on the financial condition and results of operations of the medical facilities in NewGenIvf’s network, and thus may affect its business, results of operations and financial condition.
Moreover, NewGenIvf does not currently maintain professional malpractice liability insurance for its physicians and nurses. As a result, NewGenIvf may be subject to medical disputes and claims arising under relevant laws from time to time, which could cause substantial damage to NewGenIvf if not covered by professional malpractice liability insurance. Any dispute with clients, or any legal proceeding involving the physicians of the medical facilities or medical professionals, regardless of its merit or eventual outcome, could result in significant legal costs and financial and/or reputational damages to the medical facilities and NewGenIvf and materially and adversely affect the business, financial condition and results of operations of the medical facilities in NewGenIvf’s network, and further affect its business, financial condition, results of operations and prospects.
NewGenIvf may not be successful in adapting to technological developments, which may affect its business and results of operations.
It is possible that new technologies could be developed or scientific advances made by NewGenIvf’s competitors, or elsewhere and licensed to NewGenIvf’s competitors, which cannot be replicated by NewGenIvf without significant capital expenditure or at all, or that replace or reduce the requirement for assisted reproductive services, ultrasound or specialized diagnostics. The consequences for NewGenIvf of the development of new technologies could include lower or loss of revenues, loss of market position and reduced prospects of NewGenIvf.
If its computer systems, or those of its providers, specialty pharmacies or other downstream vendors lag, fail or suffer security breaches, NewGenIvf may incur a material disruption of its services, which could materially impact its business and the results of operations.
NewGenIvf’s businesses in Thailand, Cambodia and Kyrgyzstan are increasingly dependent on critical, complex and interdependent information technology systems to support business processes as well as internal and external communications. NewGenIvf’s success is therefore dependent in part on its ability to secure, integrate, develop, redesign and enhance its (or contract with vendors to provide) technology systems that support its business strategy initiatives and processes in a compliant, secure, and cost and resource efficient manner. If NewGenIvf or its providers, specialty pharmacies or other downstream vendors have an issue with its or their respective technology systems, it may result in a disruption to its operations or downstream disruption to its relationships with its clients or its selective network of high-quality fertility specialists. Additionally, if NewGenIvf chooses to insource any of the services currently handled by a third party, it may result in technological or operational disruptions.
In addition, despite the implementation of security measures, its internal computer systems, and those of its provider clinics, specialty pharmacies or other downstream vendors, are potentially vulnerable to damage from malicious intrusion, malware, computer viruses, unauthorized access, natural disasters, terrorism, war and telecommunication and electrical failures. While NewGenIvf is not aware that it has experienced any such system failure, accident or security breach to date, if such an event were to occur and cause interruptions in its operations, it could result in a material disruption to its ability to operate and deliver its solutions. In addition, to the extent that any disruption or security breach were to result in a loss or inappropriate disclosure of confidential information, NewGenIvf could incur liability. See “— Risks Related to Government Regulation — NewGenIvf operates in a highly regulated industry and must comply with a significant number of complex and evolving requirements. Any lack of requisite approvals, licenses, or permits applicable to NewGenIvf’s business may have a material and adverse impact on NewGenIvf’s business, financial condition, and results of operations — Data Protection and Breaches.”
Risks Related to NewGenIvf’s Relationships with Third Parties
NewGenIvf’s business depends on its ability to maintain its network of high-quality fertility specialists and other healthcare providers. If NewGenIvf is unable to do so, its future growth would be limited and its business, financial condition and results of operations would be harmed.
NewGenIvf’s performance and success is dependent upon its continued ability to maintain a credentialed network of high-quality fertility specialists, including its senior management team, other key employees, as well as research and development and operation maintenance personnel, many of whom are difficult to replace. Fertility specialists could refuse to contract, demand higher payments or take other actions that could result in higher medical costs, less attractive service for its clients or difficulty meeting regulatory or accreditation requirements. Identifying high-quality fertility specialists, credentialing and negotiating contracts with them and evaluating, monitoring and maintaining its network, requires significant time and resources. Competition in the healthcare industry for qualified employees is intense. NewGenIvf may need to offer higher compensation and other benefits in order to attract and retain key personnel in the future, which could increase NewGenIvf’s compensation expenses, including stock-based compensation. NewGenIvf’s continued ability to compete effectively depends on NewGenIvf’s ability to attract new employees and to retain and motivate NewGenIvf’s existing employees. If NewGenIvf is not successful in maintaining its relationships with top fertility specialists, these fertility specialists may refuse to renew their contracts with it, and potential competitors may be effective in onboarding these or other high-quality fertility specialists to create a similarly high-quality network. There may be additional shifts in the fertility specialty provider space as the fertility market matures, and high-quality fertility specialists may become more demanding in re-negotiating to remain in its network. Its ability to develop and maintain satisfactory relationships with high-quality fertility specialists also may be negatively impacted by other factors not associated with it, such as regulatory changes impacting providers or consolidation activity among hospitals, physician groups and healthcare providers. In addition, certain organizations of physicians, such as practice management companies (which group together physician practices for administrative efficiency), may change the way in which healthcare providers do business with it and may compete directly with it, which could adversely affect its business, financial condition and results of operations. NewGenIvf intends to grant, and may continue to grant, options and other types of awards, which may result in increased share-based compensation expenses.
NewGenIvf’s Share Incentive Award will allow NewGenIvf to enhance its ability to attract and retain exceptionally qualified individuals and agents and to encourage them to acquire a proprietary interest in the company’s growth and performance. Competition for highly skilled personnel and agents is often intense and NewGenIvf may incur significant costs or may not be successful in attracting, integrating, or retaining qualified personnel and agents to fulfill NewGenIvf’s current or future needs. NewGenIvf believes that the granting of share-based awards is of significant importance to NewGenIvf’s ability to attract and retain agents, key personnel and employees, and NewGenIvf will continue to grant share-based awards in the future. As a result, NewGenIvf’s expenses associated with share-based compensation may increase, which may have an adverse effect on NewGenIvf’s results of operations.
Meanwhile, the retirement or loss of certain specialists, scientific staff or other key personnel, the activities of competitors, the introduction of a competing service that is perceived to be superior to the services provided by NewGenIvf, or other events which impact NewGenIvf’s reputation could adversely affect NewGenIvf’s relationships with fertility specialists. For example, one specialist who was previously engaged by NewGenIvf brought a lawsuit against NewGenIvf regarding disputed remuneration, which resulted in a settlement for NewGenIvf to compensate the specialist with a sum of approximately US$98,000. Also, fertility specialists’ relationship with NewGenIvf could affect their behaviors in recommending NewGenIvf’s services or referring patients to NewGenIvf, which could in turn adversely impact the number of patients treated by NewGenIvf and adversely impact on its financial performance, market position and prospects.
In addition, the perceived value of NewGenIvf’s solutions and its reputation may be negatively impacted if the services provided by fertility specialists or other healthcare providers are not satisfactory to NewGenIvf’s clients, including as a result of error that could result in litigation. For example, if fertility specialist or other healthcare provider releases sensitive information of its clients, it could incur additional expenses and give rise to litigation against NewGenIvf. Any such issue with one of its providers may expose it to public scrutiny, adversely affect its brand and reputation, expose it to litigation or regulatory action, and otherwise make its operations vulnerable. Further, if its services result in less than favorable outcomes, this could cause it to fail to meet its contractually guaranteed specified service metrics, and NewGenIvf could be obligated to provide the client with a fee reduction or a second chance for free, depending on their contract terms. The failure to maintain its selective network of high-quality fertility specialists or the failure of those specialists to meet and exceed its clients’ expectation, may result in a loss of or inability to grow or maintain its client base, which could adversely affect its business, financial condition and results of operations.
The medical facilities and professionals in NewGenIvf’s network could become the subject of litigation, allegations and other claims, and NewGenIvf is not insured against these liabilities.
NewGenIvf relies on the physicians and other medical professionals of the assisted reproductive medical facilities in its network to make proper clinical decisions regarding the diagnosis and treatment of clients. However, NewGenIvf does not have full and direct control over every step of clinical activities undertaken at each of the medical facilities. In addition, physicians and medical professionals outside NewGenIvf’s network may introduce patients to NewGenIvf and conduct medical treatments and/or procedures for such patients in NewGenIvf’s facilities. NewGenIvf enters into independent contractor agreements with such physicians and medical professionals and treats such patients as NewGenIvf’s own patients. As such, NewGenIvf will have to bear any liabilities arising from their medical treatments and/or procedures conducted in NewGenIvf’s facilities. Any incorrect clinical decision or malpractice on the part of physicians and other medical professionals (including those from outside of its network), or any failure by the medical facilities in its network to properly manage their clinical activities may result in unsatisfactory treatment outcomes, patient injury or even death, which could lead to disputes with patients and/or their families or the medical professionals, including those from outside its network. In its experience, moreover, clients of fertility treatments tend to be more demanding on the medical services received. In addition, the relevant laws governing medical disputes and claims grant claimants liberal rights in bringing claims against physicians and other medical professionals practicing in the jurisdiction. As a result, the medical facilities in its network may be subject to medical disputes and claims arising under relevant laws, from time to time, which could generate substantial damages imposed on such facilities if not covered by professional liability insurance. Any dispute with its patients and/or their families or the medical professionals, including those from outside its network, or any legal proceeding involving the physicians of the medical facilities or medical professionals, including those from outside its network, regardless of its merit or eventual outcome, could result in significant legal costs and reputational damage to the medical facilities and materially and adversely affect the business, financial condition and results of operations of the medical facilities in its network, and further affect its business, financial condition and results of operations.
The assisted reproductive medical facilities in NewGenIvf’s network have limited control over the quality of the pharmaceuticals, medical equipment, medical consumables and other supplies used in its operations, and cannot guarantee that the products in use are not defective or counterfeit. NewGenIvf also has no control over independent sub-contractors and cannot guarantee the services thereof.
The assisted reproductive medical facilities in NewGenIvf’s network procure a variety of pharmaceuticals, medical equipment, consumables and other supplies in NewGenIvf’s operations from third-party suppliers. As the medical facilities in NewGenIvf’s network do not engage in the direct manufacture of such supplies, NewGenIvf cannot assure you that such supplies are free of defects and meet relevant quality standards or, in the case of imported supplies, verify the origin of such products. In addition, there may be counterfeit pharmaceutical products manufactured without proper licenses or approvals or fraudulently mislabeled with respect to their content or manufacturer in the pharmaceutical markets. In some cases these products are very similar in appearance to the authentic products. The quality control checks and processes may not be able to identify all counterfeit pharmaceutical products in the inventory. Any sale of such products by the medical facilities in NewGenIvf’s network, regardless of its knowledge as to their authenticity, may subject the medical facilities to administrative sanctions, civil claims, negative publicity or reputational damage. NewGenIvf cannot assure you that the medical facilities in our network will be able to successfully claim full indemnity from such manufacturers of counterfeit pharmaceutical products.
NewGenIvf also cannot assure you that the medical facilities in our network will not encounter incidents relating to defective products, or that such incidents will not materially and adversely affect our network of medical facilities. If the products provided by NewGenIvf’s suppliers are defective, of poor quality or are otherwise unsafe or ineffective, the medical facilities in NewGenIvf’s network could be subject to liability claims, complaints or adverse publicity, any of which would materially and adversely affect its results of operations and reputation. NewGenIvf cannot assure you that the medical facilities in NewGenIvf’s network will find suitable replacement suppliers on commercially acceptable terms or at all.
The suppliers are also subject to extensive laws, rules and regulations. If any suppliers violate applicable laws, rules and regulations, NewGenIvf’s reputation or procurement may be materially and adversely affected. In addition, the medical facilities in NewGenIvf’s network may be exposed to reputational damages or even liabilities for defective goods provided by the suppliers or negative publicity associated with any suppliers, and the business and results of operations of the medical facilities in NewGenIvf’s network and NewGenIvf could suffer as a result.
Independent sub-contractors and/or agents that work with NewGenIvf are also subject to extensive laws, rules, and regulations. If any sub-contractor and/or agent violates any applicable laws, rules, regulations or breaches any agreements, NewGenIvf’s reputation may be materially and adversely affected and NewGenIvf may be penalized by regulatory or other parties. In addition, NewgenIvf’s clients may engage Newgen’s sub-contractors and/or agents for ongoing services or additional services following the termination of contracts with NewGenIvf. NewGenIvf has no control over the services provided by sub-contractors and cannot assure the quality of such services or ensure compliance with applicable laws, rules and regulations. In addition, the services provided by independent sub-contractors may expose NewGenIvf to public scrutiny, adversely affect its brand and reputation, expose it to litigation or regulatory action, and otherwise make its operations vulnerable if such independent sub-contractors fail to meet their contractual obligations or to comply with applicable laws or regulations.
If NewGenIvf loses its relationship with one or more key pharmaceutical manufacturers, its business and results of operations could be adversely affected.
NewGenIvf maintains contractual relationships with select pharmaceutical manufacturers in Thailand, Cambodia and Kyrgyzstan. The consolidation of pharmaceutical manufacturers, the shortages of drugs provided by such manufacturers, the termination or material alteration of its contractual relationships, or its failure to renew such contracts could have a material adverse effect on its business and results of operations. Adoption of new laws, rules or regulations or changes in, or new interpretations of, existing laws, rules or regulations, relating to any of these programs could materially adversely affect its business and results of operations.
NewGenIvf has engaged in transactions with related parties, and such transactions present potential conflicts of interest that could have an adverse effect on its business and results of operations.
NewGenIvf has entered into a number of transactions with related parties. NewGenIvf may in the future enter into additional transactions with its related parties. Interests of these related parties may not necessarily be aligned with NewGenIvf’s or The Company’s interests and the interests of its other shareholders. For example, conflicts of interest may arise in connection with decisions regarding the transaction arrangements which may be less favorable to NewGenIvf than similar arrangements negotiated with unaffiliated third parties. Conflicts of interest may also arise in connection with the exercise of contractual remedies, such as the treatment of events of default. As a result, those related party transactions, individually or in the aggregate, may have an adverse effect on NewGenIvf’s business and results of operations.
NewGenIvf may be subject to claims and allegations relating to intellectual property and other causes.
NewGenIvf may from time to time receive claims that NewGenIvf infringes on the intellectual property rights of others. Moreover, NewGenIvf may be subject to claims by third parties who maintain that NewGenIvf’s service providers’ technology infringes third-party’s intellectual property rights. If NewGenIvf fails to successfully defend against such claim or does not prevail in such litigation, it could be required to modify, redesign or cease operating, pay monetary amounts as damages or enter into royalty or licensing arrangements with the valid intellectual property holders. Any royalty or licensing arrangements that NewGenIvf may seek in such circumstances may not be available to it on commercially reasonable terms or at all. Also, if NewGenIvf acquires technology licenses from third parties, NewGenIvf’s exposure to infringement actions may increase because NewGenIvf must rely upon these third parties to verify the origin and ownership of such technology. This exposure to liability could result in disruptions in NewGenIvf’s business that could materially and adversely affect NewGenIvf’s results of operations.
Some of NewGenIvf’s employees may previously employed at other companies, including NewGenIvf’s competitors. NewGenIvf may hire additional personnel to expand its development team and technical support team as its business grows. To the extent these employees were involved in the development of content or technology similar to NewGenIvf’s at their former employers, NewGenIvf may become subject to claims that these employees or NewGenIvf has appropriated these employees’ former employers’ proprietary information or intellectual properties. If NewGenIvf fails to successfully defend such claims against itself, NewGenIvf may be exposed to liabilities which could have a material adverse effect on its business.
NewGenIvf is currently not a party to any material legal or administrative proceedings but may subject to legal or administrative actions for defamation, negligence, copyright and trademark infringement, unfair competition, breach of service terms, or other purported injuries resulting from the content NewGenIvf provides or the nature of NewGenIvf’s services. Such legal and administrative actions, with or without merits, may be expensive and time-consuming and may result in significant diversion of resources and management attention from NewGenIvf’s business operations. Furthermore, such legal or administrative actions may adversely affect NewGenIvf’s brand image and reputation.
Certain data and information in this prospectus relied on by NewGenIvf were obtained from third-party data and polls. These metrics were not independently verified by NewGenIvf and may not be accurate.
Certain numbers and information in this prospectus were obtained and provided from numerous sources including management data, third-party data or numbers generally estimated by calculating infertile couples, fertility tourism number, etc. to generally assess potential customer numbers in Asia-Pacific countries.
These metrics were not independently verified. Such databases, third-party information, and calculations may not accurately reflect actual statistics or numbers and NewGenIvf does not have access to specific rating numbers. Similarly, any statistical data in any third-party publications also include projections based on a number of assumptions. If any one or more of the assumptions underlying the market data is later found to be incorrect, actual results may differ from the projections based on these assumptions.
Risks Related to Government Regulation
NewGenIvf operates in a highly regulated industry and must comply with a significant number of complex and evolving requirements. Any lack of requisite approvals, licenses, or permits applicable to NewGenIvf’s business may have a material and adverse impact on NewGenIvf’s business, financial condition, and results of operations.
The operations of NewGenIvf are subject to various laws, rules and regulations at the national, regional and local levels in Thailand, Cambodia, Kyrgyzstan and other applicable jurisdictions. Such laws and regulations mainly relate to (i) the licensing of local and foreign medical professionals, nursing professionals, medical technology professionals, pharmaceutical professions and other applicable licensing; (ii) the licensing, registration, and accreditation of medical facilities, laboratories, including but not limited to the licensing, registration, and accreditation of persons performing related activities; (iii) the privacy and security of confidential patient medical records; (iv) the corporate practice of medicine; (v) healthcare fraud and abuse laws; (vi) the donation and transplantation of human cells, tissues and organs; (vii) potential prohibition on surrogacy or providing intermediary assistance in surrogacy; and (viii) licensing and approval of the accommodation provided as parts of the services.
NewGenIvf has attempted to structure its operations to comply with laws, regulations and other requirements applicable to it directly and to its clients and vendors, but there can be no assurance that its operations will not be challenged or impacted by regulatory authorities or enforcement initiatives, or that the relevant authorities in each jurisdiction could impose higher standards or requirements, which NewGenIvf may have difficulty to adhere to, e.g. Medical Facilities Act B.E. 2541 (1998) and Protection of a Child Born by Medically Assisted Reproductive Technology Act B.E. 2558 (2015) for Thailand jurisdiction, Law on Reproduction Rights and on Guarantees of Their Realization of July 4, 2015 No. 148, Law on status of medical worker of May 28, 2013 No. 81 and Temporary Regulation on Procedure of Licensing Private Medical Activity approved by the resolution of government of April 4, 2017 No. 203 for Kyrgyz Republic. NewGenIvf in the future may become involved in governmental investigations, audits, reviews and assessments. Any determination by a court or agency that NewGenIvf’s solutions or services violate, or cause its clients to violate, applicable laws, regulations or other requirements could subject it or its clients to civil, criminal, or administrative penalties. Such a determination also could require it to change or terminate portions of its business, disqualify it from serving clients that do business with government entities, or cause it to refund some or all of its service fees or otherwise compensate its clients. In addition, failure to satisfy laws, regulations or other requirements could adversely affect demand for its solutions and could force it to expend significant capital, research and development and other resources to address the failure. Even an unsuccessful challenge by regulatory and other authorities or parties could be expensive and time-consuming, could result in loss of business, exposure to adverse publicity, and injury to its reputation and could adversely affect its ability to retain and attract clients. If NewGenIvf fails to comply with applicable laws, regulations and other requirements, its business, financial condition and results of operations could be adversely affected. Such non-compliance could also require significant investment to address and may prove costly. There are several additional state statutes, regulations, guidance and contractual provisions related to or impacting the healthcare industry that may apply to its business activities directly or indirectly, including, but not limited to:
| | ● | Licensing and Licensed Personnel. Many countries have licensure or registration requirements for entities acting as a medical services provider. The scope of these laws differs from country to country, and the application of such laws to the activities of fertility treatment is often unclear. Given the nature and scope of the solutions and services that NewGenIvf provides, it is required to maintain the License to Operate Medical Facility Business (Sor.Por.7), the License to Manage Medical Facility Business (Sor.Por.19), License to Certify the Standard of Service relating to Medically Assisted Reproductive Technology (KorThorPhor.9), and personnel licenses, i.e., license of medical professionals, nursing professionals, medical technology professionals, pharmaceutical professions and other applicable licenses in Thailand, Approval on Opening of Medical Clinic, Approval on Opening of Pharmacy and relevant approvals to conduct IVF, embryo implant and/or transfer activities issued by the Ministry of Health of Cambodia (“Cambodia MOH”) in Cambodia and licenses to carry out private medical activities (including diagnostics and treatment gynecological diseases, supervision of pregnant women before childbirth, IVF in outpatient and day hospital conditions (for four (4) beds)) in Kyrgyzstan, respectively, and to ensure that such licenses and registrations are in good standing on an annual basis. NewGenIvf is licensed, has licensure applications pending before appropriate regulatory bodies, is exempt from licensure or registration, or is otherwise authorized under such laws in those countries in which it provides its services. These licenses require it to comply with the rules and regulations of the governmental bodies that issued such licenses. NewGenIvf’s failure to comply with such rules and regulations could result in criminal and/ or administrative penalties, the suspension of a license, or the loss of a license, all of which could negatively impact its business. First Fertility PGS had provided arrangements of accommodation without additional charges for its patients without a tourism license in Thailand, all of which was subsequently ceased in early 2023. Pursuant to the Tourism Business and Guide Act 2551 (2008) of Thailand, a maximum fine of THB500,000 may be imposed on First Fertility PGS as a result of the above activity without a tourism license in Thailand. NewGenIvf is unable to predict, however, how its services may be viewed by regulators over time, how these laws and regulations will be interpreted, or the full extent of their applicable. If a regulatory authority in any country determines that the nature of its business requires that NewGenIvf be licensed under applicable laws, it may need to restructure its business or it may need to comply with any related requirements, such as obtaining relevant license, paying additional regulatory fees and/or penalties for previous non-compliance with relevant licensing requirements, which could adversely affect its results of operation. Additionally, in extreme case, NewGenIvf may need to cease operations until it is able to obtain appropriate licensure, which may adversely affect its revenue for a period of time that it cannot estimate. |
| | ● | Patients’ Right Protection. There has been an increased awareness of patients’ rights in Thailand, Cambodia and Kyrgyzstan, especially with the issuance of the Constitution of the Kingdom of Thailand, the Act on Court Proceedings for Consumer Cases B.E. 2551 (2008) (as amended), National Health Act B.E. 2550 (2007), and other applicable laws in Thailand, the Civil Code dated December 8, 2017 as amended by the Law on Implementation of the Civil Code dated May 31, 2011, Law on Management of Donation and Transplantation of Human Cells, Tissues, and Organs (2016) and Sub-Decree No. 61 on the Code of Medical Ethics (2003) in Cambodia and Constitution of Kyrgyzstan of May 5, 2021, Civil Code, Part I of May 8, 1996 No. 15, Law on Health Protection of Civilians of Kyrgyzstan of January 9, 2005 No. 6, Law on Reproduction Rights and on Guarantees of their Realization of July 4, 2015 No. 148, Law on status of medical worker of May 28, 2013 No. 81 and other relevant applicable laws in Kyrgyzstan, which enables consumers and patients to file suits more easily against healthcare service providers. Furthermore, treatment of more complex medical conditions has no guaranteed positive outcome, which subjects it to an increased likelihood of medical malpractice suits. Such lawsuits could result in hefty compensation payments or damage to NewGenIvf’s reputation, which may have a material adverse effect on its business, financial condition, results of operations and prospects. |
Meanwhile, Thailand is considering enacting a Patient Protection Bill (the “Bill”). The Bill, if issued, is intended to alleviate disputes between patients and healthcare providers, which have an impact on the healthcare system in Thailand as a whole. The compensation outlined in the Bill will assist patients in claiming damages, thereby fostering a positive relationship between patients and healthcare providers. Consequently, the rate of disputes is expected to decrease. The provisions under the Bill would require healthcare providers to compensate patients in a timely manner, sometimes without requiring proof of wrongdoing. The Bill also contemplates setting up a patient protection fund for damages to patients pursuant to which healthcare providers have to make mandatory contributions according to the rules determined by a patient protection committee. Failure by it to comply with applicable rules and regulations could result in penalties, the loss of regulatory permits and damage to NewGenIvf’s business reputation, each of which could have a material adverse effect on its financial condition and results of operations.
Furthermore, the Protection of A Child Born By Medically Assisted Reproductive Technology Act B.E. 2558 (2015) of Thailand was promulgated with the intention to appropriately designate the legitimate parenthood status of a child born using medically assisted reproductive technology and regulate any medical scientific research on embryology and medically assisted reproductive technologies to prevent the misuse of medically assisted reproductive technologies. NewGenIvf is therefore under the supervision of a Committee of the Protection for Children Born through Medically Assisted Reproductive Technology, which is a committee established to control, inspect, supervise and formulate various policies relating to such acts. In Cambodia and Kyrgyzstan, all health establishments, including private medical clinics, are under the supervision of the Cambodia MOH and the Ministry of Health of Kyrgyzstan, respectively, which each governs and regulates the operation of medical clinics and activities of medical practitioners in respective countries. In particular, the Medical Council of Cambodia, Cambodian Council of Nurses, Cambodian Midwives Council and the Pharmaceutical Council of Cambodia, all assist the Cambodia MOH to supervise and monitor the practice of health professionals in Cambodia. IVF/embryo implant/transfer activities are subject to an approval by the Cambodia MOH.
| | ● | Privacy and Security Requirements. There are numerous laws and regulations related to the privacy and security of health information in each country. In particular, regulations promulgated pursuant to the Personal Data Protection Act B.E. 2562 (2019) of Thailand (“PDPA”), Law on Data of Personal Character of April 14, 2008 No. 58 of Kyrgyzstan (“Data Protection Law”), as well as Regulation of Registration of Personal Data Holders (Owners) approved by the Resolution of the Cabinet of Ministers of KR of November 18, 2022, Offences Code No. 128 of October 28, 2021 of Kyrgyzstan establish privacy and security standards in each country that limit the collection, use, and/ or disclosure of certain individually identifiable health information, whether directly or indirectly (excluding the information of the deceased person) and require the implementation of administrative, physical and technological safeguards to protect the privacy of protected health information and ensure the confidentiality, integrity and availability of electronic protected health information. The privacy regulations established under the PDPA and Data Protection Law also provide patients with rights related to understanding and controlling how their protected health information is collected, used and/ or disclosed. As a provider of services to entities subject to the PDPA and Data Protection Law, NewGenIvf is directly subject to certain provisions of the regulations. To the extent permitted by applicable privacy regulations and contracts with its clients, NewGenIvf is permitted to use and disclose protected health information to perform its services and for other limited purposes, but other uses and disclosures, such as marketing communications, require written authorization from the patient or must meet an exception specified under the privacy regulations. |
NewGenIvf also has downstream entities which provide it with services and are also subject to applicable regulations. If NewGenIvf or any of its downstream entities are unable to properly protect the privacy and security of protected health information entrusted to it, it could be found to have breached its contracts with its clients and be subject to investigation by the relevant supervision institution, i.e., the Office of the Personal Data Protection Committee of Thailand (the Government Authority under the PDPA), the Cambodia MOH and the State Data Protection Agency under the Cabinet of Ministers of Kyrgyzstan (the “Agency”). In the event the Office of the Personal Data Protection Committee or the Agency finds that NewGenIvf has failed to comply with applicable privacy and security standards, it could face civil, criminal, and/ or administrative penalties. In addition, the Office of the Personal Data Protection Committee performs compliance audits in order to proactively enforce the privacy and security standards. The Office of the Personal Data Protection Committee has become an increasingly active regulator and has signaled its intention to continue this trend. The Office of the Personal Data Protection Committee has the discretion to impose penalties and may require companies to enter into resolution agreements and corrective action plans which impose ongoing compliance requirements. The Office of the Personal Data Protection Committee’s enforcement activity, or audit related to incident regarding it or its downstream entity, can result in financial liability and reputational harm, and responses to such enforcement activity can consume significant internal resources. Although NewGenIvf has implemented and maintain policies, processes and compliance program infrastructure to assist it in complying with these laws and regulations and its contractual obligations, NewGenIvf cannot provide assurance regarding how these laws and regulations will be interpreted, enforced or applied to its operations. In associated with enforcement activities and potential contractual liabilities, its ongoing efforts to comply with evolving laws and regulations might also require it to make costly system purchases and/or modifications or otherwise divert significant resources to compliance initiatives from time to time.
| | ● | Other Privacy and Security Requirements. In addition, numerous other laws govern the collection, dissemination, use, access to and confidentiality of personal information. For example, the Law on E-Commerce of Cambodia (2019) places an obligation on those who electronically store private information to use all means to ensure that the information is protected by security safeguards in every reasonable circumstance to avoid the loss, access, use, modification, leakage, or disclosure of the information, except with the consent of the data owner or other lawfully authorized party. The Law on E-Commerce also prohibits individuals from dishonestly accessing, downloading, copying, extracting, leaking, deleting, modifying, or otherwise interfering with data stored by other persons. Applicable laws are contributing to increased enforcement activity and may also be subject to interpretation by various courts and other governmental authorities. |
Certain of NewGenIvf’s solutions and services involve the transmission and storage of client data in various jurisdictions, which subjects the operation of those solutions and services to privacy or data protection laws and regulations in those jurisdictions. While NewGenIvf believes those solutions and services comply with current regulatory and security requirements in the jurisdictions in which it provides these solutions and services, there can be no assurance that such requirements will not change or that it will not otherwise be subject to legal or regulatory actions. The laws and regulations are rapidly evolving and changing, and could have an adverse impact on its operations. These laws and regulations are subject to uncertainty in how they may be interpreted and enforced by government authorities and regulators. The costs of compliance with, and the other burdens imposed by, these and other laws or regulatory actions may increase its operational costs, prevent it from providing its solutions, and/or impact its ability to invest in or jointly develop its solutions. NewGenIvf also may face audits or investigations by one or more government agencies relating to its compliance with these laws and regulations.
An adverse outcome under any such investigation or audit could result in fines, penalties, other liability, or could result in adverse publicity or a loss of reputation, and adversely affect NewGenIvf’s business. Any failure or perceived failure by it or by NewGenIvf’s solutions to comply with these laws and regulations may subject it to legal or regulatory actions, damage its reputation or adversely affect its ability to provide its solutions in the jurisdiction that has enacted the applicable law or regulation. Moreover, if these laws and regulations change, or are interpreted and applied in a manner that is inconsistent with its policies and processes or the operation of its solutions NewGenIvf may need to expend resources in order to change its business operations, policies and processes or the manner in which it provides its solutions. This could adversely affect NewGenIvf’s business, financial condition and results of operations.
| | ● | Data Protection and Breaches. In recent years, there have been a number of well-publicized data breaches involving the improper dissemination of personal information of individuals both within and outside of the healthcare industry. Pursuant to the applicable data protection law of Thailand, the PDPA requires businesses to notify the data subjects and/or the government authorities upon the occurrence of a data breach. The laws are not consistent, and compliance in the event of a widespread data breach is costly. Each country also constantly amending existing laws, requiring attention to frequently changing regulatory requirements. Most countries require holders of personal information to maintain safeguards and take certain actions in response to a data breach, such as providing prompt notification of the breach to affected individuals. In some countries, these laws are limited to electronic data, but they increasingly are enacting or considering stricter and broader requirements. |
Despite NewGenIvf’s security management efforts with respect to physical and technological safeguards, employee training, vendor (and sub-vendor) controls and contractual relationships, its infrastructure, data or other operation centers and systems used in its business operations, including the internet and related systems of its vendors (including vendors to whom NewGenIvf outsources data hosting, storage and processing functions) are vulnerable to, and may from time to time experience, unauthorized access to data and/or breaches of confidential information due to a variety of causes. Techniques used to obtain unauthorized access to or compromise systems change frequently, are becoming increasingly sophisticated and complex, and are often not detected until after an incident has occurred. As a result, NewGenIvf might not be able to anticipate these techniques, implement adequate preventive measures, or immediately detect a potential compromise. If its security measures, some of which are managed by third parties, or the security measures of its service providers or vendors, are breached or fail, it is possible that unauthorized or illegal access to or acquisition, disclosure, use or processing of personal information, confidential information, or other sensitive client or employee data, including protected health information, may occur. A security breach or failure could result from a variety of circumstances and events, including third-party action, human negligence or error, malfeasance, employee theft or misuse, phishing and other social engineering schemes, computer viruses, attacks by computer hackers, failures during the process of upgrading or replacing software, databases or components thereof, power outages, hardware failures, telecommunication failures, and catastrophic events. If NewGenIvf’s security measures, or those of its service providers or vendors, were to be breached or fail, its reputation could be severely damaged, adversely affecting client or investor confidence. As a result, clients may curtail their use of or stop using its offering and its business may suffer. In addition, NewGenIvf could face litigation, damages for contract breach, penalties and regulatory actions for violation of laws or regulations applicable to data protection and significant costs for remediation and for measures to prevent future occurrences. In addition, any potential security breach could result in increased costs associated with liability for stolen assets or information, repairing system damage that may have been caused by such breaches, incentives offered to clients or other business partners in an effort to maintain the business relationships after a breach and implementing measures to prevent future occurrences, including organizational changes, deploying additional personnel and protection technologies, training employees and engaging third-party experts and consultants. Negative publicity may also result from real, threatened or perceived security breaches affecting it or its industry or clients, which could cause it to lose clients or partners and adversely affect its operations and future prospects. NewGenIvf may not carry insurance or maintain coverage sufficient to compensate for all liability and such insurance may not be available for renewal on acceptable terms or at all, and in any event, insurance coverage would not address the reputational damage that could result from a security incident.
| | ● | Fraud and Abuse Laws. NewGenIvf may be impacted directly and indirectly by certain fraud and abuse laws, including the Act Supplementing the Constitution Relating to the Prevention and Suppression of Corruption B.E. 2561 (2018) of Thailand, the Penal Code of Thailand, the Criminal Code of Cambodia, the Offences Code of October 28, 2021 No. 128 of Kyrgyzstan, the Criminal Code of October 28, 2021, No. 17 of Kyrgyzstan and the Law on prevention of corruption of August 8, 2021 No. 153 of Kyrgyzstan. Because the solutions and services NewGenIvf provides are not reimbursed by government healthcare payors, such fraud and abuse laws generally do not directly apply to its business, however, some laws may be applicable. The laws, regulations and other requirements in this area are both broad and vague and judicial interpretation can also be inconsistent. NewGenIvf reviews its practices with regulatory experts in an effort to comply with all applicable laws, regulatory and other requirements. However, NewGenIvf is unable to predict how these laws, regulations and other requirements will be interpreted or the full extent of their application, particularly to services that are not directly reimbursed by healthcare programs. Any determination by a regulatory authority that any of NewGenIvf’s activities or those of its clients or vendors violate any of these laws or regulations could subject NewGenIvf to civil or criminal penalties, require it to enter into corporate integrity agreements or similar agreements with ongoing compliance obligations, disqualify it from providing services to clients and/or have an adverse impact on its business, financial condition and results of operations. Even an unsuccessful challenge by a regulatory authority of NewGenIvf’s activities could result in adverse publicity and could require a costly response from it. |
| | ● | Consumer Protection Laws. Consumer protection laws are being applied increasingly by the Office of the Consumer Protection Board in Thailand and by the Cambodia Ministry of Health to regulate the collection, use, storage and disclosure of personal or health information, through websites or otherwise, and, in Cambodia, by the Consumer Protection Competition and Fraud Repression Directorate-General, to regulate the presentation of website content. Courts may also adopt the standards for fair information practices, which concern consumer notice, choice, security and access. |
| | ● | Restrictions on Communication. Communications with NewGenIvf’s clients increasingly may be subject to and restricted by laws and regulations governing communications via telephone, fax, text, and email. NewGenIvf also uses email and social media platforms as marketing tools. For example, NewGenIvf maintains social media accounts. As laws and regulations rapidly evolve to govern the use of these platforms and devices, the failure by it, its employees or third parties acting at its direction to abide by applicable laws and regulations in the use of these platforms and devices could adversely impact its business, financial condition and results of operations or subject it to fines or other penalties. |
| | ● | Advertisement Laws. NewGenIvf’s advertisement and announcements, in particular, the messages releasing on the Internet related to medical facilities may subject to the laws and regulations of relevant jurisdictions (and potential prohibition in Cambodia on commercial advertisement of private medical services). |
For example, in Thailand, NewGenIvf shall apply for and obtain the approval and/ or pre-approval from the relevant authority for the images, and text used in advertisements or announcements which shall be in accordance with the Medical Facility Act B.E. 2541 (1998) (and its amendments) and the Notification of the Department of Health Services Support on Rules, Procedures, Conditions, and Costs of Advertisements or Announcements of Healthcare Facilities B.E. 2562 (2019) (and its amendments) and the Operational Manual for Approval of Advertisements or Announcements relating to Healthcare Facilities. If such approval was not obtained by NewGenIvf, it could lead to significant liabilities and consequences, which could adversely impact NewGenIvf’s business, financial condition and results of operations or subject its sales and marketing director to personal liabilities.
For Cambodia, Prakas 028 on Advertisement of Private Medical, Paramedical and Medical Aid Practices dated August 23, 2004 issued by the Cambodia MOH prohibits commercial advertising of private medical services. Advertisement of private health care services is only allowed for any advertisements within the professional framework not affecting the ethics of private medical services and such advertisement requires a permit from the Cambodia MOH. In addition, the Royal Government of Cambodia has recently issued Sub-Decree 232 on the Management of Commercial Advertisements of Goods and Services on November 4, 2022 to provide the legal framework for the management of commercial advertising of goods and services for all types, forms and means in Cambodia. In light of this Sub-Decree, in addition to the permit requirement of the Cambodia MOH, a person wishing to advertise their goods and/or services in Cambodia may also apply for a compliance certificate from the Ministry of Commerce, which certifies that advertising text or content complies with the Law on Consumer Protection or other applicable regulations.
For Kyrgyzstan, the Law on Advertisements of December 24, 1998, No. 155 requires that if the activities of the advertiser subject to licensing, the advertisement of such advertiser must include the license number and the name of the authority that issued the license, except for radio advertising, where it is sufficient to state “licensed activity” on the territory of Kyrgyzstan. In advertising goods (including works and services), and other objects of advertising, cost indicators must be stated in the national currency. There are also other requirements established in relation to size, frequency, cost and other features of advertisements via different types of media.
New laws and regulations relevant to the fertility services may be introduced in the future, or the current applicable regulations may otherwise be amended or replaced requiring the assisted reproductive medical facilities in its network to conduct business with additional oversight and regulatory compliance. If NewGenIvf fails to obtain the necessary licenses, permits and approvals, NewGenIvf may be subject to fines, confiscation of revenues generated from incompliance operations, or the suspension of relevant operations. NewGenIvf may also experience adverse publicity arising from such non-compliance with government regulations that negatively impacts its brand. NewGenIvf may experience difficulties or failures in obtaining the necessary approvals, licenses, and permits for new spaces or new service offerings. If NewGenIvf fails to obtain the material licenses, NewGenIvf’s business activities could be severely delayed. In addition, there can be no assurance that NewGenIvf will be able to obtain, renew, and/or convert all of the approvals, licenses, and permits required for its existing business operations upon their expiration in a timely manner, in a cost-efficient manner or at all, which could adversely affect NewGenIvf’s business operations and financial condition.
In addition, considerable uncertainties exist in relation to the interpretation and implementation of existing and future laws and regulations governing NewGenIvf’s business activities. NewGenIvf could be found not in compliance with any future laws and regulations or of the laws and regulations currently in effect due to changes in the relevant authorities’ interpretation of those laws and regulations. It is possible that different interpretations or enforcement of these regulations could subject the current or past practices to allegations of impropriety or illegality or require the medical facilities in its network to implement changes in the facilities, equipment, personnel or services, or increase capital expenditure and operating expenses. If NewGenIvf fails to complete, obtain, or maintain any of the required licenses or approvals or make the necessary filings, NewGenIvf may be subject to various penalties, such as confiscation of unlawful gains, the imposition of fines, revocation of licenses, and the discontinuation or restriction of NewGenIvf’s operations. Any such penalties or changes in policies, regulations, or enforcement by government authorities may disrupt NewGenIvf’s operations and materially and adversely affect NewGenIvf’s business, financial condition, and results of operations.
Legal or regulatory restriction, government regulation, industry standards and other requirements create risks and challenges with respect to NewGenIvf’s compliance efforts and its business strategies and could adversely impact NewGenIvf’s business and limited the growth of NewGenIvf’s operations.
The healthcare industry is highly regulated and subject to frequently changing laws, regulations, industry standards and other requirements. Many healthcare laws and regulations are complex, and their application to specific solutions, services and relationships may not be clear. In particular, many existing healthcare laws and regulations, when enacted, did not anticipate the solutions and services that NewGenIvf provides, and these laws and regulations may be applied to its solutions and services in ways that NewGenIvf does not anticipate. Efforts to reform or revise aspects of the healthcare industry or to revise or create additional legal or and regulatory requirements could impact its operations, the use of its solutions and services, and its ability to market new solutions and services, or could create unexpected liabilities for it. NewGenIvf also may be impacted by laws, industry standards and other requirements that are not specific to the healthcare industry, such as consumer protection laws and payment card industry standards. These requirements may impact its operations and, if not followed, could result in fines, penalties and other liabilities and adverse publicity and injury to its reputation.
There is a risk that existing or future laws may be interpreted in a manner that is not consistent with the healthcare industry’s current practices and could have an adverse effect on NewGenIvf’s business, financial condition, results of operations and growth prospects.
Any litigation against NewGenIvf could be costly and time-consuming to defend and could harm its business, financial condition and results of operations.
NewGenIvf has in the past and may in the future become subject to regulatory actions, litigation, disputes, or claims of various types, legal proceedings and claims that arise in the ordinary course of business, such as claims brought by its clients or vendors in connection with commercial disputes or employment claims made by its current or former employees, as well as claims brought by relevant regulatory authorities or NewGenIvf’s competitors, patients, employees, or other third parties against NewGenIvf. NewGenIvf is unable to predict the outcome of any of these legal proceedings. Such regulatory actions, disputes, allegations, complaints, or legal proceedings may damage NewGenIvf’s reputation, evolve into litigation, or otherwise have a material adverse impact on NewGenIvf’s reputation and business. Such proceedings might result in substantial costs, regardless of the outcome, and may significantly divert management’s attention and resources from operating NewGenIvf’s business, which might seriously harm its business, financial condition and results of operations. Insurance might not cover such claims, might not provide sufficient payments to cover all the costs to resolve one or more such claims, and might not continue to be available on terms acceptable to it. A claim brought against it that is uninsured or underinsured could result in unanticipated costs, potentially harming its business, financial condition and results of operations. The outcomes of actions NewGenIvf institutes may not be successful or favorable to NewGenIvf. Lawsuits against NewGenIvf may also generate negative publicity that significantly harms NewGenIvf’s reputation, which may adversely affect NewGenIvf’s client base. NewGenIvf may also need to pay damages or settle lawsuits with a substantial amount of cash.
Acquisitions, strategic investments, partnerships, or alliances could be difficult to identify, pose integration challenges, divert the attention of management, disrupt NewGenIvf’s business, dilute stockholder value, and adversely affect its business, financial condition and results of operations.
NewGenIvf may in the future seek to acquire or invest in businesses, joint ventures, products and services, or technologies that it believes could complement or expand its platform, enhance its technical capabilities, or otherwise offer growth opportunities. Any such acquisition or investment may divert the attention of management and cause NewGenIvf to incur various expenses in identifying, investigating and pursuing suitable opportunities, whether or not the transactions are completed, and may result in unforeseen operating difficulties and expenditures. In particular, NewGenIvf may encounter difficulties assimilating or integrating the businesses, technologies, products and services, personnel or operations of the acquired companies, particularly if the key personnel of the acquired company choose not to work for it, they are operationally difficult to integrate, or NewGenIvf has difficulty retaining the clients of any acquired business due to changes in ownership, management or otherwise. These transactions may also disrupt its business, divert its resources, and require significant management attention that would otherwise be available for development of its existing business and may not benefit NewGenIvf’s business strategy, may not generate sufficient revenues to offset the associated acquisition costs or may not otherwise result in the intended benefits. Any such transactions that NewGenIvf is able to complete may not result in any synergies or other benefits it had expected to achieve, which could result in impairment charges that could be substantial. In addition, NewGenIvf may not be able to find and identify desirable acquisition targets or business opportunities or be successful in entering into an agreement with any particular strategic partner. These transactions could also result in dilutive issuances of equity securities or the incurrence of debt, which could adversely affect its results of operations. In addition, if the resulting business from such a transaction fails to meet NewGenIvf’s expectations, or it fails to successfully integrate such businesses into its own, its business, financial condition and results of operations may be adversely affected or it may be exposed to unknown risks or liabilities. Even when NewGenIvf identifies an appropriate acquisition or investment target, it may not be able to negotiate the terms of the acquisition or investment successfully, obtain financing for the proposed transaction, or integrate the relevant businesses into its existing business and operations. Strategic investments or acquisitions will involve risks commonly encountered in business relationships, including:
| | ● | difficulties in assimilating and integrating the operations, personnel, systems, data, technologies, products and services of the acquired business; |
| | ● | inability of the acquired technologies, products or businesses to achieve expected levels of revenue, profitability, productivity or other benefits; |
| | ● | difficulties in retaining, training, motivating and integrating key personnel; |
| | ● | diversion of management’s time and resources from NewGenIvf’s normal daily operations; |
| | ● | difficulties in maintaining uniform standards, controls, procedures and policies within the combined organizations; |
| | ● | difficulties in retaining relationships with customers, employees and suppliers of the acquired business; |
| | ● | risks of entering markets in which NewGenIvf have limited or no prior experience; |
| | ● | regulatory risks, including remaining in good standing with existing regulatory bodies or receiving any necessary pre-closing or post-closing approvals, as well as being subject to new regulators with oversight over an acquired business; |
| | ● | assumption of contractual obligations that contain terms that are not beneficial to NewGenIvf, require it to license or waive intellectual property rights or increase its risk for liability; |
| | ● | failure to further successfully develop the acquired technology; |
| | ● | liability for activities of the acquired business before the acquisition, including intellectual property infringement claims, violations of laws, commercial disputes, tax liabilities and other known and unknown liabilities; |
| | ● | potential disruptions to NewGenIvf’s ongoing businesses; and |
| | ● | unexpected costs and unknown risks and liabilities associated with strategic investments or acquisitions. |
Even if the transaction is consummated, NewGenIvf may only have limited control over the companies in which it only has minority stake, it cannot ensure that these companies will always comply with applicable laws and regulations in their business operations. Non-compliance of regulatory requirements by NewGenIvf’s investees may cause substantial harm to NewGenIvf’s reputations and the value of NewGenIvf’s investment. In addition, if the resulting business from such a transaction fails to meet NewGenIvf’s expectations, or it fails to successfully integrate such businesses into its own, its business, financial condition and results of operations may be adversely affected or it may be exposed to unknown risks or liabilities. If NewGenIvf is unable to effectively address these challenges, its ability to execute acquisitions as a component of its long-term strategy will be impaired, which could have an adverse effect on its growth. As a result of the above, NewGenIvf’s strategies may not be successfully implemented beyond the current markets.
Any investment might not achieve the synergies, operational or financial benefits it expects and may adversely impact NewGenIvf’s operating results. In addition, NewGenIvf cannot assure you that any future investment in or acquisition of new businesses or technology will lead to the successful development of new or enhanced products and services or that any new or enhanced products and services, if developed, will achieve market acceptance, or prove to be profitable.
Changes in NewGenIvf’s effective tax rate or tax liability may have an adverse effect on its results of operations.
NewGenIvf’s effective tax rate could increase due to several factors, including, but not limited to:
| | ● | changes in the relative amounts of income before taxes in the various jurisdictions in which NewGenIvf operates that have differing statutory tax rates; |
| | ● | changes in tax laws, tax treaties, and regulations or the interpretation of them; |
| | ● | changes to its assessment about its ability to realize its deferred tax assets that are based on estimates of its future results, the prudence and feasibility of possible tax planning strategies, and the economic and political environments in which NewGenIvf does business; |
| | ● | the outcome of future tax audits, examinations, or administrative appeals; and |
| | ● | limitations or adverse findings regarding its ability to do business in some jurisdictions. |
Any of these developments could have an adverse effect on its results of operations.
NewGenIvf’s reported financial results may be adversely affected by changes in accounting principles generally accepted in relevant jurisdictions.
Accounting principles generally accepted in Thailand, Cambodia and Kyrgyzstan are subject to interpretation by the relevant supervision institutions, and various bodies formed to promulgate and interpret appropriate accounting principles. A change in these principles or interpretations could have a significant effect on NewGenIvf’s reported results of operations and could affect the reporting of transactions already completed before the announcement of a change. The adoption of new or revised accounting principles may require it to make changes to its systems, processes and control, which could have a significant effect on its reported financial results, cause unexpected financial reporting fluctuations, retroactively affect previously reported results or require it to make costly changes to its operational processes and accounting systems upon or following the adoption of these standards.
If NewGenIvf’s estimates or judgments relating to its critical accounting policies prove to be incorrect, its results of operations could be adversely affected.
The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the amounts reported in NewGenIvf’s consolidated financial statements and accompanying notes appearing elsewhere in this prospectus. NewGenIvf bases its estimates on historical experience and on various other assumptions that it believes to be reasonable under the circumstances, as provided in the section titled “Management’s Discussion and Analysis of Financial Condition and Results of Operations of NewGenIvf — Critical Accounting Policies, Judgments and Estimates.” The results of these estimates form the basis for making judgments about the carrying values of assets, liabilities and equity, and the amount of revenue and expenses that are not readily apparent from other sources. Significant estimates and judgments used in preparing NewGenIvf’s consolidated financial statements include those related to the determination of fair value of its Class A Ordinary Shares and Warrants and revenue recognition relating to services rendered but for which no claim has yet been reported, among other things. NewGenIvf’s results of operations may be adversely affected if its assumptions change or if actual circumstances differ from those in its assumptions, which could cause its results of operations to fall below the expectations of securities analysts and investors, resulting in a decline in the market price of its Class A Ordinary Shares and Warrants.
NewGenIvf is subject to anti-corruption, anti-bribery, anti-money laundering, and similar laws, and non-compliance with such laws can subject it to criminal or civil liability and harm its business, financial condition and results of operations.
NewGenIvf is subject to the Anti-Money Laundering Act B.E. 2542 (1999) of Thailand, the Act Supplementing the Constitution Relating to the Prevention and Suppression of Corruption B.E. 2561 (2018) of Thailand, and the Penal Code of Thailand, domestic bribery laws, and other anticorruption and anti-money laundering laws in the countries in which it conducts activities. Anti-corruption and anti-bribery laws have been enforced aggressively in recent years and are interpreted broadly to generally prohibit companies, their employees and their third-party intermediaries from authorizing, offering, or providing, directly or indirectly, improper payments or benefits to recipients in the public or private sector. If NewGenIvf expands its business and sales and to the public sector, it may engage with business partners and third-party intermediaries to market its services and to obtain for it the necessary permits, licenses, and other regulatory approvals. In addition, NewGenIvf or its third-party intermediaries may have direct or indirect interactions with officials and employees of government agencies or state-owned or affiliated entities. NewGenIvf can be held liable for the corrupt or other illegal activities of these third-party intermediaries, its employees, representatives, contractors, partners and agents, even if it does not explicitly authorize such activities. Detecting, investigating, and resolving actual or alleged violations of anti-corruption laws can require a significant diversion of time, resources, and attention from senior management. In addition, noncompliance with anti-corruption, anti-bribery, or anti-money laundering laws could subject it to whistleblower complaints, investigations, prosecution, enforcement actions, sanctions, settlements, fines, damages, other civil or criminal penalties or injunctions, suspension or debarment from contracting with certain persons, reputational harm, adverse media coverage, and other collateral consequences. If any subpoenas or investigations are launched, or governmental or other sanctions are imposed, or if NewGenIvf does not prevail in any possible civil or criminal proceeding, its business, financial condition and results of operations could be harmed. In addition, responding to any action will likely result in a materially significant diversion of management’s attention and resources and significant defense costs and other professional fees, which could adversely affect its business, financial condition and results of operations.
For more information about our SEC filings, please see “Where You Can Find More Information” and “Incorporation by Reference.”
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
Some of the statements made under “Prospectus Summary,” “Risk Factors,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” “Business” and elsewhere in this prospectus constitute forward-looking statements. In some cases, you can identify forward-looking statements by terminology such as “may,” “will,” “should,” “expects,” “plans,” “anticipates,” “believes,” “estimates,” “predicts,” “potential” “intends” or “continue,” or the negative of these terms or other comparable terminology.
These forward-looking statements may include, but are not limited to, statements relating to our objectives, plans and strategies, statements that contain projections of results of operations or of financial condition, expected capital needs and expenses, statements relating to the research, development, completion and use of our products, and all statements (other than statements of historical facts) that address activities, events or developments that we intend, expect, project, believe or anticipate will or may occur in the future.
Forward-looking statements are not guarantees of future performance and are subject to risks and uncertainties. We have based these forward-looking statements on assumptions and assessments made by our management in light of their experience and their perception of historical trends, current conditions, expected future developments and other factors they believe to be appropriate.
Important factors that could cause actual results, developments and business decisions to differ materially from those anticipated in these forward-looking statements include, among other things:
| ● | our planned level of revenues and capital expenditures; |
| | ● | our ability to market and sell our products and services; |
| | ● | our plans to continue to invest in research and development to develop technology for both existing and new products; |
| | ● | our ability to maintain our relationships with suppliers, manufacturers and other partners; |
| | ● | our ability to maintain or protect the validity of our intellectual property and know-how; |
| | ● | our ability to retain key executive members; |
| | ● | our ability to internally develop and protect new inventions and intellectual property; |
| | ● | our ability to expose and educate the industry about the use of our services and products; |
| | ● | our expectations regarding our tax classifications; |
| | ● | interpretations of current laws and the passages of future laws; and |
| | ● | the impact of the coronavirus (COVID-19) pandemic and resulting government actions on us, our manufacturers, suppliers and facilities. |
These statements are only current predictions and are subject to known and unknown risks, uncertainties and other factors that may cause our or our industry’s actual results, levels of activity, performance or achievements to be materially different from those anticipated by the forward-looking statements. We discuss many of these risks in this prospectus in greater detail under the heading “Risk Factors” and elsewhere in this prospectus. You should not rely upon forward-looking statements as predictions of future events.
Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee future results, levels of activity, performance or achievements. Except as required by law, we are under no duty to update or revise any of the forward-looking statements, whether as a result of new information, future events or otherwise, after the date of this prospectus.
MANAGEMENT
The following table sets forth information regarding our executive officers and directors as of the date of this prospectus.
| Name | | Age | | Title |
| Wing Fung Alfred Siu | | 68 | | Chairman of the Board of Directors, Chief Executive Officer |
| Hei Yue Tina Fong | | 42 | | Director, Chief Marketing Officer |
| Hok Man Jefferson Au | | 43 | | Independent Director |
| Yip Eng Jeremy Foo | | 72 | | Independent Director |
| Ho Fai Chung | | 61 | | Chief Financial Officer |
Wing Fung Alfred Siu. NewGenIvf’s co-founder, Mr. Wing Fung Alfred Siu, has served as the Chairman of the Board and the Chief Executive Officer of NewGenIvf (before the Closing, Legacy NewGenIvf) since 2019. Prior to establishing Legacy NewGenIvf in 2019, Mr. Siu served as a director of First Fertility PGS Center Co., Ltd. since 2014. Mr. Siu received his master’s degree in science and bachelor’s degree in science from Stanford University.
Hei Yue Tina Fong. Ms. Fong has served as a Director and the Chief Marketing Officer of NewGenIvf (before the Closing, Legacy NewGenIvf) since 2019. Prior to establishing NewGenIvf in 2019, Ms. Fong served as a director of First Fertility PGS Center Co., Ltd. since 2014. Ms. Fong received her bachelor’s degree in marketing from Indiana University.
Ho Fai Chung. Mr. Chung has served as NewGenIvf’s Chief Financial Officer since October 10, 2024. Mr. Chung has over 30 years of working experience in Asia. He is a certified public accountant in the U.S.
Mr. Chung served as Financial Controller of King City Limited, a Hong Kong beauty salon, from January 2024 to May 2024. In this role, Mr. Chung evaluated and implemented the company’s sales system; set up employment contracts for staff members; set up the staff appraisal system for salary review, performance evaluation, and staff productivity; and set up a new management account format for the company for better evaluation of its business metrics. Prior to this role, Mr. Chung was the Accounting Manager at Beauty Land Holdings Ltd, another Hong Kong beauty salon, from May of 2017 to November of 2023. As Accounting Manager, Mr. Chung built up effective management tools to monitor and control all of the financial and accounting functions of the company and developed business strategies with the General Manager in order to achieve the company’s financial goals.
Mr. Chung started his career in an accountancy firm, Price Waterhouse (“PwC”), in Hong Kong. After five full years of working exposure in PwC, Mr. Chung joined a number of companies to take up regionalfinancial control as well as general management jobs in businesses including fashion, office products, telecommunications/internet and advertising. He had worked and based in China,Taiwan, Singapore and Malaysia before and had extensive regional financial controlling exposure in Asia.
Born in Hong Kong, Mr. Chung holds a Bachelor of Law degree from University of London and a Master’s degree in Accounting and Finance from Lancaster University (UK). Mr. Chung also holds a Master’s degree in International and Public Affairs from Hong Kong University. He is a certified public accountant in the U.S.
Hok Man Jefferson Au. Mr. Au has served as NewGenIvf’s independent director since April 3, 2024. Mr. Au has served as the Assistant Financial Controller and the Company Secretary at Coolpoint Innonism Holding Limited since May 2017 and a director of JWMG CPA Limited, Certified Public Accountants since August 2014. He previously worked as the audit supervisor at Clement C.W. Chan & Co., Certified Public Accountants from September 2010 to March 2014. Mr. Au obtained his honours diploma in accounting from Hong Kong Shue Yan University (formerly known as Hong Kong Shue Yan College) and received his Master of Science in professional accountancy from the University of London. Mr. Au is a member of the Hong Kong Institute of Certified Public Accountants and an associate of the Association of Chartered Certified Accountants.
Yip Eng Jeremy Foo. Mr. Foo has served as NewGenIvf’s independent director since April 3, 202. Mr. Foo has been a freelance organizational development and learning consultant since 2007. Mr. Foo previously served as the head of human capital practice of Changi Airports International, or CAI, from 2008 to 2011. Prior to joining CAI, Mr. Foo served as the head of institute of management and allied health sciences of National Healthcare Group, or NHG, from 2005 to 2007. Prior to joining NHG, Mr. Foo served as the head of Ministry of Defence, Singapore, or MINDEF, and served as CEO of MINDEF centre for management development, from 1998 to 2005. Prior to joining MINDEF, Mr. Foo worked in key senior positions covering strategic and operations planning, sea command, and naval leadership and professional development of Republic of Singapore Navy from 1979 to 1998. Mr. Foo obtained his Bachelor of Science from National University of Singapore and his Postgraduate Certificate in Business Administration from University of Leicester.
Election of Officers
Our executive officers are appointed by, and serve at the discretion of, the Board of Directors.
Family Relationships
Mr. Wing Fung Alfred Siu and Ms. Hei Yue Tina Fong are husband and wife. Other than as disclosed in this prospectus, none of the directors or executive officers has a family relationship as defined in Item 401 of Regulation S-K.
Involvement in Certain Legal Proceedings
To the best of our knowledge, none of our directors or executive officers has, during the past ten years, been involved in any legal proceedings described in subparagraph (f) of Item 401 of Regulation S-K. Our directors and officers have not been involved in any transactions with us or any of our affiliates or associates which are required to be disclosed pursuant to the rules and regulations of the SEC.
Diversity Matrix of Board of Directors
The following table provides the diversity statistics of the Board, as required by Rule 5606 of the Nasdaq Listing Rules:
| Board Diversity Matrix as at the date of this prospectus |
| |
| Country of Principal Executive Offices: | | | Thailand | |
| Foreign Private Issuer | | | Yes | |
| Disclosure Prohibited Under Home Country Law | | | No | |
| Total Number of Directors | | | 4 | |
| | | Female | | | Male | | | Non-Binary | | | Did Not
Disclose
Gender | |
| Part I: Gender Identity | | | | | | | | | | | | |
| Directors | | | 1 | | | | 3 | | | | — | | | | — | |
| Part II: Demographic Background | | | | | | | | | | | | | | | | |
| Underrepresented Individual in Home Country Jurisdiction | | | — | | | | — | | | | — | | | | — | |
| LGBTQ+ | | | — | | | | — | | | | — | | | | — | |
| Did Not Disclose Demographic Background | | | — | | | | — | | | | — | | | | — | |
B. Compensation
The following table sets forth the amount of compensation, including base salary, discretionary bonus, equity compensation, contractual benefits and contributions to defined contribution plans, which was paid, earned and/or accrued during the fiscal years ended December 31, 2023 and 2022, for each of the officers and directors for Legacy NewGenIvf prior to the Business Combination.
| Name | | 2023
Compensation
US$ | | | 2022
Compensation
US$ | |
| Directors and Officers | | | | | | |
| Wing Fung Alfred Siu | | | 125,000 | | | | 120,000 | |
| Hei Yue Tina Fong | | | 125,000 | | | | 120,000 | |
| Total | | $ | 250,000 | | | $ | 240,000 | |
Board Practices
Committees of the Board of Directors
We established three committees under the Board: an audit committee (“Audit Committee”), a Compensation Committee (“Compensation Committee”) and a nominating and corporate governance committee (“Nominating and Corporate Governance Committee). We have adopted a charter for each of the three committees. Each committee’s members and functions are described below.
Audit Committee. Our Audit Committee consists of Mr. Hok Man Jefferson Au and Mr. Yip Eng Jeremy Foo. We have determined that all of these individuals satisfy the “independence” requirements of NASDAQ Rule 5605 and Rule 10A-3 under the Exchange Act. Our Board has determined that Mr. Hok Man Jefferson Au qualifies as an audit committee financial expert and has the accounting or financial management expertise as required under Item 407(d)(5)(ii) and (iii) of Regulation S-K. The audit committee will oversee our accounting and financial reporting processes and the audits of the financial statements of our company. The Audit Committee will be responsible for, among other things:
| ● | establishing clear hiring policies for employees or former employees of the independent auditors; |
| ● | reviewing and recommending to the Board for approval, the appointment, reappointment or removal of the independent auditor, after considering its annual performance evaluation of the independent auditor; |
| ● | approving the remuneration and terms of engagement of the independent auditor and pre-approving all auditing and non-auditing services permitted to be performed by the Company’s independent auditors at least annually; |
| ● | obtaining a written report from the Company’s independent auditor describing matters relating to its independence and quality control procedures; |
| ● | reviewing with the independent registered public accounting firm any audit problems or difficulties and management’s response; |
| ● | discussing with the Company’s independent auditor, among other things, the audits of the financial statements, including whether any material information should be disclosed, in addition to issues regarding accounting and auditing principles and practices; |
| ● | reviewing and approving all proposed related party transactions, as defined in Item 404 of Regulation S-K under the Securities Act; |
| ● | reviewing and recommending the financial statements for inclusion within the Company’s quarterly earnings releases and to the Board for inclusion in its annual reports; |
| ● | discussing the annual audited financial statements with management and the independent registered public accounting firm; |
| ● | reviewing policies with respect to risk assessment and risk management; |
| ● | reviewing the adequacy and effectiveness of the Company’s accounting and internal control policies and procedures and any special steps taken to monitor and control major financial risk exposures; |
| ● | periodically reviewing and reassessing the adequacy of the committee charter; |
| ● | approving annual audit plans, and undertaking an annual performance evaluation of the internal audit function; |
| ● | establishing and overseeing procedures for the handling of complaints and whistleblowing; |
| ● | meeting separately and periodically with management, the internal auditors and the independent registered public accounting firm; |
| ● | monitoring compliance with the Company’s code of business conduct and ethics, including reviewing the adequacy and effectiveness of its procedures to ensure proper compliance; |
| ● | reporting periodically to the Board; and |
| ● | such other matters that are specifically delegated to the Company’s Audit Committee by the Board from time to time. |
A copy of the audit committee’s current charter is available at our corporate website at www.newgenivf.com.
Compensation Committee. Our Compensation Committee (“Compensation Committee”) consists of Mr. Wing Fung Alfred Siu, Ms. Hei Yue Tina Fong, and Mr. Yip Eng Jeremy Foo. The Chairman of the Compensation Committee is Mr. Siu. The Company has determined that Mr. Foo satisfies the “independence” requirements of Rule 5605(c)(2) of the Nasdaq Stock Market Listing Rules. The Compensation Committee assists the Board in reviewing and approving compensation structure, including all forms of compensation relating to the Company’s directors and executive officers. The Company’s Chief Executive Officer may not be present at any committee meeting during which their compensation is deliberated upon. The Compensation Committee is responsible for, among other things:
| ● | reviewing and evaluating the Company’s executive compensation and benefits policies generally; |
| ● | reviewing and recommending any incentive compensation or equity plans, programs or other similar arrangements; |
| ● | periodically reviewing and reassessing the adequacy of the Compensation Committee charter; |
| ● | selecting compensation consultant, legal counsel or other adviser only after taking into consideration all factors relevant to that person’s independence from management; |
| ● | reporting periodically to the Board; and |
| ● | such other matters that are specifically delegated to the Compensation Committee by the Board from time to time. |
A copy of the Compensation Committee’s current charter is available at our corporate website at: www.newgenivf.com.
Nominating and Corporate Governance Committee. The Company’s Nominating and Corporate Governance Committee consist of Mr. Wing Fung Alfred Siu, Ms. Hei Yue Tina Fong, and Mr. Hok Man Jefferson Au. The Chairman of the Nominating and Corporate Governance Committee is Mr. Siu. The Company has determined that Mr. Au satisfies the “independence” requirements of Rule 5605(c)(2) of the Nasdaq Stock Market Listing Rules. The Nominating and Corporate Governance Committee assists the Board of Directors in selecting individuals qualified to become the Company’s directors and in determining the composition of the Board of Directors and its committees. The Nominating and Corporate Governance Committee is responsible for, among other things:
| ● | recommending nominees to the Board for election or re-election to the Board, or for appointment to fill any vacancy or newly created directorships on the Board; |
| ● | reviewing periodically with the Board the current composition of the Board with regards to characteristics such as judgment, experience, expertise, diversity and background; |
| ● | recommending to the Board of criteria with respect to nomination or appointment of members of its Board of Directors and chairs and members of its committees or other corporate governance matters as may be required pursuant to any SEC or Nasdaq Stock Market Listing Rules, or otherwise considered desirable and appropriate; |
| ● | recommending to the Board the names of directors to serve as members of the Audit Committee and the Compensation Committee, as well as of the Nominating and Corporate Governance Committee itself; |
| ● | periodically and reassessing the adequacy of the committee charter; |
| ● | overseeing compliance with the corporate governance guidelines and code of business conduct and ethics; and |
| ● | overseeing and leading the self-evaluation of the Board in its performance and |
| ● | effectiveness as a whole. |
A copy of the Nominating and Corporate Governance Committee’s current charter is available at our corporate website at www.newgenivf.com.
Duties and Functions of Directors
Under the laws of the British Virgin Islands, the Company’s directors owe fiduciary duties to the Company, including duty to act honestly and in good faith in what the directors believe to be in the best interests of the company, duty to exercise powers for a proper purpose and directors shall not act, or agree to act, in a matter that contravenes the BVI Act or the Memorandum and Articles of Association, duty to exercise the care, diligence and skill that a reasonable director would exercise in the circumstances, and duty to avoid conflicts of interest. In fulfilling their duty of care to the Company, the Company’s directors must ensure compliance with the Company’s Memorandum and Articles of Association, as amended and restated from time to time. The Company has the right to seek damages if a duty owed by its directors is breached. In limited exceptional circumstances, a shareholder may have the right to seek damages in the Company’s name if a duty owed by the Company’s directors is breached. The functions and powers of the Board include, among other things, (i) convening shareholder meetings at such times and in such manner and places as the director considers necessary or desirable, (ii) declaring dividends, (iii) appointing directors or officers and determining their terms of offices and responsibilities, and (iv) approving the transfer of shares of the Company, including the registering of such shares in the Company’s share register.
Terms of Directors and Officers
The Company’s officers are elected by and serve at the discretion of the Board. Each director holds office for the term fixed by the resolution of shareholders or the resolution of directors appointing him until such time as his successor takes office or until the earlier of his death, resignation or removal from office by resolution of directors with or without cause or by resolution of shareholders for cause. The directors may at any time appoint any person to be a director either to fill a vacancy or as an addition to the existing directors. Where the directors appoint a person as director to fill a vacancy, the term shall not exceed the term that remained when the person who has ceased to be a director ceased to hold office. A vacancy in relation to directors occurs if a director dies or otherwise ceases to hold office prior to the expiration of his term of office.
Interested Transactions
A director may, subject to any separate requirements for Audit Committee approval under applicable laws or applicable Nasdaq Stock Market Listing Rules, vote on a matter relating to the transaction in which he or she is interested, provided that the interest of any directors in such transaction is disclosed by him or her to all other directors.
Director Agreements
We have entered into director agreements with our directors, which require us to maintain director and officer liability insurance for our directors, provide reimbursements for business related travel and accommodation and other reasonable expenses, and an annual remuneration of between $20,000 to $25,000 for our independent directors, and $240,000 for our executive directors.
Employees
As of December 31, 2023, Legacy NewGenIvf had 70 full-time employees, of which 63 are based in Thailand, Cambodia and Kyrgyzstan. NewGenIvf aims to attract and retain employees with the skills, and experience necessary to implement its growth strategy. The following table sets forth the number of its employees in Thailand, Cambodia and Kyrgyzstan by function as of December 31, 2023:
| Function | | Number of
employees | |
| Thailand | | | |
| Medical professionals | | | 16 | |
| Administrative staff and others | | | 12 | |
| Sub-total | | | 28 | |
| Cambodia | | | | |
| Medical professionals | | | 9 | |
| Administrative staff and others | | | 12 | |
| Sub-total | | | 21 | |
| Kyrgyzstan | | | | |
| Medical professionals | | | 4 | |
| Administrative staff and others | | | 10 | |
| Sub-total | | | 14 | |
| Hong Kong | | | | |
| Administrative staff and others | | | 7 | |
| Total | | | 70 | |
We believe that we maintain a good working relationship with our employees and we have not experienced any significant labor disputes.
Share Ownership
Except as specifically noted, the following table sets forth information with respect to the beneficial ownership of our ordinary shares as of the date of this annual report by:
| ● | each of our directors and executive officers; and |
| ● | each of our principal shareholders who beneficially own more than 5% of our total outstanding ordinary shares; |
The calculations in the table below are based on 10,149,386 Class A Ordinary Shares outstanding as of May 10, 2024. Unless otherwise indicated, each person has sole investment and voting power with respect to all shares shown as beneficially owned. The term “beneficial owner” of securities refers to any person who, even if not the record owner of the securities, has or shares the underlying benefits of ownership. These benefits include the power to direct the voting or the disposition of the securities or to receive the economic benefit of ownership of the securities. A person also is considered to be the “beneficial owner” of securities that the person has the right to acquire within 60 days by option or other agreement. Beneficial owners include persons who hold their securities through one or more trustees, brokers, agents, legal representatives or other intermediaries, or through companies in which they have a “controlling interest”, which means the direct or indirect power to direct the management and policies of the entity. The Company’s directors and executive officers do not have different voting rights than other shareholders of the Company.
| Name of Beneficial Owner | | Number of
Shares | | | % of Class | |
| Five Percent Holders other than our Directors and Officers | | | | | | |
| Chardan Capital Markets, LLC | | | 1,569,000 | | | | 15.46 | % |
| A SPAC Holdings Group Corp. | | | 655,000 | | | | 6.45 | % |
| Future Yield Holdings Limited | | | 750,000 | | | | 7.39 | % |
| Kit Yee Sze | | | 546,925 | | | | 5.39 | % |
| Directors and Named Executive Officers: | | | | | | | | |
| Wing Fung Alfred Siu | | | 1,779,500 | | | | 17.53 | % |
| Hei Yue Tina Fong | | | 2,326,000 | | | | 22.92 | % |
| Hok Man Jefferson Au | | | - | | | | - | |
| Yip Eng Jeremy Foo | | | - | | | | - | |
| Chiu, Wai Yip Raymond | | | - | | | | - | |
| All Directors and Executive Officers as Group | | | 4,105,500 | | | | 40.45 | % |
RELATED PARTY TRANSACTIONS
Related Party Transactions
A summary of related parties of the Company is as follows:
| | | Relationship |
| Seazen Resources Investment Limited | | Shareholder |
| Mr. Siu, Wing Fung Alfred and Ms. Fong, Hei Yue Tina | | Shareholders and directors(1) |
| Harcourt Limited | | A related company(2) |
| (1) | Ms. Fong is the spouse of Mr. Siu. |
| (2) | The directors and shareholders of Harcourt Limited are Mr. Wing Fung Alfred Siu and Ms. Hei Yue Tina Fong. |
Transaction with Seazen Resources Investment Limited
During the year ended December 31, 2022, Legacy NewGenIvf had consideration receivables from Seazen Resources Investment Limited, representing the unsettled consideration for Legacy NewGenIvf’s shares. For the years ended December 31, 2022 and 2023, the largest amount outstanding was US$319,872. As of the date of this prospectus, the balance of loans receivable from Seazen Resources Investment Limited is US$ Nil.
Separately, Legacy NewGenIvf borrowed money from Seazen Resources Investment Limited in May 2020 primarily for its general working capital purposes. The maximum borrowing was HKD5,000,000. The borrowing carried fixed interest at 18.0% per annum. Interest expense on the borrowing was US$45,988 and US$Nil during the year ended December 31, 2022 and 2023, respectively. As at December 31, 2023, the amount outstanding was nil.
Transaction with Mr. Wing Fung Alfred Siu and Ms. Hei Yue Tina Fong
Historically, certain amount of cash provided by operating activities was distributed to Mr. Wing Fung Alfred Siu and Ms. Hei Yue Tina Fong, resulting in amount due from them. As of the date of this prospectus, the aggregate balance of amount due from Mr. Siu and Ms. Fong was US$354,285 and US$2,240,872, respectively. For the years ended December 31, 2022 and 2023, the largest amount due from Mr. Siu, Wing Fung Alfred and Ms. Fong, Hei Yue Tina was US$2,240,872, and US$2,240,872, respectively. Mr. Siu and Ms. Fong intend to continue to repay the remaining outstanding amounts due pursuant to the terms and conditions of the repayment agreement dated August 14, 2023.
In addition, NewGenIvf also recorded remuneration to its directors, Mr. Siu and Ms. Fong. The remuneration to Mr. Siu, Wing Fung Alfred was US$100,000, US$120,000 and US$125,000 during the year ended December 31, 2021, 2022 and 2023, respectively. The remuneration to Ms. Fong, Hei Yue Tina was US$100,000, US$120,000 and US$125,000 during the year ended December 31, 2021, 2022 and 2023, respectively. The remuneration during the years ended December 31, 2021, 2022 and 2023 was all in the nature of the fair value of the services provided by Mr. Siu and Ms. Fong and was recorded as noncash operating expense and additional paid-in capital. Mr. Siu Wing Fung also entered into agreement to waive the balance of due from the Company US$88,151 in 2023.
Transaction with Harcourt Limited
Historically, NewGenIvf borrowed money from Harcourt Limited without interest for normal operating use in 2022 and 2023. For the years ended December 31, 2022 and 2023, the largest amount outstanding was US$110,773. As of December 31, 2022 and 2023, the borrowings payable to Harcourt Limited were US$110,773 and US$ Nil, respectively.
LISTING DETAILS
Our Ordinary Shares currently trade on Nasdaq under the symbol “NIVF.” As of the date of this prospectus, our only listed class of securities are our Ordinary Shares. All of our Ordinary Shares, including those to be offered by the Selling Shareholder pursuant to this prospectus, have the same rights and privileges. For more information, see “Description of Share Capital—Ordinary Shares.”
USE OF PROCEEDS
All of the Resale Shares will be sold by the Selling Shareholder for its own account. We will not receive any proceeds from the resale of the Resale Shares. However, we may receive up to $500,000,000 in gross proceeds under the White Lion Purchase Agreement from sales of Ordinary shares that we may elect to make to White Lion pursuant to the White Lion Purchase Agreement after the date of this prospectus subject to certain contingencies as described herein, if any, from time to time in our sole discretion, during the Commitment Period. However, we will not receive any proceeds from the issuance of Commitment Shares.
The proceeds from White Lion that we receive under the White Lion Purchase Agreement, if any, are currently expected to be used for general corporate purposes, including working capital, and for growth initiatives. We retain broad discretion over the use of the net proceeds from the sale of our Ordinary Shares under the White Lion Purchase Agreement. The precise amount and timing of the application of such proceeds will depend upon our liquidity needs and the availability and cost of other capital over which we have little or no control. As of the date hereof, we cannot specify with certainty the particular uses for the net proceeds from the sales of our Ordinary Shares, if any, to White Lion, under the White Lion Purchase Agreement.
We will incur all costs associated with this prospectus and the Registration Statement of which it is a part.
DIVIDEND POLICY
We have not declared or paid any cash dividend on our Ordinary Shares as of the date of this prospectus. We currently intend to retain any future earnings and do not expect to pay any dividends in the near future. Any further determination to pay dividends on our ordinary shares would be at the discretion of our Board of Directors, subject to applicable laws, and would depend on our financial condition, results of operations, capital requirements, general business conditions, and other factors that our Board of Directors may deem relevant.
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion and analysis of our results of operations and financial condition should be read together with ou consolidated financial statements and the notes thereto and other financial information, which are included elsewhere in this registration statement. Our financial statements have been prepared in accordance with U.S. generally accepted accounting principles (“U.S. GAAP”). In addition, our financial statements and the financial information included in this registration statement reflect our organizational transactions and have been prepared as if our current corporate structure had been in place throughout the relevant periods.
This section contains forward-looking statements. These forward-looking statements are subject to various factors, risks and uncertainties that could cause actual results to differ materially from those reflected in these forward-looking statements. Further, as a result of these factors, risks and uncertainties, the forward-looking events may not occur. Relevant factors, risks and uncertainties include, but are not limited to, those discussed in the section entitled “Business,” “Risk Factors” and elsewhere in this registration statement. Readers are cautioned not to place undue reliance on forward-looking statements, which reflect management’s beliefs and opinions as of the date of this registration statement. We are not obligated to publicly update or revise any forward -looking statements, whether as a result of new information, future events or otherwise. See “Cautionary Note Regarding Forward-Looking Statements.”
Overview
NewGenIvf is an assisted reproductive services (“ARS”) provider in Asia Pacific. Since the establishment of its first clinic in Thailand in 2014, it has established itself as a long-standing ARS provider in the region. NewGenIvf’s mission is to assist couples and individuals across Asia Pacific, regardless of fertility challenges that they may face, to fulfil their dreams of building families and to increase their access to fertility treatments. Its strategic presence in Thailand, Cambodia, and Kyrgyzstan positions the company to take advantage of opportunities across Asia Pacific.
NewGenIvf is still in the early stage of materializing its long-term objective of building a comprehensive, sophisticated and high-end ARS platform for its clients and providing personalized solutions based on NewGenIvf’s brands and client-generated services. NewGenIvf plans to offer full fertility services for fertility tourists across Asia Pacific, continue to invest in laboratories and facilities updates, increase its brand awareness and market share, as well as expand service reach through acquisitions and partnerships, which NewGenIvf believes will help expand its client base and enhance expertise attraction, and in turn strengthen NewGenIvf’s monetization capabilities.
Key Factors Affecting NewGenIvf’s Results of Operations
NewGenIvf’s results of operation are principally affected by the following factors:
Regulatory environment
The ARS market in Asia-Pacific region is highly regulated. The implementation and enforcement of laws, regulations and government policies in Thailand, Cambodia, Kyrgyzstan and other applicable jurisdictions significantly impact the design, pricing and sale of fertility services and cost of compliance for clinics across Asia Pacific. Medical facilities providing fertility services generally must be filed and registered with the relevant supervision institutions and such filing and registration must be renewed periodically. Any change in laws, regulations or policies in relation to such filing or registration could affect NewGenIvf’s ability and plans to launch new services and renew registration for existing services. The regulatory framework for medical facilities and services, especially those involving ARS, is, and will continue, evolving. Any changes in the applicable regulatory frameworks in the jurisdictions where NewGenIvf operates may materially affect its financial condition and results of operations.
Growth and competitive landscape of Asia Pacific’s ARS market
NewGenIvf’s revenue has historically been primarily derived from clients in Asia Pacific. As such, NewGenIvf’s financial performance and future growth depend primarily on the demand for ARS, as well as changes in its competitive landscape, in Asia Pacific. Population growth, infertility rates, and demand for facility treatments in the region will ultimately determine the demand for NewGenIvf’s services. According to CIC, infertility is increasingly becoming prevalent globally, primarily driven by increasing average age of first birth, as well as various lifestyle and environmental factors. Driven by an increased infertility rate and growing demand for children without birth defects, resulting from improving living standards and improved awareness about birth defects and prevention, the global ARS market is expected to continue to grow. Furthermore, according to CIC, a growing number of governments around the world has granted legal recognition to same-sex marriages, which brings more desires for having children to form a complete family. According to CIC, because of the fertility rate and recent government incentive policies, such as the Three-child Policy of China in 2021, the ARS market increased significantly in Asia Pacific. Leveraging its status as a long-standing ARS provider in Asia Pacific, NewGenIvf expects to continue to be well positioned to capture the expected growth in the demand for ARS in the area.
To date, NewGenIvf holds an exclusive license granted by a division of the Genetics and IVF Institute to use MicroSort technology in Thailand and Cambodia, which is a form of pre-conception gender selection technology for humans. While NewGenIvf expects to benefit from first-mover advantages for this technology in the two regions, market entry by potential competitors or faster-than-expected development of potential competitors may affect its market position and demand for its services and cause downward pricing pressure on its treatments, which may in turn materially and adversely affect its results of operations. Meanwhile, ARS market could also be affected by the macroeconomic environment and geopolitical events. Uncertainty in the macroeconomic environment, resulting from a range of events and trends, including the rise in global inflation and interest rates, supply chain disruptions, geopolitical pressures, including the unknown impact of current and future trade regulations, changes in Asian-Pacific relations, fluctuation in foreign exchange rates, and associated global economic conditions may result in volatility in ARS market and NewGenIvf’s operating performance. For example, NewGenIvf derives a substantial portion of its revenue from Chinese clients and as such, its failure to maintain PRC-sourced revenues and access to new and existing clients from the PRC could materially and adversely affect its results of operations and competitive position. However, the near-term growth prospects of the PRC economy are unclear due to the uncertain effects of ongoing economic stress caused by policies to contain the COVID-19 pandemic, trade and national security policies, and the elevated levels of private and public indebtedness, among others. According to the National Statistics Bureau of the PRC, growth rate of China’s GDP for the year 2022 slowed down to 3.0% on a year-on-year basis compared to the growth rate of approximately 8.4% for the year 2021. In the second quarter of 2023, China’s GDP grew only 0.8% on a quarter basis, a significant slowdown from the 2.2% quarter growth registered in the first quarter of 2023. A prolonged downturn in the PRC economy generally could materially and adversely affect NewGenIvf’s results of operations and there is a significant likelihood that NewGenIvf’s actual results over the time periods and under the scenarios covered by the projections would be different. However, China’s GDP in the third quarter of 2023 grew 4.9% on a year-on-year basis and grew 1.3% on a quarter-by-quarter basis. NewGenIvf believes that if there is a recovery of the PRC economy, it might increase the demand for NewgenIvf’s services and therefore in turn affect NewGenIvf’s results of operations.
Fluctuation of costs
NewGenIvf’s costs primarily include clinic costs, cost of goods sold, selling and marketing expenses and general and administrative expenses, details of which are set out below.
| | ● | Clinic costs. NewGenIvf’s clinic costs primarily consisted of sub-contracting charges, office supplies and staff salaries and bonus, most of which are recognized during the provision of surrogacy services. Its clinic costs represented approximately 55.7%, 65.7% of its revenue for the years ended December 31, 2023 and 2022, respectively. As NewGenIvf gradually expands the scale of its operation and presence in Asia Pacific, its clinic costs is expected to increase in the foreseeable future, which will affect its profitability. |
| | ● | Cost of goods sold. NewGenIvf’s cost of goods sold primarily consisted of purchase and direct cost for IVF treatment services and surrogacy and ancillary caring services, most of which are recognized during the provision of IVF treatment services. Its cost of goods sold represented approximately 11.6% and 8.5% of the revenue for the years ended December 31, 2023 and 2022, respectively. NewGenIvf expects its cost of goods sold to increase in the foreseeable future as it gradually grows its revenues and expand its sales network. |
| | ● | Selling and marketing expenses. NewGenIvf’s selling and marketing expenses primarily consisted of social media expense. Its selling and marketing expenses represented approximately 0.4% and 0.6% of its revenue for the years ended December 31, 2023 and 2022, respectively. NewGenIvf expects its selling and marketing expenses to increase as it plans to expand its sales and scale its operation in Asia-Pacific. |
| | ● | General and administrative expenses. NewGenIvf’s general and administrative expenses primarily consisted of depreciation in operating lease right-of-use (“ROU”) assets, l and staff salaries and director fees. Its general and administrative expenses represented approximately 24.5% and 18.4% of its revenue for the years ended December 31, 2023 and 2022, respectively. NewGenIvf expects its general and administrative expenses to increase in line with its expansion plan. |
NewGenIvf expects its cost structure to evolve as it develops and expands its business. As NewGenIvf continues to develop new services and technologies, NewGenIvf expects to incur additional costs in relation to its raw materials procurement, production and sales and marketing, among other things. Moreover, to support NewGenIvf’s business growth, it expects to increase its headcount, particularly for its lab and nurse team, and incur higher staff costs as a result.
Ability to maintain trust of clients and reputation in the industry
The success of NewGenIvf’s business will depend to a large extent on its ability to gain broad acceptance of its services from clients. Reputation is crucial in keeping existing clients and attracting new clients. NewGenIvf’s reputation depends on a number of factors, including for example the success, effectiveness, quality and pricing of its services, service offerings of its competitors, the effectiveness of its marketing efforts to drive awareness and the demand for fertility services, which eventually will affect its ability to maintain clients and attract new clients. Therefore, NewGenIvf’s success will depend to a large extent on its ability to maintain its reputation in the industry and its clients’ trust, which would affect the number of its clients and treatment cycles that will in turn affect its revenues.
NewGenIvf believes that the medical facilities in its network are increasingly recognized among clients, for their service quality, technological expertise and patient experience. NewGenIvf also hopes to keep its clients by providing discounts in treatment services and via the “success guarantee” program for egg donation services in Cambodia and surrogacy services in Kyrgyzstan, which provides treatments to clients until a success is achieved.
Based on its increasingly recognized reputation, NewGenIvf believes that there is substantial opportunity to continue to grow its revenue through attracting new clients. NewGenIvf’s addressable market is couples who want to have children, egg freezing patients, LGBT groups and couples with genetic abnormalities, particularly those in Asia Pacific. NewGenIvf believes that its current client base represents a small percentage of its total market opportunity. NewGenIvf intends to attract new clients by, among other things, making significant investments in sales and marketing to engage, educate and drive awareness of the unmet need of fertility treatment among its potential clients and by its customer-reference discounts mechanism. Additionally, NewGenIvf believes that its expanding presence has resulted in a heightened awareness of the need to offer fertility services and the value it provides to its clients, which it believes will help facilitate its growth. In addition, NewGenIvf is continuously utilizing its established client relationships to evaluate other potential services that could benefit its clients and simultaneously drive its growth.
International traveling conditions
The revenue from international clients is a critical component of NewGenIvf’s revenue. International traveling to Thailand, Cambodia and Kyrgyzstan may be affected by a number of factors, including local and global political, economic and cultural conditions. Furthermore, an outbreak, or threatened outbreak, of any severe contagious disease may also in turn significantly reduce the demand of traveling. For example, the COVID-19 pandemic has had resulted in a number of countries declaring a state of emergency and a number of countries, including the countries in Asian Pacific, imposing extensive travel restrictions. NewGenIvf’s revenue in the year 2021 was significantly adversely affected due to the impact from COVID-19 travel restrictions. In addition, a Chinese crime thriller, No More Bets, which has grossed more than $500 million at the international box office since its August 2023 release and which tells the harrowing story of characters being lured and kidnapped into a violent scam ring in an unnamed Southeast Asian country after accepting lucrative overseas job offers, and the continuing social media coverage may have brought fears and safety concerns to Chinese tourists of being scammed and kidnapped in Thailand and Cambodia. In addition, in October 2023, a 14-year-old with a gun opened fire in a luxury shopping mall in downtown Bangkok, killing two people and injuring five in one of Thailand’s most popular tourist destinations. These conditions may cause NewGenIvf difficulty in attracting clients from the PRC to travel to Thailand, Cambodia and Kyrgyzstan for NewGenIvf’s services, which could materially and adversely affect NewGenIvf’s operations and financial results.
Given the uncertainty of the local and global conditions and the countries’ future policy regarding international traveling, all of which are beyond NewGenIvf’s control, NewGenIvf’s results of operation may be materially and adversely affected by any changes in international travelling conditions.
Key Components of Results of Operations
NewGenIvf’s revenues were derived from two types of services: IVF treatment services and surrogacy and ancillary caring services.
Revenue
The following table sets forth NewGenIvf’s revenue for the periods indicated.
| | | For the Nine Months ended September 30, | |
| | | 2024 | | | 2023 | |
| | | US$ | | | US$ | |
| Revenues | | | 4,159,763 | | | | 3,616,698 | |
The following table sets forth a breakdown of NewGenIvf’s revenue by the types of services, in absolute amounts and as percentages of total revenue, for the periods indicated.
| | | For the Year ended December 31, | |
| | | 2023 | | | 2022 | |
| | | US$ | | | % | | | US$ | | | % | |
| IVF treatment services(1) | | | 4,021,696 | | | | 78.3 | | | | 2,819,163 | | | | 47.4 | |
| Surrogacy and ancillary caring services | | | 1,114,457 | | | | 21.7 | | | | 3,125,027 | | | | 52.6 | |
| Total revenues | | | 5,136,153 | | | | 100.0 | | | | 5,944,190 | | | | 100.0 | |
| (1) | Include an insignificant amount of revenue derived from consultation customers who used NewGenIvf’s non-IVF treatment and insignificant services, such as check-ups services, blood test services and other minor services. |
For the fiscal year ended December 31, 2023, the Company’s revenue was $5,136,153. For the fiscal year ended December 31, 2023, the Company’s revenue was $5,944,190.
NewGenIvf generated revenue from facilities located in various geographic regions. The following table sets forth a breakdown of NewGenIvf’s revenue based on the locations where the revenue originated, in absolute amounts and as percentages of total revenue, for the periods indicated.
| | | For the Year ended December 31, | |
| | | 2023 | | | 2022 | |
| | | US$ | | | % | | | US$ | | | % | |
| HK SAR | | | 34,038 | | | | 0.7 | | | | — | | | | — | |
| Kyrgyzstan | | | 3,123,593 | | | | 60.8 | | | | 5,060,973 | | | | 85.1 | |
| Cambodia | | | 621,619 | | | | 12.1 | % | | | 377,608 | | | | 6.4 | |
| Thailand | | | 1,356,903 | | | | 26.4 | % | | | 505,609 | | | | 8.5 | |
| Total revenues | | | 5,136,153 | | | | 100.0 | | | | 5,944,190 | | | | 100.0 | |
NewGenIvf’s revenue results are affected by, among others, changes in sales price and the fluctuation of foreign currency rates with US dollars. A 5% change in sales price would cause 5% change in NewGenIvf’s revenue. Based on the breakdown of the revenue contribution in terms of currencies used by customers for 2023, a 5% change in foreign currency rates with US dollars would cause approximately 1.3% change in NewGenIvf’s revenue. NewGenIvf’s average sales revenue from IVF treatment services per each IVF Customer (as defined below) was approximately US$ 14,951 in 2023 and average sales revenue from surrogacy and related ancillary caring services per each Surrogacy Customer was approximately US$10,926 in 2023.
For the year ended December 31, 2023, NewGenIvf served 357 customers using IVF treatment services and surrogacy and ancillary caring services, and recorded average revenue per such significant customer of approximately US$14,386.
IVF treatment services
NewGenIvf generated revenue from IVF treatment services provided at facilities that NewGenIvf operated in Thailand and Cambodia. In addition, NewGenIvf also recognized revenues from IVF treatments included in surrogacy services performed in Kyrgyzstan. NewGenIvf’s revenue from IVF treatment service amounted to US$2,819,163 and US$4,021,696, representing approximately 78.3% and 47.4% of its total revenues in 2023 and 2022, respectively. The number of IVF treatment service customers (the “IVF Customers”), which includes surrogacy and ancillary caring service customers who also use IVF treatment services, was approximately 269 in 2023, and the average sales revenue from IVF treatment services per each IVF Customer was approximately US$14,951 in 2023.
IVF treatment involves the performance of a series of medical treatment and procedures that are not separately distinct and only brings benefits to client when embryo is successfully implanted, either in the client or a surrogate mother. Therefore, revenue from IVF treatment is recognized at a point in time when it is completed in clinic. The completion of this treatment is evidenced by a written IVF report indicating successful embryo implantation.
Surrogacy and ancillary caring services
NewGenIvf also generated revenue from surrogacy and related ancillary caring services provided at facilities that NewGenIvf operated in Kyrgyzstan. NewGenIvf’s revenue from surrogacy and ancillary caring services amounted to US$1,114,457 and US$3,125,027, representing approximately 21.7% and 52.6% of its total revenues in 2023 and 2022, respectively. The decrease in revenue from 2022 to 2023 was primarily attributed to the departure of an agent in mid-2023, which agent had who introduced us customers for surrogacy and ancillary caring services, thus less income arising from surrogacy and ancillary caring services was generated. The number of surrogacy and related ancillary caring service customers (the “Surrogacy Customers”) was approximately 102 in 2023 and the average sales revenue from surrogacy and related ancillary caring services per each Surrogacy Customer was approximately US$10,926 in 2023.
In surrogacy and ancillary caring services, embryo from intending parents is implanted in the surrogate mother sub-contracted by NewGenIvf. During the pregnancy period of the surrogate mother, NewGenIvf provides ancillary caring services including maternity caring services such as regular body check and provision of vitamins, supplements and medicines to surrogate mothers, documentation service, and hotel accommodation services. Revenue from surrogacy and ancillary caring services is recognized at a point in time when the surrogate mother gives birth.
Cost of revenue
The following table sets forth NewGenIvf’s cost of revenue for the periods indicated.
| | | For the Nine Months Ended September 30, | |
| | | 2024 | | | 2023 | |
| | | US$ | | | US$ | |
| Cost of revenues | | | 2,872,004 | | | | 2,131,738 | |
The following table sets forth a breakdown of NewGenIvf’s cost of revenue by the nature of the cost, in absolute amounts and as percentages of total cost of revenues, for the periods indicated.
| | | For the Year ended December 31, | |
| | | 2023 | | | 2022 | |
| | | US$ | | | % | | | US$ | | | % | |
| Cost of revenues | | | | | | | | | | | | |
| Cost of goods sold | | | 594,984 | | | | 17.2 | | | | 502,969 | | | | 11.4 | |
| Clinic costs | | | 2,859,384 | | | | 82.8 | | | | 3,903,452 | | | | 88.6 | |
| Total cost of revenues | | | 3,454,368 | | | | 100.0 | | | | 4,406,421 | | | | 100.0 | |
Cost of goods sold. Cost of goods sold primarily consisted of purchase and direct cost for IVF treatment services and surrogacy and ancillary caring services. NewGenIvf’s cost of goods was mostly recognized during the provision of IVF treatment services.
Clinic costs. Clinic costs primarily consisted of sub-contracting charges, office supplies and staff salaries and bonus. The largest portion of clinic costs was sub-contracting charges, representing fees paid to agents who recruited surrogate mothers and assisted in the documentation, consulting and medical treatment arrangement throughout treatment procedure. NewGenIvf’s clinic costs of goods were mostly recognized during the provision of surrogacy services.
Gross profit and gross margin
The following table sets forth NewGenIvf’s gross profit in absolute amounts for the periods indicated.
| | | For the Nine Months Ended September 30, | |
| | | 2024 | | | 2023 | |
| | | US$ | | | US$ | |
| Gross profit | | | 1,287,759 | | | | 1,484,960 | |
The following table sets forth NewGenIvf’s gross profit in absolute amounts and its gross margin as percentages of total revenues, for the periods indicated.
| | | For the Year ended December 31, | |
| | | 2023 | | | 2022 | |
| | | US$ | | | % | | | US$ | | | % | |
| Gross profit | | | 1,681,785 | | | | 32.7 | % | | | 1,537,769 | | | | 25.9 | |
| Revenues | | | 5,136,153 | | | | — | | | | 5,944,190 | | | | — | |
NewGenIvf expects that gross profit and gross margin will continue to be affected by various factors including the geographic locations where treatments are performed, as well as the pricing with its clients, agent subcontracting charges and the costs of the supplies provided by major pharmaceutical companies, all of which are negotiated separately.
Operating expenses
NewGenIvf’s operating expenses consist primarily of selling and marketing expenses and general and administrative expenses. NewGenIvf’s selling and marketing expenses are primarily social media expenses. NewGenIvf’s general and administrative expenses mainly include depreciation in operating lease ROU assets, loss on disposal of plant and equipment and staff salaries.
Other income
NewGenIvf’s other income consists primarily of waiver of related party balance.
Interest expense
NewGenIvf’s interest expense is incurred in relation to its interest-bearing borrowing.
Taxation
British Virgin Islands
NewGenIvf is incorporated in the British Virgin Islands and is not subject to tax on income or capital gains under current British Virgin Islands law. In addition, upon payment of dividends to shareholders, no British Virgin Islands withholding tax will be imposed.
Hong Kong
Under the two-tiered profits tax rates regime, Hong Kong tax residents are subject to Hong Kong profits tax in respect of profits arising in or derived from Hong Kong at 8.25% for the first HK$2 million of profits of the qualifying group entity, and profits above HK$2 million will be taxed at 16.5%. The profits of group entities not qualifying for the two-tiered profits tax rates regime will continue to be taxed at a flat rate of 16.5%.
Accordingly, the Hong Kong profits tax is calculated at 8.25% on the first HK$2 million of the estimated assessable profits and at 16.5% on the remaining estimated assessable profits.
Thailand
The companies incorporated in Thailand are taxed on worldwide income. A company incorporated outside of Thailand is taxed on its profits arising from or in consequence of the business carried on in Thailand. The Thailand corporate income tax rate is 20%. A foreign company not carrying on business in Thailand is subject to a final withholding tax on certain types of assessable income (e.g., interest, dividends, royalties, rentals, and service fees) paid from or in Thailand. The rate of tax is generally 15%, except for dividends, which is 10%, while other rates may apply under the provisions of a double tax treaty.
Cambodia
The standard rate of corporate income tax for companies and permanent establishments in Cambodia who are classified as medium and large taxpayers is 20%. For companies and permanent establishments who are classified as small taxpayers, the corporate income tax rates are progressive rates from 0% to 20%. In view of the annual turnover of the company, which ranges from KHR1 billion to KHR6 billion for service and commercial sectors, the company is considered a medium-sized company.
Kyrgyzstan
NewGenIvf is subject to a corporate income tax on its aggregate annual income earned worldwide. Non-resident legal entities carrying out business activities through a permanent establishment in Kyrgyzstan are subject to profit tax on the income attributed to the activities of that permanent establishments. Profit tax is calculated at a rate of 10% of aggregate annual income less allowed deductions.
Results of Operations
Nine month period ending September 30, 2024 Compared with Nine month period ending September 30, 2023
| | | Nine Months
Ended
September 30,
2024 | | | Nine Months
Ended
September 30,
2023 | |
| Revenues | | $ | 4,159,763 | | | $ | 3,616,698 | |
| Cost of revenues | | | (2,872,004 | ) | | | (2,131,738 | ) |
| Gross profit | | | 1,287,759 | | | | 1,484,960 | |
| | | | | | | | | |
| Operating expenses | | | | | | | | |
| Selling and marketing expenses | | | (192,276 | ) | | | (11,540 | ) |
| General and administrative expenses | | | (1,166,858 | ) | | | (1,663,952 | ) |
| | | | | | | | | |
| Total operating expenses | | | (1,359,134 | ) | | | (1,675,492 | ) |
| | | | | | | | | |
| Operating income (loss) | | | (71,375 | ) | | | (190,532 | ) |
| | | | | | | | | |
| Other income (expenses), net | | | | | | | | |
| Other income, net | | | 8,404 | | | | 23,892 | |
| Interest income | | | 2,399 | | | | 396 | |
| Interest expense | | | (357,551 | ) | | | (20,922 | ) |
| Total other income (expenses), net | | | (346,748 | ) | | | 3,366 | |
| | | | | | | | | |
| Income (loss) before taxes | | | (418,123 | ) | | | (187,166 | ) |
| Provision for income taxes | | | --- | | | | (83,742 | ) |
| Net income (loss) | | | (418,123 | ) | | | (270,908 | ) |
| Less: net loss attributable to non-controlling interests | | | (1,723 | ) | | | (65,476 | ) |
| Net income (loss) attributable to the shareholders of the Company | | $ | (416,400 | ) | | | (205,432 | ) |
| | | | | | | | | |
| Other comprehensive income (loss) | | | | | | | | |
| Foreign currency translation adjustment | | | 13,879 | | | | 37,270 | |
| Total comprehensive income (loss) | | | (404,244 | ) | | | (233,638 | ) |
| Less: total comprehensive loss attributable to non-controlling interests | | | 1,498 | | | | (55,938 | ) |
| Total comprehensive income attributable to the shareholders of the Company | | $ | (405,742 | ) | | | (177,700 | ) |
| | | | | | | | | |
| Earning per share – basic | | $ | (0.04 | ) | | | (0.34 | ) |
| - diluted | | | (0.03 | ) | | | (0.34 | ) |
| Weighted average shares outstanding – Basic | | | 10,149,386 | | | | 601,830 | |
| - diluted | | | 14,770,914 | | | | 601,830 | |
Year Ended December 31, 2023 Compared with Year Ended December 31, 2022
| | | For the Year ended
December 31, | |
| | | 2023 | | | 2022 | |
| | | US$ | |
| Revenues | | | 5,136,153 | | | | 5,944,190 | |
| Cost of revenues | | | (3,454,368 | ) | | | (4,406,421 | ) |
| Gross profit | | | 1,681,785 | | | | 1,537,769 | |
| | | | | | | | | |
| Operating expenses | | | | | | | | |
| Selling and marketing expenses | | | (18,030 | ) | | | (36,194 | ) |
| General and administrative expenses | | | (1,259,364 | ) | | | (1,094,962 | ) |
| Auditors fees | | | (362,149 | ) | | | (7,908 | ) |
| Total operating expenses | | | (1,639,543 | ) | | | (1,139,064 | ) |
| | | | | | | | | |
| Operating income | | | 42,242 | | | | 398,705 | |
| | | | | | | | | |
| Other income (expenses), net | | | | | | | | |
| Other income | | | 111,837 | | | | 23,019 | |
| Interest income | | | 518 | | | | 21 | |
| Interest expense | | | (46,179 | ) | | | (77,757 | ) |
| Total other income (expenses), net | | | 66,176 | | | | (54,717 | ) |
| | | | | | | | | |
| Income before taxes | | | 108,418 | | | | 343,988 | |
| Provision for income taxes | | | — | | | | (208,141 | ) |
| Net income | | | 108,418 | | | | 135,847 | |
| Less: net loss attributable to non-controlling interests | | | (21,775 | ) | | | (322,820 | ) |
| Net income attributable to the shareholders of the Company | | | 130,193 | | | | 458,667 | |
| Other comprehensive (loss) income | | | | | | | | |
| Foreign currency translation adjustment | | | (22,704 | ) | | | (1,920 | ) |
| Total comprehensive income | | | 85,714 | | | | 133,927 | |
| Less: Total comprehensive loss attributable to non-controlling interests | | | (27,621 | ) | | | (323,458 | ) |
| Total comprehensive income attributable to the shareholders of the Company | | | 113,335 | | | | 457,385 | |
| | | | | | | | | |
| (Loss) earning per share – basic and diluted | | | 0.18 | | | | 0.80 | |
| Basic and diluted weighted average shares outstanding | | | 615,135 | | | | 575,930 | |
Revenue
NewGenIvf’s revenue for the nine months ended September 30, 2024 was $4,159,763 and was $3,616,698 for the nine months ended September 30, 2023.
NewGenIvf’s revenue decreased by approximately 13.6% from US$5,944,190 in the fiscal year ended December 31, 2022 to US$5,136,153 in the fiscal year ended December 31, 2023.
IVF treatment services
NewGenIvf’s IVF treatment service revenue increased by approximately 42.7% from US$2,819,163 in 2022 to US$4,021,696 in 2023. This increase was primarily the result of our continued expansion of clinics in Thailand which focus on IVF services.
Surrogacy and ancillary caring services
NewGenIvf’s surrogacy and ancillary caring services revenue decreased by approximately 64.3% from US$3,125,027 in 2022 to US$1,114,457 in 2023. This decrease was primarily the result of temporary caesura of surrogacy business.
Cost of revenue
NewGenIvf’s cost of revenues increased by approximately 34.7% from 2,131,738 for the nine month period ending September 30, 2023 was to 2,872,004 for the nine month period ending September 30, 2024. The reason for this increase was increased agency services costs involved in subcontracting follow-up services.
NewGenIvf’s cost of revenue decreased by approximately 21.6% from US$4,406,421 in the fiscal year ended December 31, 2022 to US$3,454,368 in the fiscal year ended December 31, 2023.
Cost of goods sold
NewGenIvf’s cost of goods sold increased by approximately 18.3% from US$502,969 in the fiscal year ended December 31, 2022 to US$594,984 in the fiscal year ended December 31, 2023, primarily attributed to the stocking arrangements prepared for 2023 exceed the original estimated demand, due to the local top management reported on board until in the middle of the year, and the procurement strategy was not immediately carried on time, which also caused procurement costs to double year-on-year.
Clinic costs
NewGenIvf’s clinic costs decreased by approximately 26.7% from US$3,903,452 in the fiscal year ended December 31, 2022 to US$2,859,384 in the fiscal year ended December 31, 2023, primarily due to the relocation arrangement, certain daily operating schedules stopped, resulting in the clinic’s service being temporarily suspended in 2023.
Gross profit
NewGenIvf’s gross profit for the nine months ended September 30, 2024 was $1,287,759, compared to its gross profit of $1,484,960 for the nine months ended September 30, 2023, representing a 13.3% decrease from the nine months ended September 30, 2023. This decrease was due primarily to business difficulties in the nine months ended September 30, 2024.
NewGenIvf’s gross profit increased by approximately 9.4% from US$1,537,769 in the fiscal year ended December 31, 2022 to US$1,681,785 in the fiscal year ended December 31, 2023, primarily attributable to a reorganizing of our cooperation model with subcontractors and the increased efficiency of our marketing services, resulting in a decrease in unit service costs per customer, directly leading to increases in gross profit margins.
NewGenIvf’s gross margin increased from 25.9% in the fiscal year ended December 31, 2022 to 32.7% in the fiscal year ended December 31, 2023.
Operating expenses
NewGenIvf’s operating expenses were $1,359,134 and $1,663,952 for the nine month period ended September 30, 2024 and the nine month period ended September 30, 2023, respectively. This represents a decrease of 18% from the nine month period ended September 30, 2023, due to no more one-time charged from legal and professional fee in connection with NewGenIvf’s de-SPAC merger which was completed on April 3, 2024, and additional legal and professional charges for a debt finance by the convertible promissory note in the amount of $291,659 and recognized in the nine months period ended September 30, 2024.
NewGenIvf’s operating expenses increased by approximately 43.9% from US$1,139,064 in the fiscal year ended December 31, 2022 to US$1,639,543 in the fiscal year ended December 31, 2023, primarily attributable to auditor fees of US$362,149 incurred in 2022 being recognised in 2023 and listing legal and professional fees of US$183,527, other than these old fees incurred, there is the similar level with last year.
Other income
NewGenIvf’s other income increased decreased from $23,892 to $8,404 from the nine month period ended September 30, 2023 to the nine month period ended September 30, 2024.
NewGenIvf’s other income increased from US$23,019 in the fiscal year ended December 31, 2022 to US$111,837 in the fiscal year ended December 31, 2023, primarily attributable to a waiving amount due to director from the company which is about US$88,151.
Interest expense
NewGenIvf’s interest expense increased by approximately 1,609%, from US$20,922 in the nine month period ended September 30, 2023 to US$357,551 in the nine month period ended September 30, 2024 as a result of interest charged and a discount on issuance of a promissory note on debt financing of US$4,300,000.
NewGenIvf’s interest expense decreased by approximately 40.6%, from US$77,757 in 2022 to US$46,179 in 2023 as a result of less interest expenses on bank and other borrowings in 2023.
Provision for income taxes
NewGenIvf’s provision for income taxes was not made as a result of 100% allowance from deferred tax assets on net operating loss in the nine months ended September, 2024.
NewGenIvf’s provision for income taxes decreased by approximately 100% from US$208,141 in 2022 to US$Nil in 2023 as a result of no assessable income generated from Thailand, Kyrgyzstan and Cambodia.
Net income
NewGenIvf’s net income decreased by approximately 20% from US$135,847 in 2022 to US$108,418 in 2023 as a result of a listing project carried out during in 2023 and a relocation of our operating clinic in Thailand, to cause the increase cost and salary of recruiting and training loacl talents. There is an additional auditor fees for the year, which is amounting to US$362,149.
Liquidity and Capital Resources
Cash flows and working capital
NewGenIvf’s principal sources of liquidity have been generated from its business operations. As of September 30, 2024 NewGenIvf had US$169,661 in cash and cash equivalents. As of December 31, 2023 and 2022, NewGenIvf had US$54,104 and U$27,556, respectively, in cash and cash equivalents. NewGenIvf had working capital (defined as total current assets deducted by total current liabilities) of a deficit of US$2,513,209 as of September 30, 2024. NewGenIvf had working capital of a surplus of US$79,000 and deficit of US$157,027, respectively, as of December 31, 2023 and 2022.
Over the years, certain amount of cash provided by operating activities was distributed to NewGenIvf’s primary shareholders, Mr. Siu, Wing Fung Alfred and Ms. Fong, Hei Yue Tina. As of December 31, 2023, NewGenIvf does not owe any amounts to shareholders. Nevertheless, NewGenIvf is able to generate sufficient cash flow from its business operations to operate and grow its business.
NewGenIvf continually seeks to monetize from positive cash flow contracts and increase revenue from its operating activities. NewGenIvf monitors its current and expected liquidity requirements to help ensure that it maintains sufficient cash balances to meet its existing and reasonably likely long-term liquidity needs.
NewGenIvf intends to finance its future working capital requirements and capital expenditures from cash generated from operating activities, in addition to funds raised from financing activities. NewGenIvf may, however, require additional cash due to changing business conditions or other future developments, including any investments or acquisitions it may decide to pursue. If its existing cash is insufficient to meet its requirements, NewGenIvf may seek to issue debt or equity securities or obtain additional credit facilities. Financing may be unavailable in the amounts NewGenIvf needs or on terms acceptable to it, if at all. Issuance of additional equity securities, including convertible debt securities, would dilute NewGenIvf’s earnings per share. The incurrence of debt would divert cash for working capital and capital expenditures to service debt obligations and could result in operating and financial covenants that restrict NewGenIvf’s operations and its ability to pay dividends to its shareholders. If NewGenIvf is unable to obtain additional equity or debt financing as required, its business operations and prospects may suffer. Please see “Risk Factors — Risks Relating to NewGenIvf’s Business and Industry — NewGenIvf requires a significant amount of capital to fund its operations and growth. If NewGenIvf cannot obtain sufficient capital on acceptable terms, its business, financial condition, and prospects may be materially and adversely affected.”
The following table presents NewGenIvf’s selected consolidated cash flow data for the periods indicated.
| | | For the Nine Months Ended
September 30, | |
| | | 2024 | | | 2023 | |
| CASH FLOWS FROM OPERATING ACTIVITIES | | | | | | |
| Net income (loss) | | $ | (418,123 | ) | | | (270,908 | ) |
| Adjustments to reconcile net (loss) income to net cash provided by operating activities: | | | | | | | | |
| Depreciation of plant and equipment | | | 19,300 | | | | 5,856 | |
| Amortization of right-of-use assets | | | 7,060 | | | | 56,902 | |
| Provision of expected credit loss allowance | | | -- | | | | 634 | |
| Changes in operating assets and liabilities: | | | | | | | | |
| Accounts receivable | | | (309,485 | ) | | | 12,870 | |
| Inventories | | | 31,479 | | | | (17,323 | ) |
| Loan to A SPAC I | | | -- | | | | (140,000 | ) |
| Deposit, prepayment and other receivables, net | | | 138,580 | | | | (8,701 | ) |
| Accrued liabilities and other payables | | | 11,741 | | | | 56,234 | |
| Legal fee payable | | | (2,007,444 | ) | | | 60 | |
| Contract liabilities | | | 463 | | | | (1,250,900 | ) |
| Operating lease liabilities | | | (89,702 | ) | | | (89,702 | ) |
| Taxes payable | | | -- | | | | 87,234 | |
| Net cash provided by/(used in) operating activities | | | (2,616,131 | ) | | | (1,557,744 | ) |
| | | | | | | | | |
| CASH FLOWS FROM INVESTING ACTIVITIES | | | | | | | | |
| Purchase of plant and equipment | | | (50,337 | ) | | | (52,386 | ) |
| Purchase of intangible assets | | | (16,381 | ) | | | - | |
| Net cash used in investing activities | | | (66,718 | ) | | | (52,386 | ) |
| | | | | | | | | |
| CASH FLOWS FROM FINANCING ACTIVITIES | | | | | | | | |
| Finance lease | | | (9,317 | ) | | | (14,019 | ) |
| Other borrowings, net | | | | | | | - | |
| Amount with related parties | | | (125,394 | ) | | | 1,353,426 | |
| Subscription receivables | | | -- | | | | 192,308 | |
| Convertible promissory note | | | 2,920,238 | | | | - | |
| Net cash provided by financing activities | | | 2,785,527 | | | | 1,531,715 | |
| | | | | | | | | |
| Net increase / (decrease) in cash and cash equivalents | | | 102,678 | | | | (78,415 | ) |
| Effect of foreign currency translation on cash and cash equivalents | | | 12,879 | | | | 87,973 | |
| Cash and cash equivalents, beginning of period | | | 54,104 | | | | 27,556 | |
| Cash and cash equivalents, end of period | | $ | 169,661 | | | | 37,114 | |
| | | | | | | | | |
| Supplementary cash flow information: | | | | | | | | |
| Taxes paid | | $ | -- | | | | - | |
| Interest paid | | $ | (357,551 | ) | | | (20,922 | ) |
| Listing fee paid | | $ | -- | | | | 976,166 | |
| | | For the Nine Months Ended
September 30, | |
| | | 2024 | | | 2023 | |
| | | US$ | |
| Net cash (used in) by operating activities | | | (2,616,131 | ) | | | (1,557,744 | ) |
| Net cash (used in) investing activities | | | (66,718 | ) | | | (52,386 | ) |
| Net cash provided by financing activities | | | 2,785,527 | | | | 1,531,715 | |
| | | | | | | | | |
| Net increase/(decrease) in cash and cash equivalents | | | 102,678 | | | | (78,415 | ) |
| Effect of foreign currency translation on cash and cash equivalents | | | 12,879 | | | | 87,973 | |
| Cash and cash equivalents, beginning of period | | | 54,104 | | | | 27,556 | |
| Cash and cash equivalents, end of period | | | 169,661 | | | | 37,114 | |
| | | For the Year ended
December 31, | |
| | | 2023 | | | 2022 | |
| | | US$ | |
| Net cash (used in)/provided by operating activities | | | (1,766,135 | ) | | | 1,710,901 | |
| Net cash used in investing activities | | | (69,848 | ) | | | (94,452 | ) |
| Net cash provided by/(used in) financing activities | | | 1,881,493 | | | | (1,633,781 | ) |
| | | | | | | | | |
| Net increase/(decrease) in cash and cash equivalents | | | 45,510 | | | | (17,332 | ) |
| Effect of foreign currency translation on cash and cash equivalents | | | (18,962 | ) | | | 16,124 | |
| Cash and cash equivalents, beginning of year | | | 27,556 | | | | 28,764 | |
| Cash and cash equivalents, end of year | | | 54,104 | | | | 27,556 | |
Operating activities
Net cash used in operating activities was US$2,616,131 for the nine month period ended September 30, 2024. The difference between NewGenIvf’s net profit of US$832,640 for the nine month period ended September 30, 2024 and the net cash used in operating activities was primarily attributable to legal fee payable, accrued liabilities and other payables.
Net cash used in operating activities was US$1,766,135 for the year ended December 31, 2023. The difference between NewGenIvf’s net profit of US$108,418 for the year ended December 31, 2023 and the net cash used in operating activities was primarily attributable to refund of payment from clients from the contract liabilities and the expenses spent on the legal and professional cost which was capitalized in the book of 2023.
Net cash provided by operating activities was US$1,710,901 for the year ended December 31, 2022. The difference between NewGenIvf’s net income of US$135,847 for the year ended December 31, 2022 and the net cash provided by operating activities was primarily attributable to (i) adjustments for depreciation and amortization of US$303,944, (ii) changes in contract liabilities of US$548,010 and (iii) changes in directors’ remuneration of US$240,000, partially offset by operating lease liabilities of US$175,132.
Investing activities
Net cash used in investing activities in the nine month period ending on September 30, 2024 was ($66,718), primarily representing NewGenIvf’s additional equipment and improvement at its Thailand clinic.
Net cash used in investing activities in 2023 was US$69,848, primarily representing purchase of plant and equipment.
Net cash used in investing activities in 2022 was US$94,452, primarily representing purchase of plant and equipment.
Financing activities
Net cash used provided by financing activities in the nine month period ending on September 30, 2024 was 2,785,527, primarily representing a debt financing by convertible promissory notes.
Net cash provided by financing activities in 2023 was US$1,881,493, primarily representing amounts from shareholders.
Net cash used in financing activities in 2022 was US$1,633,781, primarily representing amounts due from related parties.
Contractual Obligations
The following table sets forth NewGenIvf’s main contractual obligations and commitments as of December 31, 2023.
| | | December 31, | |
| | | 2023 | | | 2022 | |
| | US$ | | | US$ | |
| Lease liabilities – current portion | | | 207,128 | | | | 184,651 | |
| Lease liabilities – non-current portion | | | 118,979 | | | | 242,187 | |
| Total | | | 326,107 | | | | 426,838 | |
Off-Balance Sheet Commitments and Arrangements
NewGenIvf has not entered into any financial guarantees or other commitments to guarantee the payment obligations of any third parties, nor any derivative contracts that are indexed to its shares and classified as shareholder’s equity or that are not reflected in its consolidated financial statements. Furthermore, NewGenIvf does not have any retained or contingent interest in assets transferred to an unconsolidated entity that serves as credit, liquidity or market risk support to such entity. NewGenIvf does not have any variable interest in any unconsolidated entity that provides financing, liquidity, market risk or credit support to it or engages in leasing, hedging or product development services with it.
Holding Company Structure
NewGenIvf Group Limited is a holding company with no material operations of its own. NewGenIvf Group Limited conducts all of its operations through its subsidiaries. As a result, NewGenIvf Group Limited’s ability to pay dividends depends upon dividends paid by its subsidiaries. If our subsidiaries or any newly formed subsidiaries incur debt on their own behalf in the future, the instruments governing their debt may restrict their ability to pay dividends to the Company.
NewGenIvf Group Limited is permitted under BVI law to provide funding to its subsidiaries in Hong Kong, Thailand, Cambodia and Kyrgyzstan through loans or capital contributions without restrictions on the amount of the funds.
In addition, the Company’s subsidiaries are currently permitted to pay dividends to the Company in accordance with relevant laws and regulations. Payment of dividends requirements in a company incorporated under the laws of Thailand is governed by the Civil and Commercial Code of Thailand. For example, the company may not declare dividends if the company has incurred losses, the company must appropriate to a reserved fund at each dividend contribution of dividend of at least one-twentieth of the profits until the fund reaches one-tenth of the capital, or the dividends payment must be made to the shareholders within one (1) month from the dividend declaration date. On the capital remittance or payment of dividends to the shareholders from outside of Thailand, it is regulated by the regulations issued by the Bank of Thailand, including the Exchange Control Act B.E. 2485 (1942). The fund remittance from Thailand to a foreign jurisdiction may require an approval from the Bank of Thailand or require notifying the Bank of Thailand for such transfer, depending on the types of the remittance transactions, through the commercial bank in the country. For a company incorporated under the laws of Kyrgyzstan, under Kyrgyz regulations of dividends (net profit), the dividends can be paid once a year depending on the results of the financial year of the company.
Quantitative and Qualitative Disclosure about Market Risk
Accounts receivable
In order to minimize the credit risk, NewGenIvf’s management team monitors and ensures that follow-up action is taken to recover overdue debts. NewGenIvf considers the probability of default upon initial recognition of the asset and whether there has been a significant increase in credit risk on an ongoing basis throughout each reporting period. To assess whether there is a significant increase in credit risk, NewGenIvf compares the risk of a default occurring on the asset as at the reporting date with the risk of default as at the date of initial recognition. It considers available reasonable and supportive forwarding-looking information, such as GDP growth rate and nominal GDP per capita. Based on the impairment assessment performed by NewGenIvf, the directors considered the loss allowance for account receivables as of December 31, 2023 and December 31, 2022 is $19 and $26, respectively.
Cash and cash equivalents
NewGenIvf is exposed to concentration of credit risk on liquid funds which are deposited with several banks with high credit ratings. The credit risk on liquid funds is limited because the counterparties are banks with high credit ratings assigned by international credit-rating agencies.
Deposits and other receivables, amount due from shareholders and loan to A SPAC I
NewGenIvf assessed the impairment for deposits and other receivables, due from shareholders and loan to A SPAC I individually based on internal credit rating and ageing of these debtors which, in the opinion of the directors, have no significant increase in credit risk since initial recognition. Based on the impairment assessment performed by the Company, the directors consider the loss allowance for deposits and other receivables, due from shareholders and loan to A SPAC I as of December 31, 2023 is $14, $17,818 and Nil, respectively. The loss allowance for deposits and other receivables, due from shareholders and loan to A SPAC I as of December 31, 2022 is $141, $17,059 and Nil, respectively. The loss allowance for deposits and other receivables and amount due from shareholders as of December 31, 2021 was $115 and $6,312 and Nil, respectively.
Cash flow interest rate risk
NewGenIvf is exposed to cash flow interest rate risk through the changes in interest rates related mainly to its variable-rates bank balances.
NewGenIvf currently does not have any interest rate hedging policy in relation to fair value interest rate risk and cash flow interest rate risk. The directors monitor NewGenIvf’s exposures on an ongoing basis and will consider hedging the interest rate should the need arises.
Sensitivity analysis
The sensitivity analysis below has been determined by assuming that a change in interest rates had occurred at the end of the reporting period and had been applied to the exposure to interest rates for financial instruments in existence at that date. 1% increase or decrease is used when reporting interest rate risk internally to key management personnel and represents management’s assessment of the reasonably possible change in interest rates.
If interest rates had been 1% higher or lower and all other variables were held constant, NewGenIvf’s post tax loss for the years ended December 31, 2023 and 2022 would have increased or decreased by approximately US$122 and US$275, respectively.
Foreign currency risk
Foreign currency risk is the risk that the holding of foreign currency assets will affect NewGenIvf’s financial position as a result of a change in foreign currency exchange rates.
NewGenIvf’s monetary assets and liabilities are mainly denominated in HK$ and THB which are the same as the functional currencies of the relevant group entities. Hence, in the opinion of the directors of NewGenIvf, the currency risk of US$ is considered insignificant. NewGenIvf currently does not have a foreign currency hedging policy to eliminate currency exposures. However, the directors monitor the related foreign currency exposure closely and will consider hedging significant foreign currency exposures should the need arise.
Economic and political risks
NewGenIvf’s operations are mainly conducted in Thailand, Cambodia and Kyrgyzstan. Accordingly, NewGenIvf’s business, financial condition, and results of operations may be influenced by changes in the political, economic, and legal environments in Thailand, Cambodia and Kyrgyzstan.
NewGenIvf’s operations in Thailand, Cambodia and Kyrgyzstan are subject to special considerations and significant risks not typically associated with companies in North America and Western Europe. These include risks associated with, among other things, the political, economic and legal environment and foreign currency exchange. NewGenIvf’s results may be adversely affected by changes in the political and social conditions in Thailand, Cambodia and Kyrgyzstan, and by changes in governmental policies with respect to laws and regulations, anti-inflationary measures, currency conversion, remittances abroad, and rates and methods of taxation, among other things.
Travel restriction risk
International clients contribute a large portion of NewGenIvf’s revenue. International clients need to travel to Thailand, Cambodia and Kyrgyzstan for treatment services, where NewGenIvf’s operations are mainly conducted.
International traveling to Thailand, Cambodia and Kyrgyzstan may be affected by a number of factors, including local and global political and economic conditions. Furthermore, an outbreak, or threatened outbreak, of any severe contagious disease may also in turn significantly reduce the demand of traveling or cause extensive travel restrictions. NewGenIvf’s results may be materially and adversely affected if travel restriction was imposed or difficulties in cross-border flow arose.
Inflation risk
Management of NewGenIvf monitors changes in prices levels. Historically inflation has not materially impacted NewGenIvf’s consolidated financial statements; however, significant increases in the price of labor that cannot be passed to NewGenIvf’s customers could adversely impact its results of operations.
Critical Accounting Policies, Judgments and Estimates
NewGenIvf prepares its financial statements in conformity with U.S. GAAP, which requires NewGenIvf to make judgments, estimates and assumptions. NewGenIvf continually evaluates these estimates and assumptions based on the most recently available information, its historical experience and various other assumptions that NewGenIvf’s management believes to be reasonable under the circumstances. Since the use of estimates is an integral component of the financial reporting process, actual results could differ from its expectations as a result of changes in NewGenIvf’s estimates. Some of NewGenIvf’s accounting policies require a higher degree of judgment than others in their application and require NewGenIvf to make significant accounting estimates.
The selection of critical accounting policies, the judgments and other uncertainties affecting application of those policies and the sensitivity of reported results to changes in conditions and assumptions are factors that should be considered when reviewing NewGenIvf’s financial statements. NewGenIvf’s management believes the following accounting policies involve the most significant judgments and estimates used in the preparation of their financial statements.
Foreign currency translation
NewGenIvf’s consolidated financial statements are presented in United States dollar, which is the reporting currency of NewGenIvf. The functional currency of NewGenIvf and its subsidiaries, FFPGS (HK) Limited and Well Image Limited, are HK$. Med Holdings and FFC use THB as their functional currencies. First Fertility Phnom Penh Limited uses KHR as its functional currency and First Fertility Bishkek LLC uses USD as its functional currency.
Assets and liabilities denominated in currencies other than the reporting currency are translated into the reporting currency at the rates of exchange prevailing at the balance sheet date. Translation gains and losses are recognized in the consolidated statements of operations and comprehensive income as other comprehensive income or loss.
Transactions in currencies other than the reporting currency are measured and recorded in the reporting currency at the exchange rate prevailing on the transaction date. The cumulative gain or loss from foreign currency transactions is reflected in the consolidated statements of operations and comprehensive income as other income (other expenses).
The value of foreign currencies including, the HK$, THB, KHR and RMB, may fluctuate against the United States dollar. Any significant variations of the aforementioned currencies relative to the United States dollar may materially affect NewGenIvf’s financial condition in terms of reporting in USD. See “Note 2 — Summary of Significant Accounting Policies” for details.
Revenue recognition
NewGenIvf adopted ASC Topic 606, Revenue from Contracts with Customers, and all subsequent ASUs that modified ASC 606 on April 1, 2017 using the full retrospective method which requires it to present the financial statements for all periods as if Topic 606 had been applied to all prior periods. NewGenIvf derives revenue principally from provision of IVF treatment and surrogacy and ancillary caring services. Revenue from contracts with customers is recognized using the following five steps:
| (1) | identify its contracts with customers; |
| (2) | identify its performance obligations under those contracts; |
| (3) | determine the transaction prices of those contracts; |
| (4) | allocate the transaction prices to its performance obligations in those contracts; and |
| (5) | recognize revenue when each performance obligation under those contracts is satisfied. Revenue is recognized when promised services are transferred to the client in an amount that reflects the consideration expected in exchange for those services. |
NewGenIvf enters into service agreements with its customers that outline the rights, responsibilities, and obligations of each party. The agreements also identify the scope of services, service fees and payment terms. Agreements are acknowledged and signed by both parties. All the contracts have commercial substance, and it is probable that NewGenIvf will collect considerations from its customers for service component.
NewGenIvf derives its revenues from two types of services: (1) IVF treatment services, and (2) surrogacy and ancillary caring services.
Revenue from IVF treatment services
IVF treatment is an assisted reproductive technique where eggs and sperm are collected and fertilized in laboratory to become embryo. Fertilized embryo is then implanted in the customer or a surrogate mother. IVF treatment involves the performance of a series of medical treatment and procedures that are not separately distinct and only brings benefits to customer when embryo is successfully implanted, therefore revenue from IVF treatment is recognized at a point in time when it is completed in clinic. The completion of this treatment is evidenced by a written IVF report indicating successful embryo implantation. NewGenIvf collects payment from customer in advance for IVF treatment.
Revenue from surrogacy and ancillary caring services
NewGenIvf provides surrogacy and ancillary caring services solely in Kyrgyzstan. Embryo from blood parents is implanted to surrogate mother contracted by NewGenIvf. During pregnancy period, NewGenIvf provides ancillary caring services including regular body check and provision of vitamins, supplements and medicines to surrogate mothers. The key performance obligation is identified as a single performance obligation where a baby is born, therefore revenue from surrogacy and ancillary caring services is recognized at a point in time when surrogate mother gives birth. NewGenIvf collects approximately 40% of contract sum upfront, and remaining contract sum is collected in installments across pregnancy period of surrogate mother.
Lease
NewGenIvf adopted ASU 2016-02, “Leases” (Topic 842). Lease terms used to calculate the present value of lease payments generally do not include any options to extend, renew, or terminate the lease, as NewGenIvf does not have reasonable certainty at lease inception that these options will be exercised. NewGenIvf generally considers the economic life of its operating lease ROU assets to be comparable to the useful life of similar owned assets. NewGenIvf has elected the short-term lease exception, therefore operating lease ROU assets and liabilities do not include leases with a lease term of twelve months or less. Its leases generally do not provide a residual guarantee. The operating lease ROU asset also excludes lease incentives. Lease expense is recognized on a straight-line basis over the lease term.
As of December 31, 2022, there were approximately $0.38 million ROU assets and approximately $0.43 million in lease liabilities based on the present value of the future minimum rental payments of leases, respectively. NewGenIvf’s management believes that using an incremental borrowing rate of the Hong Kong Dollar Best Lending Rate (“BLR”) minus 0.125% was the most indicative rate of NewGenIvf’s borrowing cost for the calculation of the present value of the lease payments; the rate used by NewGenIvf was 5.0%.
As of December 31, 2023, there were approximately $0.28 million ROU assets and approximately $0.33 million in lease liabilities based on the present value of the future minimum rental payments of leases, respectively. NewGenIvf’s management believes that using an incremental borrowing rate of the Hong Kong Dollar Best Lending Rate (“BLR”) minus 0.125% was the most indicative rate of NewGenIvf’s borrowing cost for the calculation of the present value of the lease payments; the rate used by NewGenIvf was 5.0%.
Financial instruments
NewGenIvf’s financial instruments, including cash and cash equivalents, accounts and other receivables, accounts and other payables, accrued liabilities and amounts due from (to) shareholders, have carrying amounts that approximate their fair values due to their short maturities. ASC Topic 820, “Fair Value Measurements and Disclosures” requires disclosing the fair value of financial instruments held by NewGenIvf. ASC Topic 825, “Financial Instruments” defines fair value and establishes a three-level valuation hierarchy for disclosures of fair value measurement that enhances disclosure requirements for fair value measures. The carrying amounts reported in the consolidated balance sheets for cash and cash equivalents, accounts and other receivables, accounts and other payables, accrued liabilities and amounts due from (to) shareholders each qualify as financial instruments and are a reasonable estimate of their fair values because of the short period between the origination of such instruments and their expected realization and their current market rate of interest. NewGenIvf analyzes all financial instruments with features of both liabilities and equity under ASC 480, “Distinguishing Liabilities from Equity” and ASC 815. See “Note 2 — Summary of Significant Accounting Policies” for details.
Recent accounting pronouncements
In April 2019, the FASB issued ASU 2019-04, Codification Improvements to Topic 326, Financial Instruments-Credit Losses, Topic 815, Derivatives and Hedging, and Topic 825, Financial Instruments, which amends and clarifies several provisions of Topic 326. In May 2019, the FASB issued ASU 2019-05, Financial Instruments-Credit Losses (Topic 326) Targeted Transition Relief, which amends Topic 326 to allow the fair value option to be elected for certain financial instruments upon adoption. ASU 2019-10 extended the effective date of ASU 2016-13 until December 15, 2022. This standard replaces the incurred loss methodology with an expected loss methodology that is referred to as the current expected credit loss (“CECL”) methodology. CECL requires an estimate of credit losses for the remaining estimated life of the financial asset using historical experience, current conditions, and reasonable and supportable forecasts and generally applies to financial assets measured at amortized cost, including loan receivables and held-to-maturity debt securities, and some off-balance sheet credit exposures such as unfunded commitments to extend credit. Financial assets measured at amortized cost will be presented at the net amount expected to be collected by using an allowance for expected credit losses. The Company already adopted the new standard and the Company recognizes the full impact of the new standard in these consolidated balance sheets and makes related disclosures.
BUSINESS
Overview
We are an assisted reproductive services (“ARS”) provider in Asia-Pacific. Since the opening of our first clinic in Thailand in 2014, we have established ourself as a long-standing ARS provider in this region. Our strategic presence in Thailand, Cambodia, and Kyrgyzstan positions us to take advantage of opportunities across Asia-Pacific. According to China Insights Consultancy (“CIC”), from 2014 to 2022, there was a rising number of women in the key ARS-targeted age group (ages 15 to 49) in Asia Pacific and a growing trend towards later maternal age. The number of married women of reproductive age in Asia Pacific has risen from 816.4 million in 2014 to 833.2 million in 2022. Additionally, according to CIC, there was increasing social acceptance of ARS use in Asia Pacific countries such as China, India, and Thailand during the same period. For example, the number of ARS users in China has risen from 136.8 thousand in 2017 to 184.9 thousand in 2022 approximately and that in Japan has risen from 98.0 thousand in 2017 to 128.5 thousand in 2022.
According to CIC, the prevalence of infertility in Asia-Pacific developing countries is substantial. For example, the infertility rate in Thailand, India and China was about 15.4%, 13.8% and 17.8%, respectively, in 2022. In India, the infertility rate in 2020 was approximately 13.1%, representing an annual growth of 2.6%. The infertility rate in China was around 17.6% in 2020, representing an annual growth of 0.6%. Infertility is increasingly gaining society’s attention as individuals are more openly discussing their struggles. Despite the prevalence of infertility, access to treatment is often limited in the Asia Pacific region. According to CIC, financial challenges, costs of treatment, and limited availability or capacity of fertility medical care are some of the main challenges in the fertility marketplace in Asia-Pacific region. Religious, social and cultural roadblocks can also prevent hopeful couples from realizing their dream to have children. We believe that we can help address some of these key challenges of Asia-Pacific fertility industry.
History and Development of the Company
Prior to the Business Combination, on April 29, 2021, A SPAC I Acquisition Corp. (“ASCA”), was incorporated as a British Virgin Islands business company, specifically a blank check company formed for the purpose of effecting a merger, share exchange, asset acquisition, share purchase, recapitalization, reorganization or similar business combination with one or more target businesses.
The Business Combination
On February 15, 2023, ASCA entered into the Merger Agreement (as amended on June 12, 2023 and December 6, 2023, the “Merger Agreement,” and the transactions contemplated thereunder, the “Business Combination”) with A SPAC I Mini Acquisition Corp., Merger Sub, NewGenIvf Limited, a Cayman Islands exempted company (“Legacy NewGenIvf”) and certain shareholders of Legacy NewGenIvf. Pursuant to the Merger Agreement, the Business Combination was effected in two steps: (i) ASCA was reincorporated to the British Virgin Islands by merging with and into A SPAC I Mini Acquisition Corp. (such transaction, the “Reincorporation Merger”); and (ii) Merger Sub merged with and into Legacy NewGenIvf, resulting in Legacy NewGenIvf being a wholly-owned subsidiary of the Company (such second step in isolation, the “Acquisition Merger”). The surviving entity of the Business Combination, together with its subsidiaries is referred to in this prospectus as “NewGenIvf,” the “Company,” “we,” “our,” or “us,” unless the context otherwise requires.
On June 12, 2023, the parties to the Merger Agreement entered into the First Amendment to Merger Agreement (the “First Amendment”), pursuant to which Legacy NewGenIvf agreed to provide non-interest bearing loans in an aggregate principal amount of up to $560,000 (the “Loan”) to ASCA to fund any amount that would be required in order to further extend the period of time available for ASCA to consummate a business combination and for ASCA’s working capital, payment of professional, administrative and operational fees and expenses, and other purposes as mutually agreed by ASCA and Legacy NewGenIvf. Such loans were to become repayable upon the closing of the Acquisition Merger. In addition, pursuant to the First Amendment, subject to receipt of at least $140,000 as part of the Loan from NewGenIvf, ASCA agreed to waive its termination rights and the right to receive any break-up fee due to Legacy NewGenIvf’s failure to deliver audited financial statements by no later than February 28, 2023.
On December 6, 2023, the parties to the Merger Agreement entered into the Second Amendment to the Merger Agreement (the “Second Amendment”) which amended and modified the Merger Agreement to, among other things, (i) reduce the size of NewGenIvf’s board of directors following the consummation of the Business Combination to five (5) directors, two (2) of whom would be executive directors designated by NewGenIvf and three (3) of whom will be designated by NewGenIvf to serve as independent directors in accordance with Nasdaq requirements, (ii) provide for the conversion of NewGenIvf shares issued by NewGenIvf following the original date of the Merger Agreement into Class A Ordinary Shares in connection with the Acquisition Merger, and (iii) remove the condition that ASCA have in excess of $5,000,000 in net tangible assets immediately after the consummation of the Business Combination.
On April 3, 2024, the Business Combination was consummated with the Company as the surviving entity.
NewGenIvf’s Business
With a focus on providing fertility treatments to fulfil the dreams of building families, NewGenIvf mainly offers two services, namely: (i) in vitro fertilization (“IVF”) treatment service, comprising traditional IVF and egg donation; and (ii) surrogacy and ancillary caring services. Currently, we have three clinics: one clinic in Thailand, one clinic in Cambodia, and one clinic in Kyrgyzstan.
| | ● | IVF treatment service: For the years ended December 31, 2023 and 2022, we generated approximately 78.3% and 47.4%, of its revenue from IVF treatments services. We primarily provide our clients with conventional IVF/intracytoplasmic sperm injection (“ICSI”) and embryo transfer services. As technology has progressively advanced, we have been able to, through technologies and facilities provided by MicroSort technology, help fulfill the family-balancing dreams of its clients and avoiding certain gender-related hereditary diseases. IVF treatment involves the performance of a series of medical treatment and procedures that are not separately distinct and only brings benefits to clients when embryo is successfully implanted, therefore revenue from IVF treatment is recognized at a point in time when it is completed in clinic. The completion of this treatment is evidenced by a written IVF report indicating successful embryo implantation. |
| | ● | Surrogacy and ancillary caring services: We also generate revenue from surrogacy services and related ancillary caring services in Kyrgyzstan. For the years ended December 31, 2023 and 2022, we generated approximately 21.7% and 52.6%, of our revenue from surrogacy and ancillary caring services. For surrogacy services, NewGenIvf conducts implantation of embryos from biological parents in surrogate mothers. In addition, NewGenIvf provides a “success guarantee” program for egg donation services in Cambodia and surrogacy services in Kyrgyzstan. Under this optional program, patients pay additional fees of approximately 40% of the original price and can have repeated attempts of IVF cycles, egg donation services and/or surrogacy services until the procedures are successful. The additional costs to NewGenIvf are generally limited and amount to approximately 30% of the original costs because NewGenIvf’s clinics, together with the patients, can choose suitable egg donors and surrogate mothers to limit the additional costs. During the pregnancy period, NewGenIvf provides ancillary caring services including regular body check and provision of vitamins, supplements and medicines to surrogate mothers. Revenue from surrogacy and ancillary caring services is recognized at a point in time when the surrogate mother gives birth. Surrogacy services provide infertile couples with an alternative method of having children. |
For the years ended December 31, 2023 and 2022, NewGenIvf’s revenue was US$5,136,153 and US$5,944,190, and its net income was US$108,418 and US$135,847, respectively.
Market Opportunity
According to CIC, NewGenIvf’s core market for fertility services is substantial and growing rapidly, driven by, among other things, societal and cultural shifts, such as people starting families later in life and other health-related challenges which could impact couples’ and individuals’ ability to have children. In addition, NewGenIvf believes that continued overall de-stigmatization of infertility will help drive better access to, and stronger demand for, fertility treatment services, thereby further enabling the expansion of NewGenIvf’s addressable market. According to CIC, the market size of fertility treatments in Asia Pacific was increasing steadily and the potential size of the Asia fertility market is expected to reach US$37.4 billion by 2030. NewGenIvf believes its market opportunity is substantial and is continuing to grow as a result of the rising demand for fertility services, the lack of adequate offerings in the market and the increasing awareness of the challenges of infertility.
Competitive Strengths
NewGenIvf believes that the following competitive strengths have positioned it to meet growing opportunities in the fertility market across Asia-Pacific, and have differentiated it from its competitors:
Broad-range ARS Provider Offering Comprehensive Fertility Treatment Services
With almost a decade of experience in the fertility market, NewGenIvf has built a reputation in the IVF industry in Asia-Pacific. NewGenIvf has reinforced its long-standing position through expanding its service offerings and locations to address the evolving clients’ needs or requests.
NewGenIvf’s comprehensive fertility treatment offerings in Thailand, Cambodia, and Kyrgyzstan, primarily including IVF, egg donation (in Cambodia) and surrogacy services (in Kyrgyzstan), make it convenient for clients in Asia-Pacific market to have access to various fertility services but with a relatively low cost, as compared with the US market. According to CIC, the average cost per IVF cycle in the US is around US$12,000 (excluding medication), which is 65% higher than that of Asia-Pacific market. Meanwhile, the average cost per IVF cycle by NewGenIvf is around US$7,000 (excluding medication). Each of NewGenIvf’s clinics in Thailand, Cambodia, and Kyrgyzstan has its own specialty, and together, NewGenIvf is able to provide more flexibility and options to its patients. For example, NewGenIvf’s Thailand clinic focus on IVF and related ancillary services including HIV sperm washing, egg freezing, and chromosome screening. The clinic in Cambodia specializes in providing both IVF services and egg donation services. NewGenIvf opened the clinic in Kyrgyzstan in 2019, which broadened NewGenIvf’s services by being legally qualified/received approval letter from The Ministry of Health of Kyrgyzstan to offer surrogacy services. As of December 31, 2023, NewGenIvf was the one of the few ARS providers in Kyrgyzstan and one of the few companies in Kyrgyzstan that is licensed to offer surrogacy services in Kyrgyzstan.
NewGenIvf attributes its track record of success to its experienced physicians and its ability to provide comprehensive ARS services, allowing it to meet patients’ increasing demand for advanced, high-end, and sophisticated ARS, a higher standard and a wider range of advanced services.
NewGenIvf has extensive experience serving Asia-Pacific patients and a deep understanding of their general profiles. In particular, NewGenIvf has personnel speaking multiple languages, including nurses, facilitators, and translators, who are familiar with the health condition and culture of Asia-Pacific patients from different countries in the region. NewGenIvf believes that it is therefore well-positioned to benefit from market growth driven by Asia-Pacific patients travelling to its clinics for treatment.
Attractive Market with Significant Demand and Fast Growth
NewGenIvf operates in the ARS market in Asia Pacific, positioning it to leverage on an attractive market with compelling underlying growth potential. According to CIC, during the years ended December 31, 2021 and 2022, the ARS market in Asia Pacific has experienced growth underpinned by long-term demographic and social trends. These trends include a rising demand for fertility services, the lack of adequate offerings in the market and the increasing awareness of the challenges of infertility, according to CIC.
According to CIC, the Asia Pacific ARS market is a large, multi-billion dollar industry growing at a strong pace of approximately 15% in 2022 as increased awareness and acceptance of IVF and surrogacy services continue to drive demand. Additionally, according to CIC, the market is underserved as a substantial percentage of patients in need of ARS treatments go untreated. The industry also remains constrained in capacity, thereby creating challenges in providing access to ARS to the volume of patients in need. According to CIC, as of December 31, 2022, there were more than 213 million infertile couples in Asia Pacific. While there have been substantial increases in the use of ARS, according to CIC, only approximately 1.47 million ARS cycles, including IVF, and other fertility treatments, were performed in Asia Pacific in 2022. This amounts to less than 1.1% of the infertile couples in Asia Pacific being treated and only 0.7% having a child though ARS in 2022, indicating significant unmet demand for ARS.
Asia-Pacific fertility markets, in particular India and China, present a vast opportunity for ARS providers in the region. China’s ARS market has been driven by an increasing rate of infertility, the implementation of the Three-Child Policy in May 2021, a decreasing number of couples at childbearing age and increasing affordability and awareness of ARS, according to CIC. China’s ARS market size in 2021 and 2022 was US$2,105 million and US$2,069 million, respectively, and is expected to further grow to US$2.3 billion in 2023, according to CIC. India’s ARS market size increased from US$1.2 billion in 2021 to US$1.5 billion in 2022, and is expected to grow further to US$1.6 billion in 2023, according to CIC. NewGenIvf believes that its existing market presence and reputation in Thailand, Cambodia, and Kyrgyzstan well positions it to capitalize on the fast-growing Asia-Pacific fertility market.
According to CIC, the significant entry barriers in Asia-Pacific ARS industry are expected to continue to constrain supply in the industry. The industry is heavily regulated and a significant number of stringent requirements must be satisfied in order to obtain relevant licenses to conduct IVF, egg donation and surrogacy procedures in the relevant countries. NewGenIvf believes that such barriers to entry can help it maintain its market position in Asia Pacific as the fertility market in the region continues to expand.
Built on years of experience, NewGenIvf has established a strong reputation in its industry, which in turn attracted potential business partners to approach NewGenIvf to negotiate cooperations and referrals. Over the years, NewGenIvf sends representatives to medical expos mostly held in the PRC to approach potential business partners and establish new partnerships by entering into agency agreements with each agent. NewGenIvf has become a significant partner with approximately 90 fertility service agents in China as well as in India. Normally, each agency agreement has a maximum term of one year, which is renewable upon mutual agreement. Agents typically market and promote NewGenIvf’s services by word-to-mouth referrals and other measures and NewGenIvf pays the agents commission at a range of 10% to 25% of the treatment fees upon the completion of client’s treatment. Normally, agents provide potential clients’ contact information to the sales team of NewGenIvf, who then approach potential clients and provide consultation on services. Overall, approximately 50% of NewGenIvf’s patients are referrals from agents, among which approximately 80% are referrals from China and the remaining 20% from India, whereas the remaining 50% of NewGenIvf’s patients are patients who contact NewGenIvf directly through its websites from social media promotions. With its partnerships in various countries, NewGenIvf believes it is able to better benefit from the growing market opportunities.
Exclusively Licensed Technology for Family Planning and Access to Mature Fertility Technologies
NewGenIvf believes that its licenses and/or access to mature technologies contribute to its ability to identify and tailor ARS services to individual patient’s needs. These technologies include:
| | ● | MicroSort Technology: NewGenIvf holds an exclusive license granted by a division of the Genetics and IVF Institute, to use MicroSort technology in Thailand and Cambodia, which is a form of pre-conception gender selection technology for humans. MicroSort technology aims to separate male sperm cells based on which gender chromosome they contain, which results in separated semen samples that contain a higher percentage of sperm cells that carry the same gender chromosome. The technology ultimately helps couples choose the gender of their future child by choosing semen samples that predominately contain sperm with the X chromosome for a female or Y chromosome for a male. Traditionally and naturally, gender selection occurs after conception, meaning after the eggs are fertilized. As a result, some fertilized eggs will go unused. However, with MicroSort technology, NewGenIvf is able to increase the ratio of male or female embryos, based on the patient’s preference. Eggs are more likely to be fertilized according to the preferences of the parents. Other improvements that MicroSort treatment could help achieve include prevention of certain gender-related hereditary diseases. As of December 31, 2023, NewGenIvf was one of the only seven exclusive license holders of MicroSort technology world-wide. |
| | ● | Preimplantation Genetic Screening (“PGS”): PGS is used in parallel with an IVF treatment cycle. PGS is the practice of determining the presence of aneuploidy (either too many or too few chromosomes) in a developing embryo. PGS improves success rates of in vitro fertilization by ensuring the transfer of euploid embryos that have a higher chance of implantation and resulting in a live birth. PGS has improved clinical outcomes for NewGenIvf by achieving a higher implantation rate of 70.9% and reducing miscarriage rates by 26.6%. |
| | ● | Next-Generation Sequencing (“NGS”): NGS is a high-throughput technology for determining the sequence of deoxyribonucleic acid (“DNA”) or ribonucleic acid (“RNA”) to study genetic variation associated with diseases or other biological phenomena. NGS determines the sequence of a sample all at once by using parallel sequencing. Traditional Sanger sequencing determines the sequence of a sample one section at a time. Sequencing thousands of gene fragments simultaneously with NGS reduces time and cost associated with sequencing and increases the coverage quality and data output. |
| | ● | Preimplantation Genetic Diagnosis (“PGD”): Similar to PGS, PGD is also used in parallel with an IVF treatment cycle. But PGD is a process more enhanced than PGS since it scans for individual genes. PGD is the practice of evaluating embryos for specific genetic abnormalities, such as sickle cell disease or cystic fibrosis, where carrier status has been documented in each of the parents. By using this technique, physicians are able to check the genes or chromosomes for a specific genetic condition. PGD can decrease the risk of miscarriage and this technology can help women better achieve a healthy pregnancy. Individuals who suspect or know they carry genes for serious medical conditions may opt to screen for healthy embryos ahead of time. |
Well Established Brand with Reliable Reputation
The founders of NewGenIvf entered the fertility market as agents in 2011 by introducing patients in need to a Thailand clinic for fertility treatments. The founders of NewGenIvf started to operate their own clinic in Thailand in 2014 and subsequently added clinics in Cambodia and Kyrgyzstan. Since then, NewGenIvf has attracted clients from countries throughout Asia-Pacific, including Mainland China, Hong Kong, India, Thailand, Australia and Taiwan.
NewGenIvf benefits from the favourable geographic locations of its clinics, especially its clinic in Thailand. Located in central Bangkok and situated in one of the biggest shopping malls of the city, the clinic is located in close proximity to various transportation facilities and popular tourist attractions, such as the Erawan Shrine. In this regard, NewGenIvf believes that its business has benefited from, and will continue to benefit from, the convenience of its locations.
NewGenIvf has developed a relatively replicable and scalable operating model that supports high productivity at its assisted reproductive medical facilities in Asia. Under this model, NewGenIvf’s medical facilities have established standardized operating procedures to select the treatment process according to each patient’s profile. NewGenIvf’s medical and operational personnel are organized into specialized teams according to the different stages of the treatment process and different patient profiles. When patients are initially admitted or would like to seek additional medical services later on, they are assigned to one of the optimal medical teams, which NewGenIvf believes is better suited after taking into account the patient’s diagnosis and preferences. NewGenIvf believes that this model allows each team to improve its efficiency and arrange suitable physicians for patients.
The physicians of NewGenIvf have also developed and employed an operating model that seeks to increase the effectiveness of physicians by utilizing standardized workflows and operating procedures with teams of supporting nurses and medical assistants. This helps to increase the number of IVF treatment cycles that physicians can perform while providing treatment customized based on patient conditions.
With its established client service history, accumulated experience as well as its continuous upgrades and development of treatment models, NewGenIvf believes that it will be able to better monetize its brands through its business.
Experienced Management Team
The NewGenIvf management team has considerable experience in the ARS market and the broader healthcare industry. A considerable number of NewGenIvf’s management are physicians or laboratory technicians who possess extensive experience in the ARS industry and are experts in their respective fields. NewGenIvf’s Chief Executive Officer, Mr. Alfred Siu, has more than 13 years of experience in the fertility service market. Dr. Wiphawee Luangtangvarodom had over 8 years of experience as an obstetrician and gynecologist. NewGenIvf’s two lab supervisors, Ms. Anussara Phinyong, and Ms. Araya Boonchaisitthipong, each had over eight years of experience in the embryologist field. These individuals have extensive experience in managing assisted reproductive medical facilities. NewGenIvf is also led by other members of the professional management team, who are intimately involved in the operational and financial management of NewGenIvf’s Group. Leveraging their experience, NewGenIvf believes that it is well positioned to expand its network and aims to become a leader in the Asia Pacific ARS market.
Strategies
NewGenIvf’s vision is to provide tailored ARS solutions to fulfil patients’ dreams of becoming a parent. To realize this vision, NewGenIvf plans to adopt the following strategies:
Offer Broad Fertility Services for Fertility Tourists across Asia Pacific
NewGenIvf intends to provide broad fertility services for fertility tourists seeking high quality, cost effective and comprehensive fertility solutions. According to CIC, the demand for fertility tourism is driven by a variety of factors including the prevalence of infertility, the introduction of the Three-Child policy in China, the improved understanding of assisted reproductive technology and increased affordability of ARS. To address these needs, NewGenIvf plans to offer its customers a “hassle-free”, seamless and integrated ARS and hospitality arrangement experience. To complement its fertility services, NewGenIvf intends to integrate its offerings with additional services for traveling patients, most of whom are first-time fertility tourists, such as translation service, hotel arrangement and airport pickup services. NewGenIvf plans to enhance its customers’ experience by entering into exclusive cooperation arrangements with local premium hospitality providers.
Furthermore, NewGenIvf expects the easing of COVID-19 travel restrictions to contribute to an increase in tourists seeking fertility services. According to CIC, the COVID-19 pandemic led to a delay in many patients’ plans for fertility treatments, with travel restrictions and border closures impacting their ability to access care. On May 5, 2023, the WHO Director-General Dr. Tedros Adhanom Ghebreyesus announced that COVID-19 no longer constituted a public health emergency of international concern. The pent-up demand for these services is expected to be released with the lifting of the travel restrictions, leading to a surge in patients seeking fertility treatment. NewGenIvf’s believes that its strategy of offering a comprehensive approach to fertility treatments will help it capture a share of the growing market for fertility tourism in Asia Pacific.
Continue to Invest in Laboratories and Facilities
NewGenIvf believes laboratories and treatment facilities are critical to supporting its future research, development and clients experience. NewGenIvf currently operates two laboratories that offer IVF services, one in Thailand and one in Cambodia, and plans to continue to scale up its existing laboratories. NewGenIvf plans to continue to invest in upgrading its laboratories and facilities to complement its growth and expansion, which it believes will help NewGenIvf maintain an edge over its competitors with regard to technology, operational efficiency, scalability, and client experience.
NewGenIvf intends to develop advanced facilities for its existing laboratories, which will be conducting research on ARS related basic science and experiments relating to emerging technologies to improve ARS success rates and lower costs. NewGenIvf also plans to correlate its data on patient treatment protocols to the embryo physiologic data and the pregnancy success rate-related data to identify better treatment protocols to increase ARS success rates. NewGenIvf intends to continue to actively promote technological cooperation with tertiary institutions to discover ways to improve its IVF success rates. Furthermore, NewGenIvf seeks to actively deploy the technology that it possesses to expand the services it provides.
NewGenIvf has accumulated experience in treating patients over 40 years old with premature ovarian failure and patients who have had recurrent ARS implementation failure, by, for the example, injecting platelet rich plasma into the ovaries to stimulate and support growth of the follicles. NewGenIvf is also implementing certain technological advancements relevant to the ARS industry, including microfluidics, automated sperm analysers, time lapsed incubators, non-invasive preimplantation genetic testing (“PGT”) of cell-free DNA in spent media, automated systems for oocyte/embryo vitrification to reduce reagent consumption and decrease labor intensity, mitochondria replacement therapy to reconstruct oocytes by nuclear transfer of polar body genome from an MII oocyte into an enucleated donor MII cytoplasm, to increase the number of oocytes available for the treatment of infertile women, preimplantation methylome screening. There are also breakthrough developments in science including organ culture systems, induced pluripotent stem cells, embryonic stem cells, spermatogonial stem cells for creation of functional gametes, but these techniques are not yet ready for human clinical trials.
NewGenIvf also intends to develop clinically customised interior design concepts for its medical facilities, including improved service rooms, consultation rooms, reception areas, nutrition food areas, and traditional Chinese medicine (such as acupuncture) facilities.
Increase Brand Awareness and Market Share
NewGenIvf intends to maintain and strengthen its brand awareness and market share in Asia Pacific. In order to expand its reach and increase patient numbers, NewGenIvf plans to collaborate with local hospitals, companies, premium hospitality providers and other key players in the ARS industry in Asia Pacific. Additionally, NewGenIvf intends to increase brand awareness through social media promotions and marketing initiatives, and establishing its business development team with the goal of attracting new patients and partners across Asia Pacific. Meanwhile, NewGenIvf intends to provide innovative treatment services to attract more clients. For example, NewGenIvf plans to introduce IVF mental health services, which allows clients who fail in IVF treatments to access online consultation for further treatment plans such as egg donation and surrogacy. These new treatments services aim to enable NewGenIvf to attract potential clients. By adopting a comprehensive strategy to expand its market share, NewGenIvf aims to strengthen its reputation as a long-standing ARS provider and capture additional market share of the growingly ARS market in Asia-Pacific.
Expand Service Reach Through Acquisitions and Partnerships
Leveraging its reputation and footprint in its current markets, NewGenIvf intends to expand its reach, services offering and client base through strategic acquisitions and/or partnerships in Asia Pacific. Acquisitions of or by companies offering similar services could not only allow NewGenIvf to diversify its client base, but also allow it to benefit from potential economies of scale and increasing efficiency through consolidation. NewGenIvf could also leverage the acquired or acquiring company’s customer base, reputation and expertise to further improve its offerings and operations. NewGenIvf intends to focus on ARS providers in Asia Pacific which possess all conventional licenses and locally recognized brands. For the global market beyond Asia Pacific, NewGenIvf intends to expand its footprint through partnerships with other IVF clinics.
In addition, NewGenIvf plans to explore expanding its client base by offering its fertility services as part of corporate benefit programs in Asia. NewGenIvf believes that there is potential in Asia in offering fertility treatments as a benefit for employees, particularly in companies with a large number of female employees of childbearing age. By partnering with corporate clients to provide fertility benefits, NewGenIvf can increase its market reach, enhance its brand reputation, and drive client growth. NewGenIvf’s broad range of fertility services, including IVF and egg freezing, can help corporate partners differentiate their employee benefits in the competitive employment landscape, which could make them more attractive to potential employees. Additionally, by offering these services, companies can help address the growing concern of delayed childbearing, which is becoming more common among women according to CIC. NewGenIvf plans to collaborate with potential corporate clients to develop customized fertility benefit programs that cater to their specific needs, and to provide comprehensive support and counselling throughout the process.
Meanwhile, NewGenIvf also intends to attract more clients by establishing its “home country gynecologist partnership program”. Under the program, NewGenIvf may, subject to its discretion and screening process, offer treatment services to clients with reduced time requirements to be spent overseas. Depending on local laws, the potential clients may be able to complete their treatments with gynecologists NewGenIvf partners with, in their home countries.
NewGenIvf had entered into a non-binding term sheet dated June 3, 2024 (the “Term Sheet”) with COVIRIX Medical Pty Ltd (“COVIRIX”) for a proposed reverse merger (the “Proposed Transaction”). However, on September 21, 2024, COVIRIX withdrew from the Proposed Transaction, as such the Proposed Transaction was terminated with no cost to the Company.
White Lion Transaction
On November 21, 2024, the Company entered into a Common Shares Purchase Agreement (the “White Lion Purchase Agreement”) with White Lion Capital, LLC (“White Lion”) and a related Registration Rights Agreement (the “RRA”). Pursuant to the White Lion Purchase Agreement, the Company has the right, but not the obligation, to require White Lion to purchase, from time to time, up to One Hundred Million Dollars ($100,000,000) in aggregate gross purchase price of newly issued Ordinary Shares, with an automatic increase to Three Hundred Million Dollars ($300,000,000) upon any substantial M&A or Material Transaction (as defined in the White Lion Purchase Agreement) and a further option to increase to Five Hundred Million Dollars ($500,000,000) after Two Hundred and Fifty Million Dollars ($250,000,000) has been issued and sold to White Lion under the White Lion Purchase Agreement, subject to certain limitations and conditions set forth in the White Lion Purchase Agreement.
Subject to the satisfaction of certain customary conditions including, without limitation, the effectiveness of the Registration Statement registering the resale of the shares issuable pursuant to the White Lion Purchase Agreement, the Company’s right to sell shares to White Lion commenced on the date of the execution of White Lion Purchase Agreement and extends until (i) 36 months from the date of execution of the White Lion Purchase Agreement, or (ii) at the Company’s option, until 65 months from the date of the execution of the White Lion Purchase Agreement in the event that $100,000,000 of purchases under the White Lion Purchase Agreement have been completed prior to the 36 month anniversary of the Execution Date (the “Commitment Period”).
During the Commitment Period, subject to the terms and conditions of the White Lion Purchase Agreement, the Company may exercise its right to sell its Ordinary Shares. The Company may deliver a Regular Purchase Notice (as such term is defined in the White Lion Purchase Agreement), pursuant to which the Company can require White Lion to purchase up to a number of Ordinary Shares equal to the lesser of (i) $3,000,000 divided by the highest closing price of the Ordinary Shares over the most recent five (5) Business Days immediately preceding the Purchase Notice, or (ii) 40% of Average Daily Trading Volume (as such term is defined in the White Lion Purchase Agreement), subject to a maximum Investment Limit of $3,000,000.
The Company may also deliver a Rapid Purchase Notice (as such term is defined in the White Lion Purchase Agreement), pursuant to which the Company may require White Lion to purchase up to a number of Ordinary Shares equal to $3,000,000 divided by the highest closing price of the Ordinary Shares over the most recent five business days immediately prior to the receipt of the notice. White Lion may waive such limits under any notice at its discretion and purchase additional shares.
The price to be paid by White Lion for any shares that the Company requires White Lion to purchase will depend on the type of purchase notice that the Company delivers. For shares being issued pursuant to a Regular Purchase Notice, the purchase price per share will be the lower of (i) the closing price of Ordinary Shares prior to the receipt of the applicable Purchase Notice, or (ii) the product of (a) the lowest daily VWAP of the Ordinary Shares during the Regular Purchase Valuation Period (as defined in the White Lion Purchase Agreement), and (b) 98%.
For shares being issued pursuant to a Rapid Purchase Notice, the Company may opt for the purchase price per share to be (i) equal to the lowest traded price of the Ordinary Shares on the date that the notice is delivered, or (ii) 97% of the lowest traded price of the Ordinary Shares two hours following White Lion’s written confirmation of the acceptance of the Rapid Purchase Notice.
No purchase notice shall result in White Lion beneficially owning (as calculated pursuant to Section 13(d) of the Securities Exchange Act of 1934, as amended, and Rule 13d-3 thereunder) more than 4.99% (subject to increase, in the sole discretion of White Lion, to 9.99%) of the number of Ordinary Shares outstanding immediately prior to the issuance of Ordinary Shares issuable pursuant to a purchase notice.
The Company has the right to terminate the White Lion Purchase Agreement in the event of a material breach of the White Lion Purchase Agreement by White Lion. The White Lion Purchase Agreement also automatically terminates upon the earlier of (i) the end of the Commitment Period and (ii) the date that the Company commences a voluntary bankruptcy proceeding, a custodian is appointed for the Company or for all or substantially all of its property, or the Company makes a general assignment for the benefit of its creditors.
In consideration for the commitments of White Lion, as described above, the Company has agreed that it will issue to White Lion 700,000 Common Shares (“Commitment Shares”). In addition, the Company has agreed that (i) if the Company fails to sign a binding term sheet for a Material Transaction within 90 days of the execution of the White Lion Purchase Agreement, it will issue to White Lion an additional 100,000 Common Shares (ii) upon the completion of a Material Transaction (as defined in the White Lion Purchase Agreement), it will issue an additional amount of Ordinary Shares equal to $500,000 divided by the closing price of the Ordinary Shares on the date of the public filing announcing the closing of the Material Transaction; and (iii) in the event the gross investment by White Lion reaches $250,000,000, the Company shall issue an additional amount of Ordinary Shares equal to $250,000 divided by the closing price of the Ordinary Shares on the Closing Date the gross investment reaches $250,000,000. The Commitment Shares will be fully earned by White Lion regardless of termination of the White Lion Purchase Agreement.
Concurrently with the White Lion Purchase Agreement, the Company entered into the RRA with White Lion, pursuant to which the Company agreed to file, within 10 business days following the execution of the White Lion Purchase Agreement, the Registration Statement with the SEC covering the resale by White Lion of number of shares determined appropriate by the Company and permitted to be included therein in accordance with applicable SEC rules, regulations and interpretations and the Commitment Shares. The RRA also contains usual and customary damages provisions for failure to file and failure to have the Registration Statement declared effective by the SEC within the time periods specified therein.
The White Lion Purchase Agreement and the RRA contain customary representations, warranties, conditions and indemnification obligations of the parties. The representations, warranties and covenants contained in such agreements were made only for purposes of such agreements and as of specific dates, were solely for the benefit of the parties to such agreements and may be subject to limitations agreed upon by the contracting parties.
Business Model
With a focus on providing fertility treatments to fulfil couples and individuals’ dreams of raising children, NewGenIvf offers mainly two services, namely: (i) IVF treatment service, comprising traditional IVF and egg donation; and (ii) surrogacy and ancillary caring services. The following table sets forth NewGenIvf’s revenue by service offerings and as a percentage of total revenue for the periods indicated:
| | | For the Year ended December 31, | |
| | | 2023 | | | 2022 | |
| | | US$ | | | % | | | US$ | | | % | |
| IVF Treatment Service | | | 4,021,696 | | | | 78.3 | | | | 2,819,163 | | | | 47.4 | |
| Surrogacy and Ancillary Caring Services | | | 1,114,457 | | | | 21.7 | | | | 3,125,027 | | | | 52.6 | |
| Total Revenue | | | 5,136,153 | | | | 100.0 | | | | 5,944,190 | | | | 100.0 | |
IVF Treatment Service
NewGenIvf primarily provides its clients with conventional IVF/ICSI and embryo transfer services. NewGenIvf is also able to, through MicroSort technology, help fulfill the family-balancing dreams of its clients and avoiding certain gender-related hereditary diseases.
IVF treatments that NewGenIvf provides address tubal factor, ovulatory dysfunction, diminished ovarian reserve, endometriosis, uterine factor, male factor, unexplained infertility and other causes. IVF bypasses the function of the fallopian tube by achieving fertilization within a laboratory environment. Ovarian hyper-stimulation is common with IVF treatments to recruit numerous follicles to increase the chances for success. Follicles are retrieved trans-vaginally using a vaginal probe and ultrasound guidance. Anaesthesia is frequently used due to the number of follicles retrieved and the resulting discomfort experienced by the patient. The eggs are identified in the follicular fluid and combined with sperm and culture medium in culture dishes, which are placed in an incubator with a temperature and gas environment designed to mimic the condition of the fallopian tubes. Once the embryos develop, typically over a 3-to-5-day period, they are transferred to the uterine cavity. According to CIC, the average clinical pregnancy success rates, using 5-day incubation, averaged approximately 64.6% (with no PGT) for IVF, with live birth rate at approximately 28.7%.
As a long-standing IVF treatments provider in Asia-Pacific, NewGenIvf had completed over 4,000 cycles of IVF treatments from 2014 to 2023. For the years ended December 31, 2023 and 2022, the revenue from NewGenIvf’s IVF treatments was US$4,021,696 and US$2,819,163, respectively, representing 78.3% and 47.4% of its total revenue in the corresponding periods.
IVF Treatments Process
A typical IVF treatment process mainly includes two stages, the pre-IVF treatment stage and the IVF treatment stage. During the IVF treatment process, NewGenIvf also provides support services such as nutrition guidance and psychological counselling. The flow chart below shows the stages involved in a typical IVF treatment process:

At the pre-IVF treatment stage, clients attend an initial consultation, undergo pre-IVF tests, and undergo treatment for gynaecological and andrological diseases, if needed. At the initial consultation, a physician reviews the clients’ detailed medical history to collect more information relating to the potential cause of their infertility. The client then undergoes various pre-IVF tests, which may include, among other things, blood pressure, hormone level, ultrasound, infectious disease screening, uterine evaluation and male fertility test. The physician will then design treatment plans based on the client’s medical history and results of the tests. If the client is satisfied with treatment plan and the test results are acceptable to the physician, the physician will prescribe medications and start stimulation treatment.
The first step of the cycle is to boost egg production through injecting synthetic hormones. Over about one week of ovarian stimulation, clients are monitored on a regular basis with blood test and transvaginal ultrasound. If follicles have reached at least 10 mm in size, an additional antagonist drug will be added into the daily injection schedule. This is used to prevent ovulation before ovum pickup time. After another few days of ovarian simulation, if follicle growth is consistent and majority of follicles are around 16 mm to 17 mm, the final injection of a human chorionic gonadotropin will be administered. The trigger injection is the final step of the stimulation process and is for the maturation of the eggs in the follicles before they are collected. The next major step is to retrieve the eggs with a minor surgical procedure called Trans Vaginal Follicle Aspiration conducted under anaesthesia. At the same time the male partner collects the sperms for fertilizing the eggs in the laboratory by a process known as intracytoplasmic sperm injection. The fertilized embryos are cultured in the laboratory for two to six days. Embryos that grow well are biopsied and tested by PGT to detect potential genetic diseases.
The final step is to transfer the embryos into the uterus using a catheter. Within eight days after the embryo transfer, a blood test can be conducted to detect whether the implantation was successful.
MicroSort Technology
MicroSort technology is a preconception process developed by the Genetics and IVF Institute, Inc. that aims to improve the chances that the baby to be conceived will be of the desired gender and prevents certain gender-related hereditary diseases.
Semen samples usually contain equal amounts of sperm carrying the Y chromosome (which will produce a boy), and sperm carrying the X chromosome (which will produce a girl). During the MicroSort process, the sperm sample is washed to remove seminal liquid and nonmotile cells. After the washing, the sample is stained with a special fluorescent material that attaches to the DNA contained in the sperm. The stained sperm cells are analyzed one by one by a flow cytometer, in which cells pass through a laser to make the stain attach to the DNA fluoresce. The sperm containing the X chromosome (which have more DNA and therefore more stain) will shine brighter than the sperm containing the Y chromosome. The flow cytometer uses a special software to identify X and Y chromosome sperm based on their fluorescence signature. The sperm carrying the chromosome that will produce the desired gender are separated from the rest of the sample -resulting in an enriched sperm sample ready for use.
NewGenIvf holds an exclusive license granted by a division of the Genetics and IVF Institute, MicroSort International, to use the MicroSort technology in Thailand and Cambodia. MicroSort licenses for NewGenIvf’s operation in Thailand and Cambodia are each provided under a lease and service agreement. In April 2019, First Fertility PGS entered into a Lease and Services Agreement with MicroSort International to use MicroSort equipment in Thailand and in March 2019, Phnom Penh Center entered into a Lease and Services Agreement with MicroSort International to use MicroSort equipment in Cambodia (together, the “Lease and Services Agreements”). Pursuant to the Lease and Services Agreements, First Fertility PGS and Phnom Penh Center each has the exclusive right to utilize the MicroSort equipment and to market and sell MicroSort sperm sorting services in Thailand and Cambodia, respectively. MicroSort International is responsible for the maintenance of MicroSort equipment and technical and engineering support. The term of each Lease and Service Agreements is initially from 2019 to 2024, which shall be automatically renewed for one year unless a written notice of at least 180 days prior to the intended termination date is provided. The consideration under each of the Lease and Services Agreements is US$9,000 per month after six months from the effective date of the agreements. MicroSort International was entitled to a down payment of US$15,000 per agreement and the aggregated amounts received by it under the agreements was US$328,500. During the term of each lease and service agreement, MicroSort grants NewGenIvf the exclusive right in that country to utilize the MicroSort equipment and market MicroSort services. The term of each lease and service agreement is initially from 2019 to 2024, which shall be automatically renewed for one year unless a written notice at least 180 days prior to the intended termination date is provided. The flow chart below shows the process involved in MicroSort:
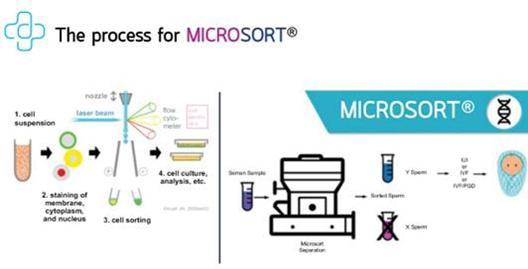
Preimplantation Genetic Screening
PGS is used in parallel with an IVF treatment cycle. PGS is the practice of determining the presence of aneuploidy (either too many or too few chromosomes) in a developing embryo. PGS improves success rates of in vitro fertilization by ensuring the transfer of euploid embryos that have a higher chance of implantation and resulting in a live birth. PGS has improved clinical outcomes for NewGenIvf by achieving a higher implantation rate of 70.9% and reducing miscarriage rates by 26.6%.
Next-Generation Sequencing
NGS is a high-throughput technology for determining the sequence of deoxyribonucleic acid DNA or RNA to study genetic variation associated with diseases or other biological phenomena. NGS determines the sequence of a sample all at once by using parallel sequencing. Traditional Sanger sequencing determines the sequence of a sample one section at a time. Sequencing thousands of gene fragments simultaneously with NGS reduces time and cost associated with sequencing and increases the coverage quality and data output.
Preimplantation Genetic Diagnosis
Similar to PGS, PGD is also used in parallel with an IVF treatment cycle. But PGD is a more enhanced process than PGS since it scans for individual genes. PGD is the practice of evaluating embryos for specific genetic abnormalities, such as sickle cell disease or cystic fibrosis, where carrier status has been documented in each of the parents. By using this technique, physicians are able to check the genes or chromosomes for a specific genetic condition. PGD can decrease the risk of miscarriage and this technology can help women achieve a healthy pregnancy. Individuals who suspect or know they carry genes for serious medical conditions may opt to screen for healthy embryos ahead of time.
Surrogacy and Ancillary Caring Services
NewGenIvf also generated revenue from surrogacy services and related ancillary caring services in Kyrgyzstan. NewGenIvf conducts implantation of embryos from biological parents in surrogate mothers. During the pregnancy period, NewGenIvf provides ancillary caring services including regular body check and provision of vitamins, supplements and medicines to surrogate mothers. Revenue from surrogacy and ancillary caring services is recognized when the surrogate mother gives birth. Surrogacy services provide infertile couples with an alternative method of having children. In general, NewGenIvf provides certain discount to clients if they wish to pursue additional services such as egg donation and surrogacy, after several cycles of IVF treatments failures due to medical reasons including, but not limited to, the poor egg quality of aged female clients.
As compared to other countries, Kyrgyzstan has the following features that allow NewGenIvf to operates its surrogacy services: (i) surrogacy is legal and regulated, which means that there are less restrictions on either intended parents or surrogate mothers, and a parent-child relationship can be requested before the child’s birth; and (ii) the costs of operation and surrogate mother is favourable, given the cost of living in Kyrgyzstan is relatively low.
In addition to the regular surrogacy services, NewGenIvf is also able to assist the clients with birth certificate applications and facilitate the application of infants’ passports and visas as supplemental services.
For the years ended December 31, 2023 and 2022, the revenue from NewGenIvf’s surrogacy and ancillary caring services was US$1,114,457 and US$3,125,027, respectively, representing 21.7% and 52.6% of its total revenue in the corresponding periods.
The flow chart below shows the stages involved in a typical surrogacy process:
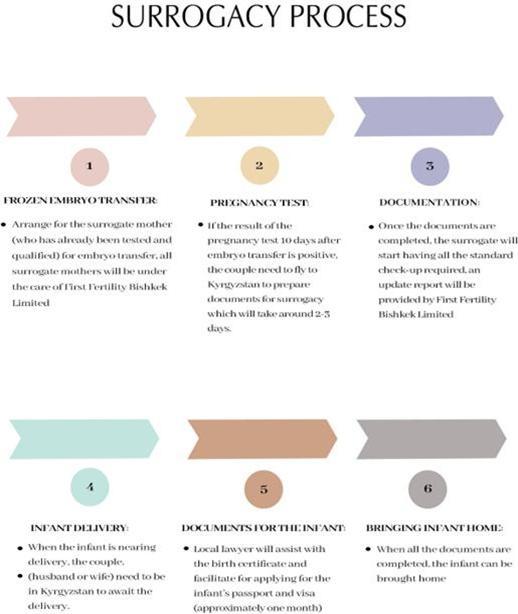
In Kyrgyzstan, NewGenIvf also provides ancillary fertility services when carrying out surrogacy services. These ancillary fertility services include: (i) maternity caring service, and (ii) documentation service.
Network of Facilities
As of December 31, 2023, NewGenIvf had one marketing and sales support office located in Hong Kong and three clinics located in Thailand, in Cambodia, and in Kyrgyzstan, respectively. The integration of the medical facilities in Thailand help NewGenIvf provide a more seamless one-stop experience to its clients. Set out below is an illustration of the locations of NewGenIvf’s clinics and marketing and sales office:
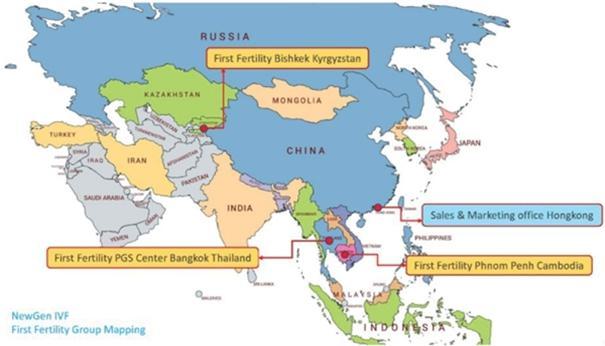
The following table sets forth the approximate aggregate average gross floor area (“G.F.A.”) of each of NewGenIvf’s clinics that were under lease and actively used for client service as of December 31, 2023:
| | | As of
December 31,
2023 | |
| | | (Square Feet) | |
| Thailand | | | |
First Fertility PGS Center Co., Ltd.
(“First Fertility PGS Center”) | | | 14,750 | |
| | | | | |
| Cambodia | | | | |
First Fertility Phnom Penh Center
(“Phnom Penh Center”) | | | 18,567 | |
| | | | | |
| Kyrgyzstan | | | | |
First Fertility Bishkek Limited Liability Company
(“First Fertility Bishkek”) | | | 2,368 | |
| | | | | |
| Aggregate G.F.A | | | 35,685 | |
To increase the scale of NewGenIvf’s operations, NewGenIvf expanded its Thailand fertility services by leasing a new property for its second clinic Erawan Consultation Clinic in May 2023. Consisting of approximately 2,500 sq. ft., Erawan Consultation Clinic is expected to open in 2024.
Currently, IVF treatments are performed in its Thailand and Cambodia clinics, egg donation services are provided in its Cambodia clinic, and surrogacy services are provided in its Kyrgyzstan clinic. The following table summarises the services available at NewGenIvf’s clinics:
| | | IVF
Treatments | | Surrogacy
Services |
| Thailand | | | | |
| First Fertility PGS Center | | √ | | × |
| | | | | |
| Cambodia | | | | |
| Phnom Penh Center | | √ | | × |
| | | | | |
| Kyrgyzstan | | | | |
| First Fertility Bishkek | | × | | √ |
The following table sets forth a breakdown of revenue from services performed at NewGenIvf’s medical centers for the periods indicated:
| | | For the Year ended December 31, | |
| | | 2023 | | | 2022 | |
| | | US$ | | | % | | | US$ | | | % | |
| HK SAR | | | 34,038 | | | | 0.7 | | | | — | | | | — | |
| Thailand | | | 1,356,903 | | | | 26.4 | | | | 505,609 | | | | 8.5 | |
| Cambodia | | | 621,619 | | | | 12.1 | | | | 377,608 | | | | 6.4 | |
| Kyrgyzstan | | | 3,123,593 | | | | 60.8 | | | | 5,060,973 | | | | 85.1 | |
| Total Revenue | | | 5,136,153 | | | | 100.0 | | | | 5,944,190 | | | | 100.0 | |
Thailand Clinic
As of December 31, 2023, NewGenIvf had one clinic in Thailand. At the clinic in Thailand, NewGenIvf offers its clients customized fertility treatment solutions including IVF/ICSI, embryo culture, hormonal blood tests, infectious diseases tests, chromosome screening by PGT, hysteroscopy, sperm analysis, sorting, washing and freezing, and egg freezing. Its medical and operational personnel are organized into specialized teams according to the different stages of the IVF treatment process and different patient profiles. When clients are admitted, they are assigned to a team which NewGenIvf believes is better suited the clients after taking into account the clients’ diagnosis and preferences. Furthermore, NewGenIvf also provides related value-added services such as nutrition guidance, psychological counselling, acupuncture, and translation interpreters to supplement the IVF treatment. NewGenIvf prides itself on providing quality and customized treatment to its clients on a day-to-day basis.
As of December 31, 2023, the clinic in Thailand had six nurses, 8 full time lab physicians and embryologists, 14 administrative staff, totaling 28 staff members.
Cambodia Clinic
NewGenIvf has one clinic, Phnom Penh Center, in Cambodia. Phnom Penh Center is staffed with one Cambodian physician, three embryologists, five nurses and twelve other staff, and offers similar IVF treatments as in Thailand and egg donation services. Phnom Penh Center operates under a license issued by Cambodia MOH for the Cambodian physician, who has entered into an agreement with Phnom Penh Center for the exclusive use of such license.
After eight years of development since its opening in 2015, Phnom Penh Center has become one of the long-standing ARS providers in Cambodia. According to CIC, it was the first to use conventional IVF technology which led to a successful birth in 2016 in Cambodia. Since its establishment, Phnom Penh Center achieved more than 1,600 IVF treatment cycles as of December 31, 2023. As of December 31, 2023, Phnom Penh Center’s IVF philosophy concentrates on three key points in the treatment process: the mother’s wellbeing, the technology used to assist mothers deliver a strong and healthy baby and the medical science used to ensure every chance of success for women in various age spectrums.
Clinic in Kyrgyzstan
NewGenIvf established First Fertility Bishkek in October 2019 in Kyrgyzstan for its surrogacy services, as Kyrgyzstan has supply of surrogate candidates at a relatively low cost and a more friendly legal environment for surrogacy services. In 2020, First Fertility Bishkek obtained the license to provide ARS and surrogacy services, becoming one of the few facilities licensed to offer ARS and one of the facilities licensed to offer surrogacy services in Kyrgyzstan as of December 31, 2023, according to CIC. In addition, NewGenIvf also provide related ancillary fertility services when carrying out surrogacy services. These ancillary fertility services include: (i) maternity caring service, and (ii) documentation service.
Physicians at First Fertility Bishkek have expertise in sourcing surrogate mothers, techniques of embryo transfers, prenatal care, baby delivery, and postnatal care. First Fertility Bishkek also collaborates closely with Phnom Penh Center in arranging shipment of frozen embryos. NewGenIvf hires local physicians and local staff. NewGenIvf also provides training for newly admitted Kyrgyzstan physicians and embryologists in Thailand. Some personnel who had relevant experience in Kyrgyzstan had also been sent from Cambodia to Kyrgyzstan to help manage such operations from time to time.
As of December 31, 2023, First Fertility Bishkek had one full-time physician, one embryologist, two nurses, and ten other staff.
Professionals
Licensed Physicians
As of December 31, 2023, NewGenIvf contracted with five licensed physicians, among which one was based in Cambodia and the other four were based in Thailand. Most of NewGenIvf’s physicians had over 10 years of experience or above. The following table summarises the number and types of such licensed physicians as of December 31, 2023.
| Country | | Licensed physician | | Licenses and
Approvals | | Effective Period | | Issuing
Authority |
| Cambodia | | Mr. Keut Serey | | Decision on permission for beauty treatment operation | | December 14, 2022 – December 14, 2026 | | The Ministry of Health of Cambodia |
| Thailand | | Dr Patsama Vichinsartvichai | | Medical Facility Operating License number 288006 | | August 12, 2022 – December 31, 2023 | | The Ministry of Health of Thailand |
| | | | | Number 30920 Medical Practitioner License | | April 1, 2004 – Indefinite | | The Ministry of Health of Thailand |
| | | | | Number 26443/2556 Reproductive Medicine Diploma | | July 1, 2013 – Indefinite | | Medical Council of Thailand |
| | | | | Certificate number obscured OB-Gyn License | | October 13, 2010 – Indefinite | | Medical Council of Thailand |
| Thailand | | Dr Keatthisak Boonsimma | | Number 31801 Medical Practitioner License | | April 1, 2005 – Indefinite | | Royal Thai College of Obstetricians and Gynaecologists of Thailand |
| | | | | Number 22624/2554 OB-Gyn License | | July 1, 2014 – Indefinite | | Medical Council of Thailand |
| | | | | Number 40962/2563 Reproductive Medicine Diploma | | July 1, 2020 – Indefinite | | Medical Council of Thailand |
| Country | | Licensed physician | | Licenses and
Approvals | | Effective Period | | Issuing
Authority |
| Thailand | | Dr Seree Teerapong | | Number 15231/2564 Reproductive Medicine License | | July 1, 2021 – Indefinite | | Medical Council of Thailand |
| | | | | Number 4576/2533 OB-Gyn License | | July 12, 1990 – Indefinite | | Medical Council of Thailand |
| | | | | Number 11544 (replacement) Medical Practitioner License | | April 12, 1984 – Indefinite | | Medical Council of Thailand |
| Thailand | | Dr Wiphawee Luangtangvarodom | | Number 38347/2562 OB-Gyn License | | August 1, 2019 – Indefinite | | Medical Council of Thailand |
| | | | | Number 43217/2564 Reproductive Medicine License | | July 1, 2021 – Indefinite | | Medical Council of Thailand |
| | | | | Number 48510 Medical Practitioner License | | April 1, 2014 – Indefinite | | Medical Council of Thailand |
Agreements with Physicians
NewGenIvf enters into independent physician agreements or employment contracts with its physicians. The terms and conditions and the format of the agreements NewGenIvf enters into with each of its physicians vary, depending on the physician’s seniority and practise nature.
Customers
For the years ended December 31, 2023 and 2022, the majority of NewGenIvf’s clients were from China (including mainland China and Hong Kong). The number of Thai and Cambodian local patients generally increased in 2022 and 2023 compared with earlier years due to the impact of COVID-19 on international travel. NewGenIvf enters into a service agreement with each of its customers that outline, among other things, the scope of services, service fees, payment terms and rights, responsibilities and obligations of each party. Customers are not entitled to enjoy the relevant services until outstanding amounts have been settled pursuant to the relevant contract. Sales to individual consumers did not vary significantly and none of the customers contribute more than 10% of NewGenIvf’s revenue for the years ended December 31, 2023 and 2022.
The following table sets forth a breakdown of NewGenIvf’s total customers by major countries (determined by the passports they provided to NewGenIvf for registration) and as a percentage of the total customers for the periods indicated(1):
| | | For the Year ended December 31, | |
| | | 2023 | | | 2022 | |
| | | First
Fertility
PGS
Center | | | Phnom
Penh
Center | | | Total | | | % | | | First
Fertility
PGS
Center | | | Phnom
Penh
Center | | | Total | | | % | |
| China(2) | | | 34 | | | | 87 | | | | 121 | | | | 42 | | | | 66 | | | | 117 | | | | 183 | | | | 72 | |
| India | | | 16 | | | | — | | | | 16 | | | | 6 | | | | 16 | | | | — | | | | 16 | | | | 6 | |
| Thailand | | | 103 | | | | — | | | | 103 | | | | 36 | | | | 25 | | | | 3 | | | | 28 | | | | 11 | |
| Cambodia | | | — | | | | 7 | | | | 7 | | | | 2 | | | | — | | | | 22 | | | | 22 | | | | 9 | |
| Others(3) | | | 31 | | | | 9 | | | | 40 | | | | 14 | | | | — | | | | 5 | | | | 5 | | | | 2 | |
| Total | | | 184 | | | | 103 | | | | 287 | | | | 100 | | | | 107 | | | | 147 | | | | 254 | | | | 100 | |
| (1) | Customers of First Fertility Bishkek are the same customers of Phnom Penh Center. |
| (2) | Include customers from mainland China and Hong Kong. |
| (3) | Include customers from Philippines, Singapore, USA, Korea, Nigeria and UK. |
In addition to significant customers using NewGenIvf’s IVF treatment services and surrogacy and ancillary caring services, NewGenIvf also has customers who only use its relatively insignificant services, such as check-ups services, blood test services and other minor services (the latter category of customers are referred to as “consultation customers”).
Sales and Marketing
For the years ended December 31, 2023 and 2022, NewGenIvf promoted brand awareness through its sales teams and, in many cases, through cooperating with third-party agencies and partners.
NewGenIvf’s sales teams have broad experience in fertility services and are responsible for identifying potential clients and managing the overall sales process. NewGenIvf’s sales team primarily relies on social media marketing, word-of-mouth referrals, recognition of its brand, printed advertisements and marketing events. NewGenIvf spends marketing expenses on placing advertisements through popular social media platforms, maintaining the official website of NewGenIvf and sending information through its official accounts on social media platforms.
Supply and Procurement
NewGenIvf’s procurement is mainly for medications, laboratory media and reagents, laboratory consumables, and blood test reagents. As of December 31, 2023 and 2022, one and four suppliers individually contributed more than 10% of the Group’s trade payable, in aggregate accounting for 30.6% and 69.8% of the Group’s trade payables, respectively. For the year ended December 31, 2023 and 2022, nil and two vendors contributed more than 10% of total purchases of the Group, in aggregate accounting for nil and 55.3% of the Group’s total purchases, respectively. NewGenIvf’s procurement team is experienced in selecting cost-effective supplies as well as selecting reliable suppliers. NewGenIvf’s major suppliers are pharmaceutical companies.
Competition
NewGenIvf believes that it is a long-standing provider of ARS in Asia Pacific that competes primarily based on the following competitive factors:
| | ● | the value and comprehensiveness of the solutions; |
| | ● | treatment that is effective and achieves desired outcomes; |
| | ● | clients’ experience, including dedicated patient education, clinical guidance and emotional support; and |
| | ● | access to a network of high-quality fertility specialists. |
NewGenIvf competes primarily with other regional fertility service providers. While NewGenIvf does not believe any single competitor offers a comparably robust and integrated fertility solution package as NewGenIvf in the regions that it operates, NewGenIvf’s competitors may compete in a variety of ways, including by providing better services, having established local connections, fulfilling evolving client needs, as well as conducting brand promotions and other marketing activities.
As NewGenIvf may introduce new ancillary services and other companies may introduce similar fertility services as NewGenIvf’s, NewGenIvf may become subject to additional competition.
Facilities
As of December 31, 2023, in addition to its clinics, NewGenIvf leased one property in Hong Kong with an aggregate square footage of approximately 8,000 for its administration support offices. NewGenIvf also operates its medical facilities as described above in “— Network of Facilities” above. NewGenIvf believes that its existing facilities are suitable and adequate to meet its current needs.
SELLING SHAREHOLDER
This prospectus relates to the possible resale from time to time by White Lion of any or all of the Ordinary Shares that may be issued by us to White Lion under the White Lion Purchase Agreement. We are registering the Resale Shares pursuant to the provisions of the RRA we entered into with White Lion on November 21, 2024, in order to permit White Lion to offer the Resale Shares for resale from time to time.
Other than the relationships described herein, to our knowledge, the Selling Shareholder has not had any material relationship with us within the past three years.
The table below presents information regarding the Selling Shareholder and the Resale Shares that it may offer from time to time under this prospectus. This table is prepared based on information supplied to us by the Selling Shareholder, and reflects holdings as of November 21, 2024. The number of shares in the column “Maximum Number of Ordinary Shares to be Sold Pursuant to this Prospectus” represents all of the Ordinary Shares that the Selling Securityholder may offer under this prospectus. The Selling Shareholder may sell some, all or none of its shares in this offering. We do not know how long the Selling Shareholder will hold the shares before selling them, and we currently have no agreements, arrangements or understandings with the Selling Shareholder regarding the sale of any of the shares.
Beneficial ownership is determined in accordance with Rule 13d-3(d) promulgated by the SEC under the Exchange Act, and includes Ordinary Shares with respect to which a Selling Shareholder has voting and investment power. The percentage of Ordinary Shares beneficially owned by the Selling Shareholder prior to the offering shown in the table below is based on an aggregate of 10,149,386 Ordinary Shares outstanding on November 21, 2024. Because the purchase price of the Common Shares issuable under the White Lion Purchase Agreement is determined on the Closing Date with respect to each purchase, the number of shares that may actually be sold by the Company under the White Lion Purchase Agreement may be fewer than the number of shares being offered by this prospectus. The fourth column assumes the sale of all of the shares offered by the Selling Securityholder pursuant to this prospectus.
The Company may require the Selling Shareholder to suspend the sales of the Resale Shares offered by this prospectus upon the occurrence of any event that makes any statement in this prospectus or the Registration Statement untrue in any material respect or that requires the changing of statements in these documents in order to make statements in those documents not misleading.
| Name of Selling Shareholder | | Ordinary
Shares
Beneficially
Owned
Prior to
Offering(1) | | | Percentage of
Ordinary Shares Beneficially Owned
Prior to
Offering(2) | | | Maximum
Number of
Ordinary
Shares
to be
Sold
Pursuant to
this
Prospectus | | | Ordinary
Shares Beneficially Owned
Immediately
After Sale of
Maximum
Number of Shares
in this
Offering(3) | | | Percentage of
Ordinary Shares Beneficially Owned Immediately
After
Sale of
Maximum
Number of Shares
in this
Offering(1)(2)(4) | |
| White Lion Capital LLC (4) | | | 0 | | | | - | | | | 40,000,000 | | | | 0 | | | | - | |
| (1) | In accordance with Rule 13d-3(d) under the Exchange Act, we have excluded from the number of shares beneficially owned prior to the offering all of the shares that White Lion may be required to purchase under the White Lion Purchase Agreement, because the issuance of such shares is solely at our discretion and is subject to conditions contained in the White Lion Purchase Agreement, the satisfaction of which are entirely outside of White Lion’s control, including the Registration Statement that includes this prospectus becoming and remaining effective. Furthermore, the purchase of Ordinary Shares is subject to certain agreed upon maximum amount limitations set forth in the White Lion Purchase Agreement. Also, the White Lion Purchase Agreement prohibits us from issuing and selling any Ordinary Shares to White Lion to the extent such shares, when aggregated with all other Ordinary Shares then beneficially owned by White Lion, would cause White Lion’s beneficial ownership of our Common Shares to exceed the 4.99% Beneficial Ownership Limitation. White Lion may increase the Beneficial Ownership Limitation up to 9.99% at its sole discretion upon sixty-one (61) days prior written notice to the Company. |
| (2) | The applicable percentage of beneficial ownership is calculated based on the total number of Ordinary Shares issued and outstanding, being 10,149,386 shares as of the date of this prospectus. |
| (3) | Assumes the sale of all of the Resale Shares offered by the Selling Shareholder pursuant to this prospectus. |
| (4) | The business address of White Lion is 17631 Ventura Blvd, Suite 1008, Encino, CA 91316. |
White Lion Transaction
On November 21, 2024, the Company entered into a Common Shares Purchase Agreement (the “White Lion Purchase Agreement”) with White Lion Capital, LLC (“White Lion”) and a related Registration Rights Agreement (the “RRA”). Pursuant to the White Lion Purchase Agreement, the Company has the right, but not the obligation, to require White Lion to purchase, from time to time, up to One Hundred Million Dollars ($100,000,000) in aggregate gross purchase price of newly issued Ordinary Shares, with an automatic increase to Three Hundred Million Dollars ($300,000,000) upon any substantial M&A or Material Transaction (as defined in the White Lion Purchase Agreement) and a further option to increase to Five Hundred Million Dollars ($500,000,000) after Two Hundred and Fifty Million Dollars ($250,000,000) has been issued and sold to White Lion under the White Lion Purchase Agreement, subject to certain limitations and conditions set forth in the White Lion Purchase Agreement.
Subject to the satisfaction of certain customary conditions including, without limitation, the effectiveness of theRegistration Statement registering the resale of the shares issuable pursuant to the White Lion Purchase Agreement, the Company’s right to sell shares to White Lion commenced on the date of the execution of White Lion Purchase Agreement and extends until (i) 36 months from the date of execution of the White Lion Purchase Agreement, or (ii) at the Company’s option, until 65 months from the date of the execution of the White Lion Purchase Agreement in the event that $100,000,000 of purchases under the White Lion Purchase Agreement have been completed prior to the 36 month anniversary of the Execution Date (the “Commitment Period”).
During the Commitment Period, subject to the terms and conditions of the White Lion Purchase Agreement, the Company may exercise its right to sell its Ordinary Shares. The Company may deliver a Regular Purchase Notice (as such term is defined in the White Lion Purchase Agreement), pursuant to which the Company can require White Lion to purchase up to a number of Ordinary Shares equal to the lesser of (i) $3,000,000 divided by the highest closing price of the Ordinary Shares over the most recent five (5) Business Days immediately preceding the Purchase Notice, or (ii) 40% of Average Daily Trading Volume (as such term is defined in the White Lion Purchase Agreement), subject to a maximum Investment Limit of $3,000,000.
The Company may also deliver a Rapid Purchase Notice (as such term is defined in the White Lion Purchase Agreement), pursuant to which the Company may require White Lion to purchase up to a number of Ordinary Shares equal to $3,000,000 divided by the highest closing price of the Ordinary Shares over the most recent five business days immediately prior to the receipt of the notice. White Lion may waive such limits under any notice at its discretion and purchase additional shares.
The price to be paid by White Lion for any shares that the Company requires White Lion to purchase will depend on the type of purchase notice that the Company delivers. For shares being issued pursuant to a Regular Purchase Notice, the purchase price per share will be the lower of (i) the closing price of Ordinary Shares prior to the receipt of the applicable Purchase Notice, or (ii) the product of (a) the lowest daily VWAP of the Ordinary Shares during the Regular Purchase Valuation Period (as defined in the White Lion Purchase Agreement), and (b) 98%.
For shares being issued pursuant to a Rapid Purchase Notice, the Company may opt for the purchase price per share to be (i) equal to the lowest traded price of the Ordinary Shares on the date that the notice is delivered, or (ii) 97% of the lowest traded price of the Ordinary Shares two hours following White Lion’s written confirmation of the acceptance of the Rapid Purchase Notice.
No purchase notice shall result in White Lion beneficially owning (as calculated pursuant to Section 13(d) of the Securities Exchange Act of 1934, as amended, and Rule 13d-3 thereunder) more than 4.99% (subject to increase, in the sole discretion of White Lion, to 9.99%) of the number of Ordinary Shares outstanding immediately prior to the issuance of Ordinary Shares issuable pursuant to a purchase notice.
The Company has the right to terminate the White Lion Purchase Agreement in the event of a material breach of the White Lion Purchase Agreement by White Lion. The White Lion Purchase Agreement also automatically terminates upon the earlier of (i) the end of the Commitment Period and (ii) the date that the Company commences a voluntary bankruptcy proceeding, a custodian is appointed for the Company or for all or substantially all of its property, or the Company makes a general assignment for the benefit of its creditors.
In consideration for the commitments of White Lion, as described above, the Company has agreed that it will issue to White Lion 700,000 Common Shares (“Commitment Shares”).
In addition, the Company has agreed that (i) if the Company fails to sign a binding term sheet for a Material Transaction within 90 days of Execution Date, it will issue an additional 100,000 Common Shares to the Investor; (ii) upon the completion of a Material Transaction (as defined in the White Lion Purchase Agreement), it will issue an additional amount of Ordinary Shares equal to $500,000 divided by the closing price of the Ordinary Shares on the date of the public filing announcing the closing of the Material Transaction; and (iii) in the event the gross investment by White Lion reaches $250,000,000, the Company shall issue an additional amount of Ordinary Shares equal to $250,000 divided by the closing price of the Ordinary Shares on the Closing Date the gross investment reaches $250,000,000. The Commitment Shares will be fully earned by White Lion regardless of termination of the White Lion Purchase Agreement.
Concurrently with the White Lion Purchase Agreement, the Company entered into the RRA with White Lion, pursuant to which the Company agreed to file, within 10 business days following the execution of the White Lion Purchase Agreement, the Registration Statement with the SEC covering the resale by White Lion of the number of shares determined appropriate by the Company and permitted to be included therein in accordance with applicable SEC rules, regulations and interpretations and the Commitment Shares. The RRA also contains usual and customary damages provisions for failure to file and failure to have the Registration Statement declared effective by the SEC within the time periods specified therein.
The White Lion Purchase Agreement and the RRA contain customary representations, warranties, conditions and indemnification obligations of the parties. The representations, warranties and covenants contained in such agreements were made only for purposes of such agreements and as of specific dates, were solely for the benefit of the parties to such agreements and may be subject to limitations agreed upon by the contracting parties.
PLAN OF DISTRIBUTION
The Ordinary Shares offered by this prospectus are being offered by the Selling Shareholder. The shares may be sold or distributed from time to time by the Selling Shareholder directly to one or more purchasers or through brokers, dealers, or underwriters who may act solely as agents at market prices prevailing at the time of sale, at prices related to the prevailing market prices, at negotiated prices, or at fixed prices, which may be changed. The sale of the shares offered by this prospectus could be effected in one or more of the following methods:
| ● | ordinary brokers’ transactions; |
| ● | transactions involving cross or block trades; |
| ● | through brokers, dealers, or underwriters who may act solely as agents; |
| ● | “at the market” into an existing market for our common stock; |
| ● | in other ways not involving market makers or established business markets, including direct sales to purchasers or sales effected through agents; |
| ● | in privately negotiated transactions; or |
| ● | any combination of the foregoing. |
In order to comply with the securities laws of certain states, if applicable, the shares may be sold only through registered or licensed brokers or dealers. In addition, in certain states, the shares may not be sold unless they have been registered or qualified for sale in the state or an exemption from the state’s registration or qualification requirement is available and complied with.
White Lion is an “underwriter” within the meaning of Section 2(a)(11) of the Securities Act.
White Lion has informed us that it intends to use one or more registered broker-dealers to effectuate all sales, if any, of our Ordinary Shares that it may acquire from us pursuant to the White Lion Purchase Agreement. Such sales will be made at prices and at terms then prevailing or at prices related to the then current market price. Each such registered broker-dealer will be an underwriter within the meaning of Section 2(a)(11) of the Securities Act. White Lion has informed us that each such broker-dealer may receive commissions from White Lion and, if so, such commissions will not exceed customary brokerage commissions.
Brokers, dealers, underwriters or agents participating in the distribution of the shares offered by this prospectus may receive compensation in the form of commissions, discounts, or concessions from the purchasers, for whom the broker-dealers may act as agent, of the shares sold by the Selling Shareholder through this prospectus. The compensation paid to any such particular broker-dealer by any such purchasers of Ordinary Shares sold by the Selling Shareholder may be less than or in excess of customary commissions. Neither we nor the Selling Shareholder can presently estimate the amount of compensation that any agent will receive from any purchasers of Ordinary Shares sold by the Selling Shareholder.
We know of no existing arrangements between the Selling Shareholder or any other shareholder, broker, dealer, underwriter or agent relating to the sale or distribution of the Ordinary Shares offered by this prospectus.
We may from time to time file with the SEC one or more supplements to this prospectus or amendments to the Registration Statement of which this prospectus forms a part to amend, supplement or update information contained in this prospectus, including, if and when required under the Securities Act, to disclose certain information relating to a particular sale of shares offered by this prospectus by a Selling Shareholder, including the names of any brokers, dealers, underwriters or agents participating in the distribution of such shares by such Selling Shareholder, any compensation paid by such Selling Shareholder to any such brokers, dealers, underwriters or agents, and any other required information.
We will pay the expenses incident to the registration under the Securities Act of the offer and sale of the Ordinary Shares covered by this prospectus by White Lion.
We also have agreed to indemnify White Lion and certain other persons against certain liabilities in connection with the offering of shares offered hereby, including liabilities arising under the Securities Act or, if such indemnity is unavailable, to contribute amounts required to be paid in respect of such liabilities. White Lion has agreed to indemnify us against liabilities under the Securities Act that may arise from certain written information furnished to us by White Lion specifically for use in this prospectus or, if such indemnity is unavailable, to contribute amounts required to be paid in respect of such liabilities. Insofar as indemnification for liabilities arising under the Securities Act may be permitted to our directors, officers, and controlling persons, we have been advised that in the opinion of the SEC this indemnification is against public policy as expressed in the Securities Act and is therefore, unenforceable.
We estimate that the total expenses for the offering will be approximately $30,593.
White Lion has represented to us that at no time prior to the date of the White Lion Purchase Agreement has White Lion, any of its affiliates or any entity managed or controlled by White Lion engaged in or effected, directly or indirectly, for its own principal account, any short sale (as such term is defined in Rule 200 of Regulation SHO of the Exchange Act) of our Common Shares that establishes a net short position with respect to our Common Shares. White Lion has agreed that, during the Commitment Term, none of White Lion, any of its affiliates nor any entity managed or controlled by White Lion will enter into or effect, directly or indirectly, any of the foregoing transactions for its own principal account or for the principal account of any other such entity.
We have advised White Lion that it is required to comply with Regulation M promulgated under the Exchange Act. With certain exceptions, Regulation M precludes White Lion, any affiliated purchasers, and any broker-dealer or other person who participates in the distribution from bidding for or purchasing, or attempting to induce any person to bid for or purchase any security which is the subject of the distribution until the entire distribution is complete. Regulation M also prohibits any bids or purchases made in order to stabilize the price of a security in connection with the distribution of that security. All of the foregoing may affect the marketability of the securities offered by this prospectus.
This offering will terminate on the date that all shares of our Ordinary Shares offered by this prospectus have been sold by the Selling Shareholder.
DESCRIPTION OF SHARE CAPITAL
Class A and Class B Ordinary Shares
General
The Memorandum and Articles of Association authorize the issuance of a maximum of 200,000,000 Class A Ordinary Shares, 200,000 Class B Ordinary Shares and 1,000,000 preferred shares with no par value (“Preferred Shares”). As of the date of this prospectus, we have 159,724,031 shares of Class A Ordinary Shares outstanding, no Class B Ordinary Shares outstanding, and no Preferred Shares outstanding. All of our outstanding Class A Ordinary Shares are validly issued, and fully paid. Our Class A Ordinary Shares and Class B Ordinary Shares are not redeemable and are not subject to any preemptive right.
Dividends.
The holders of our Class A Ordinary Shares and Class B Ordinary Shares are entitled to such dividends as may be declared by its Board of Directors subject to its Memorandum and Articles of Association and applicable law. No dividend may be declared and paid unless the Board of Directors determine that, immediately after the payment, the value of the Company assets will exceed its liabilities and the Company will be able to pay its debts as and when they fall due. Holders of Class A ordinary shares and Class B ordinary shares will be entitled to the same amount of dividends, if declared.
Voting Rights.
Subject to the rights of the Preferred Shares’ holders, in respect of all matters subject to a member’s vote, each Class A Ordinary Share and Class B Ordinary Share is entitled to one vote at a meeting of the members or on any resolution of members, and all ordinary shares vote together as one class. Voting at any shareholder meeting is by show of hands unless a poll is demanded by the chairman.
A quorum required for a meeting of shareholders consists of one or more shareholders holding not less than one-half of the votes attaching to the issued and outstanding shares entitled to vote on resolutions of shareholders to be considered at the meeting present in person or by proxy or, if a corporation or other non-natural person, by its duly authorized representative. As a BVI business company, the Company is not obliged by the BVI Act to call shareholders’ annual general meetings. The Memorandum and Articles of Association provide that the Company may (but is not obliged to) in each calendar year hold a general meeting as its annual general meeting in which case the Company will specify the meeting as such in the notices calling it, and the annual general meeting will be held at such time and place as may be determined by its directors. Each general meeting, other than an annual general meeting, shall be an extraordinary general meeting.
Shareholders’ annual general meetings and any other general meetings of the Company’s shareholders may be convened by any director or, upon a requisition of shareholders holding at the date of deposit of the requisition not less than 30 percent of the votes attaching to the issued and outstanding shares entitled to vote at general meetings in respect of the matter for which the meeting is requested, in which case the directors are obliged to convene such meeting and to put the resolutions so requisitioned to a vote at such meeting; however, the Memorandum and Articles of Association do not provide its shareholders with any right to put any proposals before any annual general meetings or any extraordinary general meetings not called by such shareholders. Advance notice of at least ten (10) days and not more than sixty (60) days is required for the convening of the Company’s annual general meeting and other general meetings unless such notice is waived in accordance with its articles of association.
Any resolution to be passed at a meeting by the shareholders requires the affirmative vote of a simple majority of the votes attaching to the shares cast by those shareholders entitled to vote who are present in person or by proxy at a general meeting.
Liquidation.
As permitted by the BVI Act and the Memorandum and Articles of Association, the Company may be voluntarily liquidated under Part XII of the BVI Act by resolution of directors and resolution of shareholders if the Company’s assets exceed the Company’s liabilities and the Company is able to pay the Company’s debts as they fall due. The Company may also be wound up in circumstances where the Company is insolvent in accordance with the terms of the BVI Insolvency Act (As Revised).
If the Company is wound up and the assets available for distribution among the Company’s shareholders are more than sufficient to repay all amounts paid to the Company on account of the issue of shares immediately prior to the winding up, the excess shall be distributable pari passu among those shareholders in proportion to the amount paid up immediately prior to the winding up on the shares held by them, respectively. If the Company is wound up and the assets available for distribution among the shareholders as such are insufficient to repay the whole of the amounts paid to the Company on account of the issue of shares, those assets shall be distributed so that, to the greatest extent possible, the losses shall be borne by the shareholders in proportion to the amounts paid up immediately prior to the winding up on the shares held by them, respectively. If the Company is wound up, the liquidator appointed by the Company may, in accordance with the BVI Act, divide among the Company’s shareholders in specie or kind the whole or any part of the Company’s assets (whether they shall consist of property of the same kind or not) and may, for such purpose, set such value as the liquidator deems fair upon any property to be divided and may determine how such division shall be carried out as between the shareholders or different classes of shareholder
Redemption, Repurchase and Surrender of Ordinary Shares.
The Company may issue shares on terms that such shares are subject to redemption, at the Company’s option or at the option of the holders thereof, on such terms and in such manner as may be determined, before the issue of such shares, by the Board of Directors. The Company may also repurchase any of its shares provided that the Company may not purchase, redeem or otherwise acquire its own shares without the consent of the shareholder whose Shares are to be purchased, redeemed or otherwise acquired unless the Company is permitted or required by the Act or any other provision in the Memorandum or Articles to purchase, redeem or otherwise acquire the Shares without such consent.
Variations of Rights of Shares.
If at any time the Company’s share are divided into different classes or series of shares, the rights attached to any class or series of shares (unless otherwise provided by the terms of issue of the shares of that class or series), whether or not the Company is being wound-up, may be varied by a resolution passed at a meeting by the holders of more than fifty percent of the issued shares of that class that have voted (and are entitled to vote thereon) in relation to any such resolution, unless otherwise provided by the terms of issue of such class. The rights conferred upon the holders of the shares of any class issued shall not, unless otherwise expressly provided by the terms of issue of the shares of that class, be deemed to be varied by the creation or issue of further shares ranking pari passu with such existing class of shares.
Inspection of Books and Records.
Holders of our Class A Ordinary Shares and Class B Ordinary Shares have no general right under BVI law to inspect or obtain copies of the Company’s list of shareholders or its corporate records. However, the Company will provide its shareholders with annual audited financial statements. See “Where You Can Find Additional Information.”
Issuance of Additional Shares.
The Memorandum and Articles of Association authorize the Board of Directors to issue additional ordinary shares from time to time as the Board of Directors shall determine, to the extent of available authorized but unissued shares. The Memorandum and Articles of Association also authorize the Board of Directors to establish from time to time one or more series of preferred shares and to determine, with respect to any series of preferred shares, the terms and rights of that series, including:
| ● | the designation of the series; |
| ● | the number of shares of the series; |
| ● | the dividend rights, dividend rates, conversion rights, voting rights; and |
| ● | the rights and terms of redemption and liquidation preferences. |
The Board of Directors may issue preferred shares without action by its shareholders to the extent authorized but unissued. Issuance of these shares may dilute the voting power of holders of Class A Ordinary Shares and Class B Ordinary Shares.
Anti-Takeover Provisions
Some provisions of the Memorandum and Articles of Association may discourage, delay or prevent a change of control of the Company or management that shareholders may consider favorable, including (i) provisions that authorize the Board of Directors to issue preferred shares in one or more series and to designate the price, rights, preferences, privileges and restrictions of such preferred shares without any further vote or action by its shareholders; and (ii) provisions providing that directors may not be removed by the shareholders except for cause.
Preferred Shares
Subject to applicable law and the Memorandum and Articles of Association, the Board of Directors may issue Preferred Shares with such preferred rights as they shall determine. The rights, privileges, restrictions and conditions attaching to the Preferred Shares shall be stated in the Memorandum and Articles of Association, which shall be amended accordingly prior to the issue of such Preferred Shares.
TAXATION
The following is a general summary of the material U.S. federal income tax consequences relevant to an investment in our Ordinary Shares. The discussion is not intended to be, nor should it be construed as, legal or tax advice to any particular prospective purchaser. The discussion is based on laws and relevant interpretations thereof as of the date of this annual report, all of which are subject to change or different interpretations, possibly with retroactive effect. The discussion does not address U.S. state or local tax laws. You should consult your own tax advisors with respect to the consequences of acquisition, ownership and disposition of our Ordinary Shares.
This discussion is based on provisions of the Code, the Treasury Regulations promulgated thereunder (whether final, temporary, or proposed), administrative rulings of the IRS, and judicial decisions, all as in effect on the date hereof, and all of which are subject to differing interpretations or change, possibly with retroactive effect. This discussion does not purport to be a complete analysis or listing of all potential U.S. federal income tax considerations that may apply to a securityholder of the Company as a result of the ownership and disposition of the Company Securities. In addition, this discussion does not address all aspects of U.S. federal income taxation that may be relevant to particular holders nor does it take into account the individual facts and circumstances of any particular holder that may affect the U.S. federal income tax consequences to such holder, and accordingly, is not intended to be, and should not be construed as, tax advice. This discussion does not address the U.S. federal 3.8% Medicare tax imposed on certain net investment income or any aspects of U.S. federal taxation other than those pertaining to the income tax, nor does it address any tax consequences arising under any U.S. state and local, or non-U.S. tax laws, or, except as discussed here, any tax reporting obligations of a holder of the Company Securities. Holders should consult their own tax advisors regarding such tax consequences in light of their particular circumstances.
No ruling has been requested or will be obtained from the IRS regarding the U.S. federal income tax consequences discussed below; thus, there can be no assurance that the IRS will not challenge the U.S. federal income tax treatment described below or that, if challenged, such treatment will be sustained by a court.
This summary is limited to considerations relevant to U.S. Holders that hold the Company Securities as “capital assets” within the meaning of section 1221 of the Code (generally, property held for investment). This discussion does not address all aspects of U.S. federal income taxation that may be important to holders in light of their individual circumstances, including holders subject to special treatment under the U.S. tax laws, such as, for example:
| | ● | banks or other financial institutions, underwriters, or insurance companies; |
| | ● | traders in securities who elect to apply a mark-to-market method of accounting; |
| | ● | real estate investment trusts and regulated investment companies; |
| | ● | tax-exempt organizations, qualified retirement plans, individual retirement accounts, or other tax- deferred accounts; |
| | ● | expatriates or former citizens or long-term residents of the United States; |
| | ● | subchapter S corporations, partnerships or other pass-through entities or investors in such entities; |
| | ● | any holder that is not a U.S. Holder; |
| | ● | dealers or traders in securities, commodities or currencies; |
| | ● | persons subject to the alternative minimum tax; |
| | ● | U.S. persons whose “functional currency” is not the U.S. dollar; |
| | ● | persons who receive stock of the Company through the issuance of restricted share under an incentive plan or through a tax-qualified retirement plan or otherwise as compensation; |
| | ● | U.S. shareholders of controlled foreign corporations, as those terms are defined in Sections 951(b) and 957(a), respectively; |
| | ● | persons who own (directly or through attribution) 5% or more (by vote or value) of the outstanding Class A Ordinary Shares (excluding treasury shares); |
| | ● | holders holding ASCA securities, or, after the Business Combination, the Company Securities, as a position in a “straddle,” as part of a “synthetic security” or “hedge,” as part of a “conversion transaction,” or other integrated investment or risk reduction transaction. |
As used in this prospectus, the term “U.S. Holder” means a beneficial owner of the Company Securities, that is, for U.S. federal income tax purposes:
| | ● | an individual who is a citizen or resident of the United States; |
| | ● | a corporation (or other entity that is classified as a corporation for U.S. federal income tax purposes) that is created or organized in or under the laws of the United States or any State thereof or the District of Columbia; |
| | ● | an estate the income of which is subject to U.S. federal income tax regardless of its source; or |
| | ● | a trust (i) if a court within the United States is able to exercise primary supervision over the administration of the trust and one or more U.S. persons have the authority to control all substantial decisions of the trust, or (ii) that has a valid election in effect under applicable Treasury Regulations to be treated as a U.S. person for U.S. federal income tax purposes. |
If a partnership, including for this purpose any entity or arrangement that is treated as a partnership for U.S. federal income tax purposes, holds the Company Securities, the U.S. federal income tax treatment of a partner in such partnership will generally depend on the status of the partner and the activities of the partnership. A holder that is a partnership and the partners in such partnership should consult their own tax advisors with regard to the U.S. federal income tax consequences of ownership and disposition of the Company Securities.
THIS SUMMARY DOES NOT PURPORT TO BE A COMPREHENSIVE ANALYSIS OR DESCRIPTION OF ALL POTENTIAL U.S. FEDERAL INCOME TAX CONSEQUENCES OF OWNERSHIP AND DISPOSITION OF THE COMPANY SECURITIES. IN ADDITION, THE U.S. FEDERAL INCOME TAX TREATMENT OF THE BENEFICIAL OWNERS OF THE COMPANY SECURITIES MAY BE AFFECTED BY MATTERS NOT DISCUSSED HEREIN AND DEPENDS IN SOME INSTANCES ON DETERMINATIONS OF FACT AND INTERPRETATIONS OF COMPLEX PROVISIONS OF U.S. FEDERAL INCOME TAX LAW FOR WHICH NO CLEAR PRECEDENT OR AUTHORITY MAY BE AVAILABLE. HOLDERS OF THE COMPANY SECURITIES SHOULD CONSULT WITH THEIR TAX ADVISORS REGARDING THE PARTICULAR TAX CONSEQUENCES TO THEM OF THE OWNERSHIP AND DISPOSITION OF THE COMPANY SECURITIES, INCLUDING THE APPLICABILITY AND EFFECTS OF U.S. FEDERAL, STATE, LOCAL, AND OTHER TAX LAWS.
Distribution on the Class A Ordinary Shares
Subject to the PFIC rules discussed below “— Passive Foreign Investment Company Status,” the gross amount of any distribution on the Class A Ordinary Shares that is made out of the Company’s current and accumulated earnings and profits (as determined for U.S. federal income tax purposes) will generally be taxable to a U.S. Holder as ordinary dividend income on the date such distribution is actually or constructively received by such U.S. Holder. Any such dividends paid to corporate U.S. Holders generally will not qualify for the dividends-received deduction that may otherwise be allowed under the Code.
Dividends received by non-corporate U.S. Holders, including individuals, from a “qualified foreign corporation” may be eligible for reduced rates of taxation, provided that certain holding period requirements and other conditions are satisfied. For these purposes, a non-U.S. corporation will be treated as a qualified foreign corporation with respect to dividends paid by that corporation on shares that are readily tradable on an established securities market in the United States. U.S. Treasury Department guidance indicates that shares listed on Nasdaq will be considered readily tradable on an established securities market in the United States. Even if the Class A Ordinary Shares are listed on Nasdaq, there can be no assurance that the Class A Ordinary Shares will be considered readily tradable on an established securities market in future years. Non-corporate U.S. Holders that do not meet a minimum holding period requirement or that elect to treat the dividend income as “investment income” pursuant to Section 163(d)(4) of the Code (dealing with the deduction for investment interest expense) will not be eligible for the reduced rates of taxation regardless of the Company’s status as a qualified foreign corporation. In addition, the rate reduction will not apply to dividends if the recipient of a dividend is obligated to make related payments with respect to positions in substantially similar or related property. This disallowance applies even if the minimum holding period has been met. Finally, the Company will not constitute a qualified foreign corporation for purposes of these rules if it is a PFIC for the taxable year in which it pays a dividend or for the preceding taxable year. See the discussion below under “— Passive Foreign Investment Company Status.
The amount of any dividend paid in foreign currency will be the U.S. dollar value of the foreign currency distributed by the Company, calculated by reference to the exchange rate in effect on the date the dividend is includible in the U.S. Holder’s income, regardless of whether the payment is in fact converted into U.S. dollars on the date of receipt. Generally, a U.S. Holder should not recognize any foreign currency gain or loss if the foreign currency is converted into U.S. dollars on the date the payment is received. However, any gain or loss resulting from currency exchange fluctuations during the period from the date the U.S. Holder includes the dividend payment in income to the date such U.S. Holder actually converts the payment into U.S. dollars will be treated as ordinary income or loss. That currency exchange income or loss (if any) generally will be income or loss from U.S. sources for foreign tax credit limitation purposes.
To the extent that the amount of any distribution made by the Company on the Class A Ordinary Shares exceeds the Company’s current and accumulated earnings and profits for a taxable year (as determined under U.S. federal income tax principles), the distribution will first be treated as a tax-free return of capital, causing a reduction in the adjusted basis of the U.S. Holder’s the Class A Ordinary Shares, and to the extent the amount of the distribution exceeds the U.S. Holder’s tax basis, the excess will be taxed as capital gain recognized on a sale or exchange as described below under “— Sale, Exchange, Redemption or Other Taxable Disposition of the Company Securities.”
Sale, Exchange, Redemption or Other Taxable Disposition of the Company Securities
Subject to the discussion below under “— Passive Foreign Investment Company Status,” a U.S. Holder will generally recognize gain or loss on any sale, exchange, redemption, or other taxable disposition of the Class A Ordinary Shares and the Warrants in an amount equal to the difference between the amount realized on the disposition and such U.S. Holder’s adjusted tax basis in such the Class A Ordinary Shares or Warrants. Any gain or loss recognized by a U.S. Holder on a taxable disposition of the Class A Ordinary Shares or Warrants will generally be capital gain or loss and will be long-term capital gain or loss if the holder’s holding period in the Class A Ordinary Shares or Warrants exceeds one year at the time of the disposition. Preferential tax rates may apply to long-term capital gains of non-corporate U.S. Holders (including individuals). The deductibility of capital losses is subject to limitations. Any gain or loss recognized by a U.S. Holder on the sale or exchange of the Class A Ordinary Shares or the Warrants will generally be treated as U.S. source gain or loss.
Exercise or Lapse of a Warrant
Except as discussed below with respect to the cashless exercise of a Warrant, a U.S. Holder generally will not recognize gain or loss upon the acquisition of an ordinary share of the Company on the exercise of a Warrant for cash. A U.S. Holder’s tax basis in an ordinary share received upon exercise of the Warrant generally will be an amount equal to the sum of the U.S. Holder’s tax basis in the Warrant exchanged therefor and the exercise price. The U.S. Holder’s holding period for an ordinary share received upon exercise of the Warrant will begin on the date following the date of exercise (or possibly the date of exercise) of the Warrants and will not include the period during which the U.S. Holder held the Warrants. If a Warrant is allowed to lapse unexercised, a U.S. Holder generally will recognize a capital loss equal to such holder’s tax basis in the Warrant.
The tax consequences of a cashless exercise of a warrant are not clear under current tax law. A cashless exercise may be tax-free, either because the exercise is not a gain realization event or because the exercise is treated as a recapitalization for U.S. federal income tax purposes. In either tax-free situation, a U.S. Holder’s basis in the Class A Ordinary Shares received would equal the holder’s basis in the Warrant. If the cashless exercise were treated as not being a gain recognition event, a U.S. Holder’s holding period in the Class A Ordinary Shares would be treated as commencing on the date following the date of exercise (or possibly the date of exercise) of the Warrant. If the cashless exercise were treated as a recapitalization, the holding period of the Class A Ordinary Share would include the holding period of the Warrant.
It is also possible that a cashless exercise could be treated in part as a taxable exchange in which gain or loss would be recognized. In such event, a U.S. Holder would recognize gain or loss with respect to the portion of the exercised Warrants treated as surrendered to pay the exercise price of the Warrants (the “surrendered warrants”). The U.S. Holder would recognize capital gain or loss with respect to the surrendered warrants in an amount generally equal to the difference between (i) the fair market value of the Class A Ordinary Shares that would have been received with respect to the surrendered warrants in a regular exercise of the Warrants and (ii) the sum of the U.S. Holder’s tax basis in the surrendered warrants and the aggregate cash exercise price of such warrants (if they had been exercised in a regular exercise). In this case, a U.S. Holder’s tax basis in the Class A Ordinary Shares received would equal the U.S. Holder’s tax basis in the Warrants exercised plus (or minus) the gain (or loss) recognized with respect to the surrendered warrants. A U.S. Holder’s holding period for the Class A Ordinary Shares would commence on the date following the date of exercise (or possibly the date of exercise) of the Warrant.
Due to the absence of authority on the U.S. federal income tax treatment of a cashless exercise, there can be no assurance which, if any, of the alternative tax consequences and holding periods described above would be adopted by the IRS or a court of law. Accordingly, U.S. Holders should consult their tax advisors regarding the tax consequences of a cashless exercise.
Passive Foreign Investment Company Status
Certain adverse U.S. federal income tax consequences could apply to a U.S. Holder if the Company or any of its subsidiaries is treated as a PFIC for any taxable year during which the U.S. Holder holds the Company Securities. A non-U.S. corporation will be classified as a PFIC for any taxable year (a) if at least 75% of its gross income in a taxable year, including its pro rata share of the gross income of any entity in which it is considered to own at least 25% of the interest by value, is passive income, or (b) if at least 50% of its assets in a taxable year of the foreign corporation, ordinarily determined based on fair market value and averaged quarterly over the year, including its pro rata share of the assets of any entity in which it is considered to own at least 25% of the interest by value, are held for the production of, or produce, passive income. Passive income generally includes dividends, interest, rents and royalties (other than rents or royalties derived from the active conduct of a trade or business) and gains from the disposition of passive assets.
If the Company is not a PFIC in the 2024 taxable year, such U.S. Holder would likely recognize gain (but not loss if the Reincorporation Merger qualifies as a “reorganization”) upon the exchange of ASCA securities for The Company securities pursuant to the Reincorporation Merger. The gain (or loss) would be computed as described above under “— If the Reincorporation Merger Does Not Qualify as a Reorganization.” Any such gain recognized by such U.S. Holder on the exchange of ASCA securities for The Company securities would be allocated ratably over the U.S. Holder’s holding period for the ASCA securities. Such amounts allocated for the current taxable year and any taxable year prior to the first taxable year in which ASCA was a PFIC would be treated as ordinary income, and not as capital gain, in the U.S. Holder’s taxable year, and such amounts allocated to each other taxable year beginning with the year that ASCA became a PFIC would be taxed at the highest tax rate in effect for each year to which the gain was allocated, together with a special interest charge on the tax attributable to each such year.
Whether the Company is a PFIC for any taxable year is a factual determination that depends on, among other things, the composition of the Company’s income and assets, the market value of its assets, and potentially the composition of the income and assets of one or more of the Company’s subsidiaries and the market value of their assets in that year. Whether a Company subsidiary is a PFIC for any taxable year is likewise a factual determination that depends on, among other things, the composition of the subsidiary’s income and assets and the market value of such assets in that year. One or more changes in these factors may cause the Company and/or one or more of its subsidiaries to become a PFIC for a taxable year even though it has not been a PFIC for one or more prior taxable years. Whether the Company or a subsidiary is treated as a PFIC for U.S. federal income tax purposes is a factual determination that must be made annually at the close of each taxable year and, thus, is subject to significant uncertainty. Moreover, there can be no assurance that the Company will timely provide a PFIC annual information statement for 2024 or going forward. The failure to provide such information on an annual basis could preclude U.S. Holders from making or maintaining a “qualified electing fund” election under Section 1295 of the Code.
If the Company were determined to be a PFIC for any taxable year (or portion thereof) that is included in the holding period of a U.S. Holder of Class A Ordinary Shares, the U.S. Holder did not make a valid “mark-to-market” election, such U.S. Holder generally will be subject to special rules with respect to:
| | ● | any gain recognized by the U.S. Holder on the sale or other disposition of the Company Securities (including a redemption treated as a sale or exchange); and |
| | ● | any “excess distribution” made to the U.S. Holder (generally, any distributions to such U.S. Holder during a taxable year of the U.S. Holder that are greater than 125% of the average annual distributions received by such U.S. Holder in respect of the Class A Ordinary Shares during the three preceding taxable years of such U.S. Holder or, if shorter, such U.S. Holder’s holding period for such ordinary shares). |
Under these rules:
| | ● | the U.S. Holder’s gain or excess distribution will be allocated ratably over the U.S. Holder’s Company Securities; |
| | ● | the amount allocated to the U.S. Holder’s taxable year in which the U.S. holder recognized gain or received the excess distribution, or to the period in the U.S. Holder’s holding period before the first day of the Company’s first taxable year in the Company is a PFIC, will be taxed as ordinary income; |
| | ● | the amount allocated to other taxable years (or portions thereof) of the U.S. Holder and included in its holding period will be taxed at the highest tax rate in effect for that year and applicable to the U.S. Holder; and |
| | ● | the interest charge generally applicable to underpayments of tax will be imposed in respect of the tax attributable to each such other taxable year of the U.S. Holder. |
Although a determination as to the Company’s PFIC status will be made annually, an initial determination that the Company is a PFIC will generally apply for subsequent years to a U.S. Holder who held Company Securities while the Company was a PFIC, whether or not the Company meets the test for PFIC status in those subsequent years.
If a U.S. Holder, at the close of its taxable year, owns shares in a PFIC that are treated as marketable stock, the U.S. Holder may make a mark-to-market election with respect to such shares for such taxable year. If the U.S. Holder makes a valid mark-to-market election for the first taxable year of the U.S. Holder in which the U.S. Holder holds (or is deemed to hold) the Class A Ordinary Shares and for which the Company is determined to be a PFIC, such holder generally will not be subject to the PFIC rules described above in respect to the Class A Ordinary Shares as long as such shares continue to be treated as marketable stock. Instead, in general, the U.S. Holder will include as ordinary income each year that the Company is treated as a PFIC the excess, if any, of the fair market value of its Class A Ordinary Shares at the end of its taxable year over the adjusted basis in its Class A Ordinary Shares. The U.S. Holder also will be allowed to take an ordinary loss in respect of the excess, if any, of the adjusted basis of its Class A Ordinary Shares over the fair market value of its Class A Ordinary Shares at the end of its taxable year (but only to the extent of the net amount of previously recognized income as a result of the mark-to-market election). The U.S. Holder’s adjusted tax basis in its Class A Ordinary Shares will be adjusted to reflect any such income or loss amounts, and any further gain recognized on a sale or other taxable disposition of the Class A Ordinary Shares in a taxable year in which the Company is treated as a PFIC will be treated as ordinary income. Special tax rules may also apply if a U.S. Holder makes a mark-to-market election for a taxable year after the first taxable year in which the U.S. Holder holds (or is deemed to hold) its Class A Ordinary Shares and for which the Company is treated as a PFIC. Currently, a mark-to-market election may not be made with respect to the Warrants.
The mark-to-market election is available only for stock that is regularly traded on a national securities exchange that is registered with the SEC, including Nasdaq (on which the Company Securities are traded), or on a foreign exchange or market that the IRS determines has rules sufficient to ensure that the market price represents a legitimate and sound fair market value. Such stock generally will be “regularly traded” for any calendar year during which such stock is traded, other than in de minimis quantities, on at least 15 days during each calendar quarter, but no assurances can be given in this regard with respect to the Class A Ordinary Shares. U.S. Holders should consult their own tax advisors regarding the availability and tax consequences of a mark-to-market election in respect of the Class A Ordinary Shares under their particular circumstances.
If the Company is a PFIC and, at any time, has a foreign subsidiary that is classified as a PFIC, U.S. Holders generally would be deemed to own a portion of the shares of such lower-tier PFIC, and generally could incur liability for the deferred tax and interest charge described above if the Company were to receive a distribution from, or dispose of all or part of the Company’s interest in, the lower-tier PFIC (even though such U.S. Holder would not receive the proceeds of those distributions or dispositions) or the U.S. Holders otherwise were deemed to have disposed of an interest in the lower-tier PFIC. A mark-to-market election generally would not be available with respect to such lower-tier PFIC. U.S. Holders are urged to consult their own tax advisors regarding the tax issues raised by lower-tier PFICs.
A U.S. Holder that owns (or is deemed to own) shares in a PFIC during any taxable year of the U.S. Holder, may have to file an IRS Form 8621 (whether or not a mark-to-market election is or has been made) with such U.S. Holder’s U.S. federal income tax return and provide any such other information as may be required by the U.S. Treasury Department. Failure to do so, if required, will extend the statute of limitations until such required information is furnished to the IRS.
The rules dealing with PFICs and mark-to-market elections are very complex and are affected by various factors in addition to those described above. Accordingly, U.S. Holders of Company Securities should consult their own tax advisors concerning the application of the PFIC rules to the Company Securities under the U.S. Holders’ particular circumstances.
Information Reporting and Backup Withholding
In general, information reporting requirements may apply to dividends received by U.S. Holders of the Class A Ordinary Shares (including constructive dividends), and the proceeds received on sale or other taxable disposition of the Class A Ordinary Shares or Warrants effected within the United States (and, in certain cases, outside the United States), in each case, other than U.S. Holders that are exempt recipients (such as corporations). Backup withholding (currently at a rate of 24%) may apply to such amounts if the U.S. Holder fails to provide an accurate taxpayer identification number (generally on an IRS Form W-9 provided to the paying agent or the U.S. Holder’s broker) or is otherwise subject to backup withholding.
Certain U.S. Holders holding specified foreign financial assets with an aggregate value in excess of the applicable dollar threshold are required to report information to the IRS relating to the Company Securities, subject to certain exceptions (including an exception for the Company Securities held in accounts maintained by U.S. financial institutions), by attaching a complete IRS Form 8938, Statement of Specified Foreign Financial Assets, with their tax return, for each year in which they hold the Company Securities. In addition to these requirements, U.S. Holders may be required to annually file FinCEN Report 114 (Report of Foreign Bank and Financial Accounts) with the U.S. Department of Treasury. U.S. Holders should consult their own tax advisors regarding information reporting requirements relating to their ownership of the Company Securities.
Backup withholding is not an additional tax. Any amounts withheld under the backup withholding rules may be allowed as a refund or credit against a holder’s U.S. federal income tax liability, if any, provided the required information is timely furnished to the IRS.
This summary does not contain a detailed description of all the United States federal income tax consequences that may be applicable to you in light of your particular circumstances and, except as set forth below with respect to PRC tax considerations, does not address the effects of any state, local or non-United States tax laws. If you are considering the purchase, ownership or disposition of our Ordinary Shares, you should consult your own tax advisors concerning the United States federal income tax consequences to you in light of your particular situation as well as any consequences arising under the laws of any other taxing jurisdiction.
LEGAL MATTERS
Certain legal matters as to U.S. federal securities law concerning this offering will be passed upon for us by Han Kun Law Offices, LLP. Certain legal matters as to BVI law will be passed upon for us by Ogier. Han Kun Law Offices LLP may rely upon Ogier with respect to matters governed by BVI law.
EXPERTS
The financial statements of NewGenIvf Limited as of December 31, 2023 and for the year then ended included in this prospectus have been so included in reliance on the report of Onestop Assurance PAC, an independent registered public accounting firm, given on the authority of said firm as an expert in auditing and accounting. The financial statements of NewGenIvf Limited as of December 31, 2022 and 2021 and for the years then ended included in this prospectus have been so included in reliance on the report of WWC, P.C., an independent registered public accounting firm, given on the authority of said firm as an expert in auditing and accounting.
EXPENSES
The following are the estimated expenses of the issuance and distribution of the securities being registered under the Registration Statement of which this prospectus forms a part, all of which will be paid by us. With the exception of the SEC registration fee, all amounts are estimates and may change:
| SEC registration fee | | $ | 4,593 | |
| Printer fees and expenses | | $ | 1,500 | * |
| Legal fees and expenses | | $ | 45,000 | * |
| Miscellaneous | | $ | 5,000 | * |
| Total | | $ | 56,093 | |
ENFORCEABILITY OF CIVIL LIABILITIES
We are incorporated under the laws of the British Virgin Islands with limited liability. We are incorporated in the British Virgin Islands because of certain benefits associated with being a British Virgin Islands company, such as political and economic stability, an effective judicial system, a favorable tax system, the absence of exchange control or currency restrictions and the availability of professional and support services. However, the British Virgin Islands has a less developed body of securities laws as compared to the United States and provides protections for investors to a lesser extent. In addition, British Virgin Islands companies may not have standing to sue before the federal courts of the United States.
Substantially all of our assets are located outside the United States. In addition, a majority of our directors and officers are nationals and/or residents of countries other than the United States, and all or a substantial portion of such persons’ assets are located outside the United States. As a result, it may be difficult for investors to effect service of process within the United States upon us or such persons or to enforce against them or against us, judgments obtained in United States courts, including judgments predicated upon the civil liability provisions of the securities laws of the United States or any state thereof.
We have appointed Cogency Global Inc., as our agent to receive service of process with respect to any action brought against us in the United States District Court for districts in the State of New York under the federal securities laws of the United States or of any State of the United States or any action brought against us in the Supreme Court of the State of New York under the securities laws of the State of New York.
The British Virgin Islands courts are unlikely:
| ● | to recognize or enforce against the Company, judgments of courts of the U.S. based on certain civil liability provisions of U.S. securities laws where that liability is in respect of penalties, taxes, fines or similar fiscal or revenue obligations of the company; and |
| ● | to impose liabilities against the Company, in original actions brought in the British Virgin Islands, based on certain civil liability provisions of U.S. securities laws that are penal in nature. |
There is no statutory enforcement in the British Virgin Islands of judgments obtained in the U.S., however, the courts of the British Virgin Islands will in certain circumstances recognize such a foreign judgment and treat it as a cause of action in itself which may be sued upon as a debt at common law so that no retrial of the issues would be necessary, provided that:
| | ● | the U.S. court issuing the judgment had jurisdiction in the matter and the company either submitted to such jurisdiction or was resident or carrying on business within such jurisdiction and was duly served with process; |
| | | |
| | ● | the judgment is final and for a liquidated sum; |
| | | |
| | ● | the judgment given by the U.S. court was not in respect of penalties, taxes, fines or similar fiscal or revenue obligations of the company; |
| | ● | in obtaining judgment there was no fraud on the part of the person in whose favor judgment was given or on the part of the court; |
| | ● | recognition or enforcement of the judgment in the British Virgin Islands would not be contrary to public policy; and |
| | | |
| | ● | the proceedings pursuant to which judgment was obtained were not contrary to natural justice. |
In appropriate circumstances, a British Virgin Islands Court may give effect in the British Virgin Islands to other kinds of final foreign judgments such as declaratory orders, orders for performance of contracts and injunctions.
WHERE YOU CAN FIND ADDITIONAL INFORMATION
We have filed with the SEC a registration statement on Form F-1 under the Securities Act relating to this registration of the Ordinary Shares to be sold by the Selling Shareholder. This prospectus, which is part of the Registration Statement, does not contain all of the information contained in the Registration Statement. The rules and regulations of the SEC allow us to omit certain information from this prospectus that is included in the Registration Statement. Statements made in this prospectus concerning the contents of any contract, agreement or other document are summaries of all material information about the documents summarized, but are not complete descriptions of all terms of these documents. If we filed any of these documents as an exhibit to the Registration Statement, you may read the document itself for a complete description of its terms.
We are subject to the periodic reporting and other information requirements of the Exchange Act as applicable to a “Foreign Private Issuer,” and we will file annual reports and other information from time to time with the SEC in accordance with such requirements. Our filings with the SEC are also available to the public through the SEC’s website at www.sec.gov.
As a foreign private issuer, we are exempt under the Exchange Act from, among other things, the rules prescribing the furnishing and content of proxy statements, and our officers, directors and principal shareholders are exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act. In addition, we will not be required under the Exchange Act to file periodic reports and financial statements with the SEC as frequently or as promptly as U.S. companies whose securities are registered under the Exchange Act.
We maintain a corporate website at www.newgenivf.com. Information contained on, or that can be accessed through, our website does not constitute a part of this prospectus. We have included our website address in this prospectus solely as an inactive textual reference. We will post on our website any materials required to be so posted on such website under applicable corporate or securities laws and regulations, including, posting any XBRL interactive financial data required to be filed with the SEC and any notices of general meetings of our shareholders.
40,000,000 Ordinary Shares

NewGenIvf Group Limited
PROSPECTUS
, 2024
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 6. Indemnification of Directors, Officers and Employees
British Virgin Islands law does not limit the extent to which a company’s memorandum and articles of association may provide for indemnification of officers and directors, except to the extent any such provision may be held by the British Virgin Islands courts to be contrary to public policy, such as to provide indemnification against willful default, civil fraud or the consequences of committing a crime. Under our Memorandum and Articles of Association, we may indemnify its directors, officers and liquidators against all expenses, including legal fees, and against all judgments, fines and amounts paid in settlement and reasonably incurred in connection with civil, criminal, administrative or investigative proceedings to which they are party or are threatened to be made a party by reason of their acting as our director, officer or liquidator. To be entitled to indemnification, these persons must have acted honestly and in good faith with a view to the best interest of the registrant and, in the case of criminal proceedings, they must have had no reasonable cause to believe their conduct was unlawful.
Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers or persons controlling the registrant pursuant to the foregoing provisions, the registrant has been informed that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable.
Item 7. Recent Sales of Unregistered Securities
Set forth below are the sales of all securities by the Company since the closing of the Business Combination which were not registered under the Securities Act. The Company believes that each of such issuances was exempt from registration under the Securities Act in reliance on Section 4(a)(2) of the Securities Act.
On April 3, 2024, the date of Closing, the Company issued to certain investors 295,000 Ordinary Shares which were converted from the ordinary shares of Legacy NewGenIvf pursuant to the terms of the Securities Purchase Agreement by and between A SPAC I Mini Acquisition Corp. and certain investors named therein (the “February Buyers”) on February 29, 2024. Pursuant to this Securities Purchase Agreement, A SPAC I Mini Acquisition Corp agreed to issue and sell to the February Buyers, in a private placement, an aggregate of up to $3,500,000 principal amount of convertible notes, consisting of one or more tranches: (i) an initial tranche of an aggregate principal amount of promissory notes of up to $1,750,000 and including an original issue discount of up to aggregate $122,500, and (ii) subsequent tranches of an aggregate principal amount of promissory notes of up to $1,750,000 and including an original issue discount of up to aggregate $122,500. The closing of the Initial Tranche took place on April 3, 2024. The Company also closed on a subsequent tranche of the aforementioned promissory notes in the principal amount of $250,000 by issuing and selling to the February Buyers shortly after the closing of the Business Combination, resulting in an aggregate principal amount of notes of $2,000,000 sold to the February Buyers. The form of these promissory notes is included as Exhibit 4.2 of the Form 6-K filed on April 4, 2024. The aforementioned Securities Purchase Agreement is included as Exhibit 4.1 of the Form 6-K filed on April 4, 2024.
2024 Debt Financing
On August 7, 2024, the Company entered into a Securities Purchase Agreement (“Securities Purchase Agreement”) with certain investors named therein (collectively, the “Buyers”), pursuant to which, amongst other things: (i) the Company agreed to sell, at an initial closing (and such initial closing, the “Initial Closing”), (a) a senior convertible note (the “Initial Note”) in the aggregate original principal amount not exceeding $1,100,000, convertible into Ordinary Shares pursuant to its terms, (b) a warrant to purchase 1,325,301 Ordinary Shares (such warrant, the “Series A Warrant”), and (c) a warrant to purchase 180,722 Ordinary Shares (the “Series B Warrant”); and (ii) the Company may require each Buyer (or each Buyer may require the Company, as applicable) to participate in the sale of (a) one or more additional convertible notes (which aggregate original principal amount for all additional convertible notes shall not exceed $9,500,000) (the “Additional Notes”) and (b) related Warrants. Subject to the satisfaction or waiver of the certain conditions to closing set forth in the Securities Purchase Agreement, the Company may require that the Buyers purchase (i) an Additional Note in the aggregate principal amount of $500,000 (the “First Mandatory Additional Note”) and (ii) an Additional Note in the aggregate principal amount of $1,500,000 (the “Second Mandatory Additional Note”).
In connection with the issuance and sale of the Notes, the Series A Warrants and the Series B Warrants, the Company entered into a registration rights agreement with the Buyers (the “Registration Rights Agreement”), pursuant to which the Company agrees to provide certain registration rights with respect to such securities. The Securities Purchase Agreement is filed as Exhibit 4.1 of the Company’s current report on Form 6-K dated August 16, 2024 and is incorporated by reference herein. The Initial Note is filed as Exhibit 10.25 of this registration statement.
Additionally, in connection with the Securities Purchase Agreement, the Company entered into amendment and exchange agreements with certain holders of its convertible promissory notes (the “Existing Notes” and each of such amendment and exchange agreements, an “Amendment and Exchange Agreement”), pursuant to which the Company exchanged the Existing Notes by issuing, among other things, (i) senior convertible notes in the aggregate principal amount of $2,700,000 (the “Exchange Notes”, and, together with the Initial Note and the Additional Notes, the “Notes”) and (b) a series of warrants to initially acquire up to a certain number of Ordinary Shares to the holders of the Existing Notes set forth therein (the “Exchange Warrants” and the Series A Warrants and the Series B Warrants, the “Warrants”). The Exchange Notes are in substantially similar form to the Initial Notes, which are discussed further below.
The Exchange Warrants expire nine years after the issuance date and are initially exercisable for an aggregate of 3,253,012 Ordinary Shares, subject to adjustment as provided therein. The Exchange Warrants may be exercised at any time after the exchange date, subject to the Maximum Percentage (as defined below). The initial exercise price of the Exchange Warrants is $0.913 per share, subject to adjustment as provided therein. If at the time of exercise of the Exchange Warrants, there is no effective registration statement registering our Ordinary Shares underlying such warrants, such warrants may be exercised on a cashless basis pursuant to their terms. The form of the Amendment and Exchange Agreement, the form of Exchange Notes, and the form of Exchange Warrants are filed as Exhibits 4.2, 4.3, and 4.4 to the Form 6-K dated August 16, 2024, and are incorporated herein by reference.
A holder may not convert any portion of the Notes and/or exercise any portion of the Warrants to the extent that, after giving effect to such conversion and/or exercise, as applicable, such holder (together with certain related parties) would beneficially own in excess of 4.99%, or the “Maximum Percentage”, of our Ordinary Shares outstanding immediately after giving effect to such conversion and/or exercise, as applicable. The Maximum Percentage may be raised or lowered to any other percentage not in excess of 9.99%, at the option of the holder, except that any increase will only be effective upon 61 days’ prior notice to us.
Initial Closing
On August 12, 2024, the Company and the Buyers consummated the Initial Closing. The Initial Note sold in connection with the Securities Purchase Agreement bears an interest rate of 14.75% per annum and is convertible into the Company’s Ordinary Shares as follows: the Conversion Amount (as defined below) into validly issued, fully paid and non-assessable Ordinary Shares at the Conversion Rate (as defined below); subject to adjustment as provided therein. No fractional Ordinary Shares are issuable upon any such conversion. A holder may convert the Initial Notes at any time after the initial issuance date of such Initial Note; subject to the Maximum Percentage.
The form of the Initial Note is included as Exhibit A of the Securities Purchase Agreement filed as Exhibit 4.1 in the Form 6-K dated August 15, 2024.
“Conversion Amount” means 110% of the sum of (A) the portion of the principal of the Initial Note to be converted, redeemed or otherwise with respect to which such determination is being made, (B) accrued and unpaid interest with respect to such aggregate principal amount owed on the Initial Note (and as reduced pursuant to redemption, conversion or otherwise, the “Principal”), (C) the Make-Whole Amount (as defined below), if any, (D) accrued and unpaid Late Charges (as defined below) with respect to the Principal on the Initial Note, Make-Whole Amount and Interest, and (E) any other unpaid amounts pursuant to the Transaction Documents (as defined in the Securities Purchase Agreement), if any.
“Conversion Rate” means the amount of Ordinary Shares issuable upon conversion of any Conversion Amount pursuant to the Initial Note determined by dividing (x) such Conversion Amount by (y) the Conversion Price.
“Conversion Price” means, as of any Conversion Date or other date of determination, $0.83, subject to adjustment as provided in the Initial Note.
“Late Charge” means a late charge incurred and payable by the Company in an amount equal to interest on such amount at the rate of eighteen percent (18%) per annum from the date such amount was due until the same is paid in full.
“Make-Whole Amount” means, as of any given date and as applicable, in connection with any conversion, redemption or other repayment under the Initial Note, an amount equal to the amount of additional interest that would accrue under the Initial Note at the interest rate then in effect assuming for calculation purposes that the outstanding Principal of the Initial Note as of the Closing Date remained outstanding through and including the maturity date.
At the Initial Closing, the Company also sold to the Buyers Series A Warrants to purchase an aggregate 1,325,301 Ordinary Shares and Series B Warrants to purchase an aggregate 180,722 Ordinary Shares.
Series A Warrants.
The Series A Warrants expire nine years after the issuance date and are initially be exercisable for an aggregate of 1,325,301 Ordinary Shares (the “Series A Warrant Shares”), subject to adjustment as provided therein. If immediately after any Additional Closing Date (as defined in the Securities Purchase Agreement) (each an “Adjustment Date”), the aggregate number of Ordinary Shares then issuable upon exercise of the Series A Warrant is less than the Maximum Eligibility Number (as defined below) immediately after such Adjustment Date the aggregate number of Ordinary Shares issuable upon exercise of the Series A Warrant will automatically increase to such Maximum Eligibility Number (in each case, without regard to any limitations on exercise contained therein and without regard to any prior exercises of such Series A Warrant). The Series A Warrants may be exercised at any time after the issuance date, subject to the Maximum Percentage. The initial exercise price of the Series A Warrants is $0.913 per share, subject to adjustment as provided therein. If at the time of exercise of the Series A Warrants, there is no effective registration statement registering the shares of our Ordinary Shares underlying such warrants, such warrants may be exercised on a cashless basis pursuant to their terms.
“Maximum Eligibility Number” means as of any time of determination, the number of Series A Warrant Shares then in effect (subject to adjustment as provided therein), but shall automatically increase on each Additional Closing Date, by such aggregate number of Ordinary Shares equal to the Additional Share Amount (as defined below).
“Additional Share Amount” means, with respect to any given Additional Closing (as defined in the Securities Purchase Agreement), such aggregate number of Ordinary Shares equal to 100% of the quotient of (x) the sum of (I) the aggregate principal amount of Additional Notes purchased by such holder at such Additional Closing and (II) the Make-Whole Amount with respect to such Additional Notes purchased by such holder at such Additional Closing, divided by (y) the applicable Additional Share Price (as defined below).
“Additional Share Price” means the lower of (i) the exercise price then in effect and (ii) (x) the lower of the Conversion Price as of such Additional Closing Date and (y) the VWAP of the Ordinary Shares as of the Trading Day immediately prior to such applicable Additional Closing Date
Series B Warrants
The Series B Warrants expire nine years after the issuance date and are initially be exercisable for an aggregate of 180,722 Ordinary Shares, subject to adjustment as provided therein. The Series B Warrants may be exercised at any time on or after February 12, 2025 (the “Initial Exercise Eligibility Date”), subject to the Maximum Percentage. If on the Initial Exercise Eligibility Date, the Ordinary Shares then issuable upon exercise of the Series B Warrants is not equal to the quotient of $150,000 divided by the Closing Bid Price (as defined therein) of the Ordinary Shares as of the Trading Day ended immediately prior to the Initial Exercise Eligibility Date (the “Adjusted Warrant Number”), on the Initial Exercise Eligibility Date the Ordinary Shares then issuable upon exercise of the Series B Warrant will automatically adjust to the Adjusted Warrant Number. The initial exercise price of the Series B Warrants were a prepaid, except for a nominal exercise price of $0.001 per share, subject to adjustment as provided therein.
The form of the Series A Warrant and form of the Series B Warrant are included as Exhibit B of the Securities Purchase Agreement included as Exhibit 4.1 in the Form 6-K filed on August 16, 2024, which is incorporate herein by reference.
Second Tranche
On August 28, 2024, the Company closed on the second tranche of the 2024 Debt Financing pursuant to the terms of the Securities Purchase Agreement. Under the second tranche, the Company sold the First Mandatory Additional Note in the aggregate principal amount of $500,000. The First Mandatory Additional Note is in substantially similar form to the Initial Note. Upon issuance of the First Mandatory Additional Note, the number of Ordinary Shares issuable upon exercise of the Series A Warrant automatically increased to 2,172,959. The First Mandatory Additional Note is included as Exhibit 4.1 in the Form 6-K filed on August 30, 2024.
The Company may receive up to approximately $29,483,257 in additional net proceeds from the 2024 Debt Financing if (i) all remaining Additional Notes in the aggregate principal amount of $9,000,000 (the “Remaining Additional Notes”) issuable pursuant to the Securities Purchase Agreement are sold and (ii) the Series A Warrants are increased by 17,698,976 assuming that (A) all Remaining Additional Notes are sold and (B) an Additional Share Price is equal to $0.509 and (iii) all of the Warrants are exercised in full, based on the following assumptions: (w) approximately $8,370,000 in net proceeds are received from the sale of Remaining Additional Notes, assuming that all Remaining Additional Notes are sold, (x) approximately $18,143,076 in net proceeds are received from the exercise of the Series A Warrants, assuming that the Series A Warrants are exercised in full at an exercise price of $0.913 and the number of Ordinary Shares issuable upon exercise of the Series A Warrants equals 19,871,935, (y) approximately $181 in net proceeds are received from the exercise of the Series B Warrants, assuming that the Series B Warrants are exercised in full at an exercise price of $0.001 per share, and (z) approximately $2,970,000 in net proceeds are received from the exercise of the Exchange Warrants, assuming that the Exchange Warrants are exercised in full at an exercise price of $0.913. The foregoing is subject to adjustment as set forth in the respective Notes and Warrants, as applicable.
Financial Statement Schedules:
All financial statement schedules have been omitted because either they are not required, are not applicable or the information required therein is otherwise set forth in the Company’s financial statements and related notes thereto.
Item 8. Exhibits and Financial Statement Schedules
| Exhibit No. | | Description |
| 3.1 | | Amended and Restated Memorandum and Articles of Association of PubCo (incorporated by reference to Annex B of PubCo’s registration statement on Form F-4 (File No. 333-275208), filed with the Securities and Exchange Commission on October 27, 2023) |
| 3.2 | | Amended and Restated Memorandum and Articles of Association of the Company dated September 23, 2024 (incorporated by reference to the Exhibit 3.2 of the Company’s registration statement on Form F-1 (File No. 333-281964), filed with the Securities and Exchange Commission on September 30, 2024). |
| 4.1 | | Specimen Class A Ordinary Share Certificate of the Company (incorporated by reference to Exhibit 2.1 of the report on Form 20-F filed with the Securities and Exchange Commission on April 9, 2024) |
| 4.2 | | Specimen Warrant Certificate of the Company (incorporated by reference to Exhibit 2.2 of the report on Form 20-F filed with the Securities and Exchange Commission on April 9, 2024) |
| 4.3 | | Warrant Agreement, dated February 14, 2022, by and between ASCA and Continental Stock Transfer & Trust Company (incorporated by reference to Exhibit 4.2 to ASCA’s Current Report on Form 8-K filed with the Securities and Exchange Commission on February 18, 2022) |
| 4.4 | | Form of Assumption of Warrant Agreement (incorporated by reference to Exhibit 4.7 to the Company’s registration statement on Form F-4 (File No. 333-275208), filed with the Securities and Exchange Commission on October 27, 2023) |
| 5.1* | | Opinion of Ogier |
| 10.1 | | Merger Agreement, dated as of February 15, 2023, by and among ASCA, NewGenIvf Limited, certain shareholders of NewGenIvf Limited, A SPAC I Mini Acquisition Corp., and A SPAC I Mini Sub Acquisition Corp. (incorporated by reference to Exhibit 2.1 to ASCA’s Current Report on Form 8-K filed with the Securities and Exchange Commission on February 16, 2023) |
| 10.2 | | First Amendment to the Merger Agreement, dated June 12, 2023, by and among ASCA, NewGenIvf Limited, Principal Shareholders, A SPAC I Mini Acquisition Corp. and A SPAC I Mini Sub Acquisition Corp. (incorporated by reference to Exhibit 2.1 to ASCA’s Current Report on Form 8-K filed with the Securities and Exchange Commission on June 13, 2023) |
| 10.3 | | Second Amendment to the Merger Agreement, dated December 6, 2023, by and among ASCA, NewGenIvf Limited, Principal Shareholders, A SPAC I Mini Acquisition Corp. and A SPAC I Mini Sub Acquisition Corp. (incorporated by reference to Exhibit 2.1 to ASCA’s Current Report on Form 8-K filed with the Securities and Exchange Commission on December 6, 2023) |
| 10.4 | | Third Amendment to the Merger Agreement, dated March 1, 2024, by and among ASCA, NewGenIvf Limited, Principal Shareholders, A SPAC I Mini Acquisition Corp. and A SPAC I Mini Sub Acquisition Corp. (incorporated by reference to Exhibit 2.1 to ASCA’s Current Report on Form 8-K filed with the Securities and Exchange Commission on March 6, 2024) |
| 10.5 | | Stock Escrow Agreement, dated February 14, 2022 by and between ASCA and Continental Stock Transfer & Trust Company (incorporated by reference to Exhibit 10.5 to ASCA’s Current Report on Form 8-K filed with the Securities and Exchange Commission on February 18, 2022) |
| 10.6 | | Voting and Support Agreement, dated as of February 15, 2023, by and among A SPAC I Acquisition Corp., A SPAC I Mini Acquisition Corp., NewGenIvf Limited, and certain shareholders of NewGenIvf Limited (incorporated by reference to Exhibit 10.1 to ASCA’s Current Report on Form 8-K filed with the Securities and Exchange Commission on February 16, 2023) |
| 10.7 | | Form of Amended and Restated Registration Rights Agreement (incorporated by reference to Exhibit 10.2 to ASCA’s Current Report on Form 8-K filed with the Securities and Exchange Commission on February 16, 2023) |
| 10.8 | | Form of Lock-Up Agreement (incorporated by reference to exhibit 4.8 of the Company’s report on Form 20-F filed with the SEC on April 9, 2024) |
| 10.9 | | Securities Purchase Agreement, dated February 29, 2024, by and among ASCA, The Company, Legacy NewGenIvf, the Buyers and Merger Sub (incorporated by reference to Exhibit 10.1 to ASCA’s Current Report on Form 8-K filed with the Securities and Exchange Commission on March 6, 2024) |
| 10.10 | | Form of Note between The Company and the Buyers (incorporated by reference to Exhibit 10.2 to ASCA’s Current Report on Form 8-K filed with the Securities and Exchange Commission on March 6, 2024) |
| 10.11 | | Acknowledgement Agreement, dated March 1, 2024, by and among ASCA, Legacy NewGenIvf and Chardan (incorporated by reference to Exhibit 10.3 to ASCA’s Current Report on Form 8-K filed with the Securities and Exchange Commission on March 6, 2024) |
| 10.12 | | Power Generator Lease Contract, dated January 10, 2021, between BD & H TECH Co., LTD. and First Fertility Phnom Penh Ltd (English Translation) (incorporated by reference to Exhibit 10.19 to the Company’s registration statement on Form F-4 (File No. 333-275208), filed with the Securities and Exchange Commission on October 27, 2023) |
| 10.13 | | Property Lease Contract, dated June 22, 2020, between SOK HEANG and First Fertility Phnom Penh Ltd (English Translation) (incorporated by reference to Exhibit 10.20 to the Company’s registration statement on Form F-4 (File No. 333-275208), filed with the Securities and Exchange Commission on October 27, 2023) |
| 10.14 | | MicroSort Lease and Services Agreement, dated March 29, 2019, between First Fertility Phnom Penh Ltd and MicroSort International (incorporated by reference to Exhibit 10.21 to the Company’s registration statement on Form F-4 (File No. 333-275208), filed with the Securities and Exchange Commission on October 27, 2023) |
| 10.15 | | Management and Administrative Services Agreement, dated November 1, 2022, between First Fertility PGS Center Ltd and Med Holdings Ltd (incorporated by reference to Exhibit 10.22 to the Company’s registration statement on Form F-4 (File No. 333-275208), filed with the Securities and Exchange Commission on October 27, 2023) |
| 10.16 | | MicroSort Lease and Services Agreement, dated April, 8, 2019, between First Fertility PGS Center Ltd. and MicroSort International (incorporated by reference to Exhibit 10.23 to the Company’s registration statement on Form F-4 (File No. 333-275208), filed with the Securities and Exchange Commission on October 27, 2023) |
| 10.17 | | Medical Consulting Service Agreement, dated January 1, 2021, between First Fertility PGS Center Ltd and First Fertility Phnom Penh Ltd (incorporated by reference to Exhibit 10.24 to the Company’s registration statement on Form F-4 (File No. 333-275208), filed with the Securities and Exchange Commission on October 27, 2023) |
| 10.18 | | Receivables Purchase Agreement, dated December, 28, 2022, between First Fertility PGS Center Ltd and Mr. Siu, Wing Fung Alfred (incorporated by reference to Exhibit 10.25 to the Company’s registration statement on Form F-4 (File No. 333-275208), filed with the Securities and Exchange Commission on October 27, 2023) |
| 10.19 | | Master Services Agreement, dated December 21, 2022, between First Fertility PGS Center Ltd and First Fertility Phnom Penh Ltd (incorporated by reference to Exhibit 10.26 to the Company’s registration statement on Form F-4 (File No. 333-275208), filed with the Securities and Exchange Commission on October 27, 2023) |
| 10.20 | | Form of Agreement for Storage of Embryos, Eggs, and Sperms Service between First Fertility PGS Center Ltd and Reproductive Expert Co Ltd (incorporated by reference to Exhibit 10.27 to the Company’s registration statement on Form F-4 (File No. 333-275208), filed with the Securities and Exchange Commission on October 27, 2023) |
| 10.21 | | Form of NewGenIvf Group Limited 2024 Share Incentive Plan (incorporated by reference to exhibit 4.21 of the Company’s report on Form 20-F filed with the SEC on April 9, 2024) |
| 10.22 | | Securities Purchase Agreement between A SPAC I Mini Acquisition Corp. and JAK Opportunities VI LLC dated February 29, 2024 (incorporated by reference to Exhibit 4.1 of the Company’s current report on Form 6-K filed with the SEC on April 4, 2024) |
| 10.23 | | Form of Note between A SPAC I Mini Acquisition Corp. and JAK Opportunities VI LLC dated February 29, 2024 (incorporated by reference to Exhibit 4.2 of the Company’s current report on Form 6-K filed with the SEC on April 4, 2024) |
| 10.24 | | Securities Purchase Agreement between the Company and certain buyers dated August 7, 2024 (incorporated by reference to Exhibit 4.1 of the Company’s current report on Form 6-K filed with the SEC on August 16, 2024) |
| 10.25 | | Form of Note between the Company and JAK Opportunities VI LLC dated August 7, 2024 (incorporated by reference to Exhibit 10.25 of the Company’s registration statement on Form F-1 (File No. 333-281964), filed with the Securities and Exchange Commission on September 6, 2024 |
| 10.26 | | Form of Note between the Company and JAK Opportunities VI LLC dated August 28, 2024 (incorporated by reference to Exhibit 4.1 of the Company’s current report on Form 6-K filed with the SEC on August 30, 2024) |
| 10.27* | | Common Stock Purchase Agreement between the Company and White Lion Capital, LLC dated November 21, 2024 |
| 10.28* | | Registration Rights Agreement between the Company and White Lion Capital, LLC dated November 21, 2024 |
| 16.1 | | Letter from WWC, P.C. regarding Item 16F of Form 20-F (incorporated by reference to Exhibit 16.1 of the Company’s annual report on Form 20-F filed with the SEC on August 20, 2024) |
| 21.1 | | List of Subsidiaries (incorporated by reference to Exhibit 8.1 of the Company’s annual report on Form 20-F filed with the SEC on August 20, 2024) |
| 23.1* | | Consent Letter from WWC, P.C. |
| 23.2* | | Consent Letter from Onestop Assurance PAC |
| 23.3* | | Consent of Ogier (included in Exhibit 5.1) |
| 24.1* | | Power of Attorney |
| 97.1 | | Clawback Policy of the Company. (incorporated by reference to Exhibit 97.1 of the Company’s annual report on Form 20-F filed with the SEC on August 20, 2024) |
| 101.INS* | | Inline XBRL Instance Document. |
| 101.SCH* | | Inline XBRL Taxonomy Extension Schema Document. |
| 101.CAL* | | Inline XBRL Taxonomy Extension Calculation Linkbase Document. |
| 101.DEF* | | Inline XBRL Taxonomy Extension Definition Linkbase Document. |
| 101.LAB* | | Inline XBRL Taxonomy Extension Label Linkbase Document. |
| 101.PRE* | | Inline XBRL Taxonomy Extension Presentation Linkbase Document. |
| 104* | | Cover Page Interactive Data File (formatted as Inline XBRL and contained in Exhibit 101) |
| 107* | | Fee Table |
Item 9. Undertakings
| (a) | The undersigned Registrant hereby undertakes: |
| (1) | To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement: |
| i. | To include any prospectus required by section 10(a)(3) of the Securities Act of 1933; |
| ii. | To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement; |
| iii. | To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement. |
| (2) | That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
| (3) | To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering. |
| (4) | To file a post-effective amendment to the registration statement to include any financial statements required by Item 8.A. of Form 20-F at the start of any delayed offering or throughout a continuous offering. Financial statements and information otherwise required by Section 10(a)(3) of the Act need not be furnished, provided that the registrant includes in the prospectus, by means of a post-effective amendment, financial statements required pursuant to this paragraph (a)(4) and other information necessary to ensure that all other information in the prospectus is at least as current as the date of those financial statements. Notwithstanding the foregoing, with respect to registration statements on Form F-3, a post-effective amendment need not be filed to include financial statements and information required by Section 10(a)(3) of the Act or Item 8.A of Form 20-F if such financial statements and information are contained in periodic reports filed with or furnished to the Commission by the registrant pursuant to section 13 or section 15(d) of the Securities Exchange Act of 1934 that are incorporated by reference in the Form F-3. |
| (5) | That, for the purpose of determining liability of the Registrant under the Securities Act of 1933 to any purchaser in the initial distribution of the securities: |
The undersigned Registrant undertakes that in a primary offering of securities of the undersigned Registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser:
| (i) | Any preliminary prospectus or prospectus of the undersigned Registrant relating to the offering required to be filed pursuant to Rule 424; |
| (ii) | Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned Registrant; |
| (iii) | The portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned Registrant; and |
| (iv) | Any other communication that is an offer in the offering made by the undersigned Registrant to the purchaser. |
| (b) | Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the U.S. Securities and Exchange Commission such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question of whether such indemnification by it is against public policy as expressed in the Act and will be governed by the final adjudication of such issue. |
| (c) | The undersigned Registrant hereby undertakes that: |
| i. | That for purposes of determining any liability under the Securities Act of 1933, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule 424(b)(1) or (4), or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective |
| ii. | That for the purpose of determining any liability under the Securities Act of 1933, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant certifies that it has reasonable grounds to believe that it meets all of the requirements for filing on Form F-1 and has duly caused this registration statement on Form F-1 to be signed on its behalf by the undersigned, thereunto duly authorized, on November 22, 2024.
| NEWGENIVF GROUP LIMITED | |
| | | |
| By: | /s/ Wing Fung Alfred Siu | |
| | Wing Fung Alfred Siu | |
| | Chief Executive Officer | |
POWER OF ATTORNEY
KNOW ALL MEN BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Wing Fung Alfred Siu his true and lawful attorney-in-fact, with full power of substitution and resubstitution for him and in his name, place and stead, in any and all capacities to sign any and all amendments including post-effective amendments to this registration statement, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, hereby ratifying and confirming all that said attorney-in-fact or his substitute, each acting alone, may lawfully do or cause to be done by virtue thereof.
Pursuant to the requirements of the Securities Act of 1933, this registration statement on Form F-1 has been signed by the following persons in the capacities and on the dates indicated.
| Signature | | Title | | Date |
| | | | | |
| /s/ Wing Fung Alfred Siu | | Chief Executive Officer and Director, | | November 22, 2024 |
| Wing Fung Alfred Siu | | (Principal Executive Officer) | | |
| | | | | |
| /s/ Hei Yue Tina Fong | | Director, Chief Marketing Officer | | November 22, 2024 |
| Hei Yue Tina Fong | | | | |
| | | | | |
| /s/ Hok Man Jefferson Au | | Independent Director | | November 22, 2024 |
| Hok Man Jefferson Au | | | | |
| | | | | |
| /s/ Yip Eng Jeremy Foo | | Independent Director | | November 22, 2024 |
| Yip Eng Jeremy Foo | | | | |
| | | | | |
| /s/ Ho Fai Chung | | Chief Financial Officer | | November 22, 2024 |
| Ho Fai Chung | | | | |
SIGNATURE OF AUTHORIZED REPRESENTATIVE IN THE UNITED STATES
Pursuant to the Securities Act of 1933, as amended, the undersigned, the duly authorized representative in the United States of America of NewGenIvf Group Limited, has signed this registration statement in New York, NY on November 22, 2024.
| | COGENCY GLOBAL INC. |
| | |
| | By: | /s/ Colleen A. De Vries |
| | Name: | Colleen A. De Vries |
| | Title: | Senior Vice President on behalf of Cogency Global Inc. |
NEWGENIVF LIMITED
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
| To: | The Board of Directors and Shareholders of NewGenIvf Limited |
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of NewGenIvf Limited and its subsidiaries (collectively the “Company”) as of December 31, 2021 and 2022, and the related consolidated statements of operations and comprehensive income (loss), changes in shareholders’ equity (deficit), and cash flows in each of the years for the two-year period ended December 31, 2022, and the related notes (collectively referred to as the financial statements). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2021 and 2022, and the results of its operations and its cash flows for each of the years in the two-year period ended December 31, 2022, in conformity with accounting principles generally accepted in the United States of America.
Restatement of Previously Issued Financial Statements
As discussed in Note 2, the Company has restated its consolidated financial statements as of December 31, 2021 and 2022, and for the years then ended.
Correction of errors in the classification of subscription receivable
The Company had previously erroneously presented subscription receivable as an asset; that classification was incorrect. According to Article 5-02.29 of Regulation S-X, subscription receivable should be presented as a deduction from equity rather than an asset. The Company has reassessed the classification of subscription receivable and has determined that it should be deducted from equity.
Recognition of directors’ remuneration for principal shareholders
The Company has previously recorded no directors’ remuneration to Mr. Siu Wing Fung, Alfred and Ms. Fong Hei Yue, Tina, who are concurrently directors and principal shareholders of the Company. The absent of cost recognition was incorrect. According to SAB Topics 1:B and 5.T., principal shareholders not receiving compensation for their time and effort serving as directors are making a capital contribution to the Company. The Company has reassessed the fair value of services rendered by these directors and has determined that it should be recorded as an operating expense and additional paid-in capital.
Emphasis of Matter — Going Concern
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As of December 31, 2021, the Company had a working capital deficit and shareholders’ deficit, accordingly, these factors gave rise to substantial doubt that the Company would continue as a going concern. As of December 31, 2022, the Company had an improvement in its capital position where the Company had net positive shareholders’ equity position, but the Company still had a working capital deficit; accordingly, the Company had not alleviated the substantial doubt that it would continue as a going concern. Management closely monitors the Company’s financial position and result of operations and has prepared a plan that includes raising additional capital and implementing improvements to increase profitability to address this substantial doubt. Details of this plan are also found in Note 1. These financial statements do not include any adjustments that might result from the outcome of this uncertainly.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ WWC, P.C.
WWC, P.C.
Certified Public Accountants
PCAOB ID No.1171
San Mateo, California
September 28, 2023
We have served as the Company’s auditor since 2022.
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
| To: | The Board of Directors and Shareholders of Newgenivf Limited |
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheet of Newgenivf Limited and its subsidiaries (collectively, the “Company”) as of December 31, 2023, the related consolidated statements of operations and comprehensive income, shareholders’ equity, and cash flows for the year ended December 31, 2023, and the related notes to the consolidated financial statements and schedule (collectively, the financial statements). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2023, and the results of its operations and its cash flows for the year ended December 31, 2023, in conformity with accounting principles generally accepted in the United States of America.
Material Uncertainty relating to Going Concern
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the financial statements, the Company had bank balance of $54,104 as of December 31, 2023 and for the year ended December 31, 2023, the Company had operating cash outflows of $1,766,135. This raises substantial doubt about its ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 1. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty. Our opinion is not modified in respect of this matter.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audit. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audit, we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audit included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audit also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audit provides a reasonable basis for our opinion.
/s/ Onestop Assurance PAC
We have served as the Company’s auditor since 2024.
Singapore
August 16, 2024
NEWGENIVF LIMITED
CONSOLIDATED BALANCE SHEETS AS OF DECEMBER 31, 2023 AND 2022
(Stated in US Dollars)
| | | 2023 | | | 2022 | |
| ASSETS | | | | | | | | |
| Current assets | | | | | | | | |
| Cash and cash equivalents | | $ | 54,104 | | | $ | 27,556 | |
| Accounts receivable, net | | | 9,374 | | | | 13,000 | |
| Inventories | | | 126,264 | | | | 46,910 | |
| Deposits, prepayment, other receivables and deferred IPO cost, net | | | 517,429 | | | | 70,285 | |
| Loan to A SPAC I | | | 140,000 | | | | — | |
| Due from shareholders | | | 354,285 | | | | 2,240,872 | |
| Total current assets | | | 1,201,456 | | | | 2,398,623 | |
| | | | | | | | | |
| Non-current assets | | | | | | | | |
| Plant and equipment, net | | | 162,157 | | | | 122,673 | |
| Right-of-use assets, net | | | 283,847 | | | | 383,670 | |
| Total non-current assets | | | 446,004 | | | | 506,343 | |
| | | | | | | | | |
| TOTAL ASSETS | | $ | 1,647,460 | | | $ | 2,904,966 | |
| | | | | | | | | |
| LIABILITIES AND SHAREHOLDERS’ EQUITY | | | | | | | | |
| Current liabilities | | | | | | | | |
| Accounts payable | | $ | 172,626 | | | $ | 104,651 | |
| Accrued liabilities and other payables | | | 241,613 | | | | 289,777 | |
| Contract liabilities | | | 7,937 | | | | 1,360,168 | |
| Due to a related party | | | — | | | | 110,773 | |
| Operating lease liabilities, current | | | 207,128 | | | | 184,651 | |
| Finance lease liabilities, current | | | 6,446 | | | | 18,758 | |
| Taxes payable | | | 486,706 | | | | 486,872 | |
| Total current liabilities | | | 1,122,456 | | | | 2,555,650 | |
| | | | | | | | | |
| Non-current liabilities | | | | | | | | |
| Operating lease liabilities, non-current | | | 118,979 | | | | 242,187 | |
| Finance lease liabilities, non-current | | | — | | | | 6,446 | |
| Total non-current liabilities | | | 118,979 | | | | 248,633 | |
| | | | | | | | | |
| Total liabilities | | $ | 1,241,435 | | | $ | 2,804,283 | |
| | | | | | | | | |
| Shareholders’ equity | | | | | | | | |
| Ordinary shares, $0.01 par value, 5,000,000 shares authorized; 698,123 and 601,830 shares issued and outstanding as of December 31, 2023 and 2022, respectively | | $ | 6,981 | | | $ | 6,018 | |
| Subscription receivable | | | (2,967,100 | ) | | | (319,872 | ) |
| Additional paid-in capital | | | 4,324,834 | | | | 1,458,941 | |
| Accumulated deficit | | | (461,351 | ) | | | (591,544 | ) |
| Accumulated other comprehensive (loss) income | | | (7,288 | ) | | | 9,570 | |
| Equity attributable to the shareholders of the Company | | | 896,076 | | | | 563,113 | |
| Non-controlling interests | | | (490,051 | ) | | | (462,430 | ) |
| Total shareholders’ equity | | | 406,025 | | | | 100,683 | |
| | | | | | | | | |
| TOTAL LIABILITIES AND SHAREHOLDERS’ EQUITY | | $ | 1,647,460 | | | $ | 2,904,966 | |
The accompanying notes are an integral part of these consolidated financial statements.
NEWGENIVF LIMITED
CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE INCOME (LOSS)
FOR THE YEARS ENDED DECEMBER 31, 2023, 2022 AND 2021
(Stated in US Dollars)
| | | 2023 | | | 2022 | | | 2021 | |
| Revenues | | $ | 5,136,153 | | | $ | 5,944,190 | | | $ | 4,118,120 | |
| Cost of revenues | | | (3,454,368 | ) | | | (4,406,421 | ) | | | (3,093,340 | ) |
| Gross profit | | | 1,681,785 | | | | 1,537,769 | | | | 1,024,780 | |
| | | | | | | | | | | | | |
| Operating expenses | | | | | | | | | | | | |
| Selling and marketing expenses | | | (18,030 | ) | | | (36,194 | ) | | | (24,693 | ) |
| General and administrative expenses | | | (1,259,364 | ) | | | (1,094,962 | ) | | | (801,329 | ) |
| Audit fees | | | (362,149 | ) | | | (7,908 | ) | | | - | |
| | | | | | | | | | | | | |
| Total operating expenses | | | (1,639,543 | ) | | | (1,139,064 | ) | | | (826,022 | ) |
| | | | | | | | | | | | | |
| Operating income | | | 42,242 | | | | 398,705 | | | | 198,758 | |
| | | | | | | | | | | | | |
| Other income (expenses), net | | | | | | | | | | | | |
| Other income, net | | | 111,837 | | | | 23,019 | | | | 45,652 | |
| Interest income | | | 518 | | | | 21 | | | | 63 | |
| Interest expense | | | (46,179 | ) | | | (77,757 | ) | | | (88,289 | ) |
| Total other income (expenses), net | | | 66,176 | | | | (54,717 | ) | | | (42,574 | ) |
| | | | | | | | | | | | | |
| Income before taxes | | | 108,418 | | | | 343,988 | | | | 156,184 | |
| Provision for income taxes | | | — | | | | (208,141 | ) | | | (294,716 | ) |
| Net income (loss) | | | 108,418 | | | | 135,847 | | | | (138,532 | ) |
| Less: net loss attributable to non-controlling interests | | | (21,775 | ) | | | (322,820 | ) | | | (137,999 | ) |
| Net income (loss) attributable to the shareholders of the Company | | $ | 130,193 | | | $ | 458,667 | | | | (533 | ) |
| | | | | | | | | | | | | |
| Other comprehensive income (loss) | | | | | | | | | | | | |
| Foreign currency translation adjustment | | | (22,704 | ) | | | (1,920 | ) | | | 7,751 | |
| Total comprehensive income (loss) | | | 85,714 | | | | 133,927 | | | | (130,781 | ) |
| Less: total comprehensive loss attributable to non-controlling interests | | | (27,621 | ) | | | (323,458 | ) | | | (136,396 | ) |
| Total comprehensive income attributable to the shareholders of the Company | | $ | 113,335 | | | $ | 457,385 | | | | 5,615 | |
| | | | | | | | | | | | | |
| Earning per share – basic and diluted | | $ | 0.18 | | | $ | 0.80 | | | | (0.00 | ) |
| Basic and diluted weighted average shares outstanding | | | 615,135 | | | | 575,930 | | | | 560,000 | |
The accompanying notes are an integral part of these consolidated financial statements.
NEWGENIVF LIMITED
CONSOLIDATED STATEMENTS OF CHANGES IN SHAREHOLDERS’ EQUITY (DEFICIT)
FOR THE YEARS ENDED DECEMBER 31, 2023, 2022 AND 2021
(Stated in US Dollars)
| | | Number of
shares | | | Ordinary
shares | | | Subscription
receivable | | | Additional
paid-in
capital | | | Accumulated
deficit | | | Accumulated
other
comprehensive
income/(loss) | | | Total
attributable
to the
shareholders
of the
Company | | | Non- controlling
interests | | | Total | |
| Balance, January 1, 2021 | | | 560,000 | | | $ | 5,600 | | | $ | — | | | $ | 57,821 | | | $ | (1,049,678 | ) | | $ | 4,704 | | | $ | (981,553 | ) | | $ | (2,576 | ) | | $ | (984,129 | ) |
| Net loss | | | — | | | | — | | | | — | | | | — | | | | (533 | ) | | | — | | | | (533 | ) | | | (137,999 | ) | | | (138,532 | ) |
| Foreign currency translation adjustment | | | — | | | | — | | | | — | | | | — | | | | — | | | | 6,148 | | | | 6,148 | | | | 1,603 | | | | 7,751 | |
| Directors’ remuneration | | | — | | | | — | | | | — | | | | 200,000 | | | | — | | | | — | | | | 200,000 | | | | — | | | | 200,000 | |
| Balance, December 31, 2021 | | | 560,000 | | | $ | 5,600 | | | $ | — | | | $ | 257,821 | | | $ | (1,050,211 | ) | | $ | 10,852 | | | $ | (775,938 | ) | | $ | (138,972 | ) | | $ | (914,910 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Balance, January 1, 2022 | | | 560,000 | | | $ | 5,600 | | | $ | — | | | $ | 257,821 | | | $ | (1,050,211 | ) | | $ | 10,852 | | | $ | (775,938 | ) | | $ | (138,972 | ) | | $ | (914,910 | ) |
| Net income (loss) | | | — | | | | — | | | | — | | | | — | | | | 458,667 | | | | — | | | | 458,667 | | | | (322,820 | ) | | | 135,847 | |
| Foreign currency translation adjustment | | | — | | | | — | | | | — | | | | — | | | | — | | | | (1,282 | ) | | | (1,282 | ) | | | (638 | ) | | | (1,920 | ) |
| Directors’ remuneration | | | — | | | | — | | | | — | | | | 240,000 | | | | — | | | | — | | | | 240,000 | | | | — | | | | 240,000 | |
| Issuance of shares | | | 41,830 | | | | 418 | | | | (319,872 | ) | | | 961,120 | | | | — | | | | — | | | | 641,666 | | | | — | | | | 641,666 | |
| Balance, December 31, 2022 | | | 601,830 | | | $ | 6,018 | | | $ | (319,872 | ) | | $ | 1,458,941 | | | $ | (591,544 | ) | | $ | 9,570 | | | $ | 563,113 | | | $ | (462,430 | ) | | $ | 100,683 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Balance, January 1, 2023 | | | 601,830 | | | $ | 6,018 | | | $ | (319,872 | ) | | $ | 1,458,941 | | | $ | (591,544 | ) | | $ | 9,570 | | | $ | 563,113 | | | $ | (462,430 | ) | | $ | 100,683 | |
| Net (loss) income | | | — | | | | — | | | | — | | | | — | | | | 130,193 | | | | — | | | | 130,193 | | | | (21,775 | ) | | | 108,418 | |
| Foreign currency translation adjustment | | | — | | | | — | | | | — | | | | — | | | | — | | | | (16,858 | ) | | | (16,858 | ) | | | (5,846 | ) | | | (22,704 | ) |
| Settlement of subscription receivable | | | — | | | | — | | | | 219,628 | | | | — | | | | — | | | | — | | | | 219,628 | | | | — | | | | 219,628 | |
| Issuance of shares | | | 96,293 | | | | 963 | | | | (2,866,856 | ) | | | 2,865,893 | | | | — | | | | — | | | | — | | | | — | | | | — | |
| Balance, December 31, 2023 | | | 698,123 | | | $ | 6,981 | | | $ | (2,967,100 | ) | | $ | 4,324,834 | | | $ | (461,351 | ) | | $ | (7,288 | ) | | $ | 896,076 | | | $ | (490,051 | ) | | $ | 406,025 | |
The accompanying notes are an integral part of these consolidated financial statements.
NEWGENIVF LIMITED
CONSOLIDATED STATEMENTS OF CASH FLOWS
FOR THE YEARS ENDED DECEMBER 31, 2023, 2022 AND 2021
(Stated in US Dollars)
| | | 2023 | | | 2022 | | | 2021 | |
| | | | | | | | | | |
| CASH FLOWS FROM OPERATING ACTIVITIES | | | | | | | | | | | | |
| Net income (loss) | | $ | 108,418 | | | $ | 135,847 | | | $ | (138,532 | ) |
| Adjustments to reconcile net (loss) income to net cash provided by operating activities: | | | | | | | | | | | | |
| Depreciation of plant and equipment | | | 31,173 | | | | 100,533 | | | | 166,709 | |
| Amortization of right-of-use assets | | | 198,535 | | | | 203,411 | | | | 175,830 | |
| Loss on disposal of plant and equipment | | | — | | | | 114,013 | | | | — | |
| Provision of expected credit loss allowance | | | 625 | | | | 10,777 | | | | 6,717 | |
| Interest expense | | | 46,179 | | | | — | | | | — | |
| Waiver of related party balance | | | (88,151 | ) | | | — | | | | — | |
| Directors’ remuneration | | | — | | | | 240,000 | | | | 200,000 | |
| Legal and professional fee | | | 27,320 | | | | — | | | | — | |
| Provision for income taxes | | | — | | | | 208,141 | | | | — | |
| Changes in operating assets and liabilities: | | | | | | | | | | | | |
| Accounts receivable | | | 1,166 | | | | 129,922 | | | | 56,183 | |
| Inventories | | | (80,665 | ) | | | (7,219 | ) | | | 1,352 | |
| Deposit and other receivables, net | | | (448,266 | ) | | | (15,197 | ) | | | 10,987 | |
| Accounts payable | | | 71,362 | | | | 58,752 | | | | (60,989 | ) |
| Accrued liabilities and other payables | | | (51,167 | ) | | | 190,689 | | | | 79,853 | |
| Contract liabilities | | | (1,352,231 | ) | | | 548,010 | | | | 812,158 | |
| Operating lease liabilities | | | (230,433 | ) | | | (175,132 | ) | | | (148,677 | ) |
| Finance lease liabilities | | | — | | | | (19,476 | ) | | | (19,476 | ) |
| Tax paid | | | — | | | | (12,170 | ) | | | 290,887 | |
| Net cash (used in) provided by operating activities | | | (1,766,135 | ) | | | 1,710,901 | | | | 1,433,002 | |
| | | | | | | | | | | | | |
| CASH FLOWS FROM INVESTING ACTIVITIES | | | | | | | | | | | | |
| Purchase of plant and equipment | | | (69,848 | ) | | | (94,452 | ) | | | (16,575 | ) |
| Net cash used in investing activities | | | (69,848 | ) | | | (94,452 | ) | | | (16,575 | ) |
| | | | | | | | | | | | | |
| CASH FLOWS FROM FINANCING ACTIVITIES | | | | | | | | | | | | |
| Amount due from A SPAC I | | | (140,000 | ) | | | — | | | | — | |
| Finance lease | | | (9,317 | ) | | | (19,476 | ) | | | (17,221 | ) |
| Other borrowings, net | | | — | | | | 128,204 | | | | 512,821 | |
| Issuance of shares | | | 192,308 | | | | — | | | | — | |
| Interest paid | | | (24,704 | ) | | | — | | | | — | |
| Amount with related parties | | | 1,863,206 | | | | (1,742,509 | ) | | | (2,039,969 | ) |
| Net cash provided by (used in) financing activities | | | 1,881,493 | | | | (1,633,781 | ) | | | (1,544,369 | ) |
| | | | | | | | | | | | | |
| Net increase/(decrease) in cash and cash equivalents | | | 45,510 | | | | (17,332 | ) | | | (127,942 | ) |
| Effect of foreign currency translation on cash and cash equivalents | | | (18,962 | ) | | | 16,124 | | | | 50,514 | |
| Cash and cash equivalents, beginning of year | | | 27,556 | | | | 28,764 | | | | 106,192 | |
| Cash and cash equivalents, end of year | | $ | 54,104 | | | $ | 27,556 | | | | 28,764 | |
| | | | | | | | | | | | | |
| Supplementary cash flow information: | | | | | | | | | | | | |
| Taxes paid | | $ | - | | | $ | (12,170 | ) | | | (3,829 | ) |
| Interest paid | | $ | (24,704 | ) | | $ | (55,469 | ) | | | (65,582 | ) |
The accompanying notes are an integral part of these consolidated financial statements.
NEWGENIVF LIMITED
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2023, 2022 AND 2021
(Stated in US Dollars)
NOTE 1 — ORGANIZATION AND PRINCIPAL ACTIVITIES
NewGenIvf Limited (the “Company” or the “Group”) was incorporated under the laws of the Cayman Islands on January 16, 2019 as an investment holding company.
The following is an organization chart of the Company and its subsidiaries:

As of December 31, 2023, the Company’s subsidiaries are detailed in the table as follows:
| Name | | Background | | Ownership % | | Principal activity |
| FFPGS (HK) Limited | | ● A Hong Kong company ● Incorporated on December 19, 2019 | | 100% | | Marketing and administrative services |
| Well Image Limited | | ● A Hong Kong company ● Incorporated on July 11, 2008 | | 100% | | Investment holding |
| Med Holdings Limited (“Med Holdings”) (Note) | | ● A Thailand company ● Incorporated on January 21, 2015 | | 49%* | | Investment holding |
| First Fertility PGS Center Limited (“FFC”) (Note) | | ● A Thailand company ● Incorporated on March 6, 2014 | | 74% | | Provision of IVF treatment |
| First Fertility Phnom Penh Limited (“FFPP”) | | ● A Cambodia company ● Incorporated on August 10, 2015 | | 100% | | Provision of IVF treatment |
NEWGENIVF LIMITED
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2023, 2022 AND 2021
(Stated in US Dollars)
NOTE 1 — ORGANIZATION AND PRINCIPAL ACTIVITIES (cont.)
| Name | | Background | | Ownership % | | Principal activity |
| First Fertility Bishkek LLC (“FFB”) | | ● A Kyrgyzstan company ● Incorporated on October 11, 2019 | | 100% | | Provision surrogacy and ancillary caring services |
| * | Where less than 50% of the equity of an investee is held, the Company (through its subsidiaries) holds significantly more voting rights than any other vote holder or organized company of vote holders. An assessment has been made, taking into account all the factors relevant to the relationship with the investee, to ascertain control has been established and the investee should be consolidated as a subsidiary of the Company. |
Note:
According to the Foreign Business Act (the “FBA”), the majority shareholdings of limited company incorporated in Thailand is required to be owned by Thai nationals.
With reference to the capital structure and voting rights structure of ordinary shares and preference shares (the “Share Structure”) of Med Holdings and FFC, all the preference share capital is owned by a Thai national. The ordinary shares and preference shares have the same rights and status in all respects except for the distribution of profits by way of dividends with details as follow:
| (a) | Dividends from profits of Med Holdings and FFC shall be allocated to the holders of preference shares at a rate fixed from time to time by the board of directors prior to allocating to the holders of ordinary shares. In any event, such dividends to be allocated to the holders of preference shares shall not exceed 15% of the total amount of dividends declared from time to time; |
| (b) | After allocation of dividends as per (a) above, the rest of the dividends shall be distributed equally amongst the holders of ordinary shares according to their shareholding ratio; |
| (c) | The holders of preferred shares shall be entitled to dividends only in respect of the years for which the Company has declared a dividend payment, and there shall be no cumulative dividends; and |
| (d) | Dividends allocated to the holders of preferred shares in each year shall be limited at the rate as stated in (a) only. No additional dividends shall be paid to the holders of preferred shares. |
Based upon the management’s judgement on the Shares Structure, as the Company is able to exercise majority voting power in any board meeting, the Company accounts for Med Holdings and FFC as subsidiaries on the ground that the Company is able to control Med Holdings and FFC by exercising its majority voting power in any board meetings.
NEWGENIVF LIMITED
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2023, 2022 AND 2021
(Stated in US Dollars)
NOTE 1 — ORGANIZATION AND PRINCIPAL ACTIVITIES (cont.)
Group reorganization
Pursuant to a group reorganization (the “group reorganization”) to rationalize the structure of the Company and its subsidiary companies (herein collectively referred to as the “Group”) in preparation for the listing of its shares, the Company becomes the holding company of the Group on February 2, 2023. As the Group were under same control of the shareholders and their entire equity interests were also ultimately held by the shareholders immediately prior to the group reorganization, the consolidated statements of income and comprehensive income, consolidated statements of changes in shareholders’ equity and consolidated statements of cash flows are prepared as if the current group structure had been in existence throughout the three-year period ended December 31, 2023, or since the respective dates of incorporation/establishment of the relevant entity, where this is a shorter period.
The consolidated balance sheets as of December 31, 2023 and 2022 present the assets and liabilities of the aforementioned companies now comprising the Group which had been incorporated/established as of the relevant balance sheet date as if the current group structure had been in existence at those dates based on the same control aforementioned. The Company eliminates all significant intercompany balances and transactions in its consolidated financial statements.
The movement in the Company’s authorized share capital and the number of ordinary shares outstanding and issued in the Company are also detailed in Note 10.
Going concern
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As of December 31, 2023, the Company had bank balance of $54,104 and may have challenge to settle its obligations when payment become due. The Company is always closely monitoring the market opportunities and is currently in the process of exercising various fundraising projects with various potential investors to improve the Company’s cash flow position for its operation and short-term payables. One fundraising project was completed on April 3, 2024. As of April 4, 2024, the Company settled $2 million to any payment with respect to accounts payable, but not, directly or indirectly, for (i) except for expenses relating to the Business Combination, the satisfaction of any indebtedness of the Company or any of its Subsidiaries, (ii) the redemption or repurchase of any securities of the Company or any of its Subsidiaries, or (iii) the settlement of any outstanding litigation as at December 31, 2023. The Company secured funding subsequent to year-end with total of $2 million, and that the Company received $2 million funding to date. Please refer to Note 20 – Subsequent Events for further information. The Company can make no assurance that required financings will be available for the amounts needed, or on terms commercially acceptable to the Company, if at all. If one or all of these events does not occur or subsequent capital raises are insufficient to bridge financial and liquidity shortfall, there would likely be a material adverse effect on the Company and its financial statements.
NOTE 2 — SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Principles of consolidation and basis of preparation
The accompanying consolidated financial statements reflect the accounts of the Company and all of its subsidiaries in which a controlling interest is maintained. All inter-company balances and transactions have been eliminated in consolidation.
Management has prepared the accompanying consolidated financial statements and these notes in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”). The Company maintains its general ledger and journals with the accrual method accounting.
NEWGENIVF LIMITED
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2023, 2022 AND 2021
(Stated in US Dollars)
NOTE 2 — SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.)
Use of estimates
The preparation of the consolidated financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosures of contingent assets and liabilities as of the date of the consolidated financial statements and the reported amounts of revenues and expenses during the periods presented. Significant estimates required to be made by management include, but are not limited to, contingent tax liability for Kyrgyzstan. Actual results could differ from those estimates, and as such, differences may be material to the consolidated financial statements.
Foreign currency translation
The accompanying consolidated financial statements are presented in United States dollar (“$”), which is the reporting currency of the Company. The functional currency of the Company and its subsidiaries, FFPGS (HK) Limited and Well Image Limited, are Hong Kong dollar (“HK$”). Med Holdings and FFC use Thai baht (“THB”) as their functional currencies. First Fertility Phnom Penh Limited uses Cambodian riel (“KHR”) as its functional currency and First Fertility Bishkek LLC uses United States dollar (“USD”) as its functional currency.
Assets and liabilities denominated in currencies other than the reporting currency are translated into the reporting currency at the rates of exchange prevailing at the balance sheet date. Translation gains and losses are recognized in the consolidated statements of operations and comprehensive income as other comprehensive income or loss.
Transactions in currencies other than the reporting currency are measured and recorded in the reporting currency at the exchange rate prevailing on the transaction date. The cumulative gain or loss from foreign currency transactions is reflected in the consolidated statements of operations and comprehensive income as other income (other expenses).
The value of foreign currencies including, the HK$, THB, KHR and RMB, may fluctuate against the United States dollar. Any significant variations of the aforementioned currencies relative to the United States dollar may materially affect the Company’s financial condition in terms of reporting in USD. The following table outlines the currency exchange rates that were used in preparing the accompanying consolidated financial statements:
| | | | | 2023 | | | 2022 | | | 2021 | |
| Period-end | | $: HK$ | | | 7.8000 | | | | 7.8000 | | | | 7.8000 | |
| Period average | | $: HK$ | | | 7.8000 | | | | 7.8000 | | | | 7.8000 | |
| Period-end | | $: THB | | | 34.2265 | | | | 34.6153 | | | | 33.1964 | |
| Period average | | $: THB | | | 34.7867 | | | | 35.1428 | | | | 32.1003 | |
| Period-end | | $: KHR | | | 4,080.0304 | | | | 4,114.3335 | | | | 4,068.9577 | |
| Period average | | $: KHR | | | 4,105.4181 | | | | 4,083.7043 | | | | 4,065.8164 | |
| Period-end | | $: RMB | | | 7.0971 | | | | 6.9091 | | | | 6.3551 | |
| Period average | | $: RMB | | | 7.0835 | | | | 6.4569 | | | | 6.4368 | |
NEWGENIVF LIMITED
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2023, 2022 AND 2021
(Stated in US Dollars)
NOTE 2 — SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.)
Cash and cash equivalents
Cash and cash equivalents include cash on hand, deposits held at call with financial institutions, other short-term deposits with original maturities of three months or less that are readily convertible to known amounts of cash and which are subject to an insignificant risk of changes in value.
Deposits, prepayment, other receivables and deferred IPO cost, net
Deposits, prepayment, other receivables and deferred Initial Public Offering (“IPO”) cost, net primarily include deposits paid to suppliers, prepaid expenses, the prepaid professional fee which meets the definition of deferred IPO cost, and other deposits.
Deferred IPO costs consist of underwriting, legal, accounting and other expenses incurred through the balance sheet date that are directly related to the Initial Public Offering and that were charged to shareholders’ equity upon the completion of the Initial Public Offering.
Plant and equipment, net
Plant and equipment are stated at cost less accumulated depreciation. Depreciation is provided over their estimated useful lives, using the straight-line method. The Company typically applies a salvage value of 0%. The estimated useful lives of the plan and equipment are as follows:
| Furniture and fixtures | | 3 – 5 years |
| Leasehold improvements | | the lesser of useful life or term of lease |
| Medical instruments | | 3 – 10 years |
| Motor vehicle | | 3 – 5 years |
| Office equipment | | 3 – 5 years |
The cost and related accumulated depreciation of assets sold or otherwise retired are eliminated from the accounts, and any gain or loss are included in the Company’s results of operations. The costs of maintenance and repairs are expensed as incurred. Significant renewals and betterments that extend the useful life of an assets are capitalized.
Impairment of long-lived assets
The Company evaluates the long-lived assets for impairment whenever events or changes in circumstances indicate that the carrying amount of assets may not be recoverable. Impairment may become obsolete from a difference in the industry, introduction of new technologies, or if the Company has inadequate working capital to utilize the long-lived assets to generate adequate profits. Impairment is present if the carrying amount of an asset is less than its expected future undiscounted cash flows.
If an asset is considered impaired, a loss is recognized based on the amount by which the carrying amount exceeds the fair market value of the asset. Assets to be disposed of are reported lower the carrying amount or fair value less cost to sell.
NEWGENIVF LIMITED
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2023, 2022 AND 2021
(Stated in US Dollars)
NOTE 2 — SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.)
Inventories
Inventories are stated at the lower of cost and net realizable value. Costs are determined on a first-in, first-out basis. Net realizable value is based on the estimated selling prices less any estimated costs to be incurred to completion and disposal. A provision for excess and obsolete inventory will be made based primarily on forecasts of product demand. The excess balance determined by this analysis becomes the basis for excess inventory charge and the written-down value of the inventory becomes its cost. Written-down inventory would not be reversed if market conditions improve.
Other borrowings
Other borrowings are recognized initially at fair value, net of debt issuance costs incurred. Other borrowings are subsequently stated at amortized cost; any difference between the proceeds (net of debt issuance costs) and the redemption value is recognized in the consolidated statements of operations over the period of the borrowings using the effective interest method.
Ordinary shares
The Company’s ordinary shares are stated at par value of $0.01 per ordinary share. The difference between the consideration received, net of issuance cost, and the par value is recorded in additional paid-in capital.
Revenue recognition
The Company adopted ASC Topic 606, Revenue from Contracts with Customers, and all subsequent ASUs that modified ASC 606 on April 1, 2017 using the full retrospective method which requires the Company to present the financial statements for all periods as if Topic 606 had been applied to all prior periods. The Company derives revenue principally from provision of In vitro fertilization (“IVF”) treatment and surrogacy and ancillary caring services. Revenue from contracts with customers is recognized using the following five steps:
| (1) | identify its contracts with customers; |
| (2) | identify its performance obligations under those contracts; |
| (3) | determine the transaction prices of those contracts; |
| (4) | allocate the transaction prices to its performance obligations in those contracts; and |
| (5) | recognize revenue when each performance obligation under those contracts is satisfied. Revenue is recognized when promised services are transferred to the client in an amount that reflects the consideration expected in exchange for those services. |
The Company enters into service agreements with its customers that outline the rights, responsibilities, and obligations of each party. The agreements also identify the scope of services, service fees, and payment terms. Agreements are acknowledged and signed by both parties. All the contracts have commercial substance, and it is probable that the Company will collect considerations from its customers for service component.
NEWGENIVF LIMITED
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2023, 2022 AND 2021
(Stated in US Dollars)
NOTE 2 — SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.)
Revenue recognition (cont.)
The Company derives its revenues from two sources: (1) revenue from IVF treatment, and (2) revenue from surrogacy and ancillary caring services.
Revenue from IVF treatment
In vitro fertilization (“IVF”) treatment is an assisted reproductive technique where eggs and sperm are collected and fertilized in laboratory to become embryo. Fertilized embryo is then implanted to the customer or a surrogate mother. IVF treatment involves the performance of a series of medical treatment and procedures that are not separately distinct and only brings benefits to customer when embryo is successfully implanted, therefore revenue from IVF treatment is recognized at a point in time when it is completed in clinic. The completion of this treatment is evidenced by a written IVF report indicating successful embryo implantation. The Company collects payment from customer in advance for IVF treatment. The amount of revenue recognized from contract liabilities to the Company’s result of operations can be found in Note 8 below.
Revenue from surrogacy and ancillary caring services
The Company provides surrogacy and ancillary caring services solely in Kyrgyzstan. Embryo from blood parents is implanted to surrogate mother contracted by the Company. During pregnancy period, the Company provides ancillary caring services including regular body check and provision of vitamins, supplements and medicines to surrogate mothers. The key performance obligation is identified as a single performance obligation where a baby is born, therefore revenue from surrogacy and ancillary caring services is recognized at a point in time when surrogate mother gives birth. The Company collects approximately 40% of contract sum upfront, and remaining contract sum is collected in installments across pregnancy period of surrogate mother. The amount of revenue recognized from contract liabilities to the Company’s result of operations can be found in Note 8 below.
Contract related assets and liabilities are classified as current assets and current liabilities. Significant balance sheet accounts related to the revenue cycle are as follows:
Account receivables, net
Accounts receivable, net are stated at the original amount less an allowance for expected credit loss on such receivables. The allowance for expected credit loss is estimated based upon the Company’s assessment of various factors including historical experience, the age of the accounts receivable balances, current general economic conditions, future expectations and customer specific quantitative and qualitative factors that may affect the Company’s customers’ ability to pay. An allowance is also made when there is objective evidence for the Company to reasonably estimate the amount of probable loss.
NEWGENIVF LIMITED
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2023, 2022 AND 2021
(Stated in US Dollars)
NOTE 2 — SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.)
Revenue recognition (cont.)
Contract liabilities
Contract liabilities represent considerations received from customers in advance of satisfying the Company’s performance obligations under the contract. These amounts are expected to be earned within 12 months and are classified as current liabilities.
Expected credit loss
ASU No. 2016-13, Financial Instruments — Credit Losses (Topic 326): Measurement of Credit Losses on Financial Instruments requires entities to use a current lifetime expected credit loss methodology to measure impairments of certain financial assets. Using this methodology will result in earlier recognition of losses than under the current incurred loss approach, which requires waiting to recognize a loss until it is probable of having been incurred. There are other provisions within the standard that affect how impairments of other financial assets may be recorded and presented, and that expand disclosures. Expected credit losses are probability-weighted estimates of credit losses. Credit losses are measured at the present value of all cash shortfalls (i.e., the difference between the cash flows due to the entity in accordance with the contract and the cash flows that the Company expects to receive). ECLs are discounted at the effective interest rate of the financial asset.
Retirement benefits
Retirement benefits in the form of mandatory government-sponsored defined contribution plans are charged to either expense as incurred or allocated to wages as part of cost of revenues.
Segment information
Operating segments are identified as components of an enterprise about which separate discrete financial information is available for evaluation by the chief operating decision maker (the “CODM”), or decision making group, in making decisions on how to allocate resources and assess performance. The Company operates and manages in one operating segment. The Company defines its CODM as Mr. Siu Wing Fund Alfred, the Company’s Chief Executive Officer. Since the Company operates in one operating segment, all required financial segment information can be found in the consolidated financial statements. The long-lived assets and revenue from external customers as of December 31, 2023, 2022 and 2021 by geographical area are presented in Note 13.
NEWGENIVF LIMITED
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2023, 2022 AND 2021
(Stated in US Dollars)
NOTE 2 — SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.)
Leases
The Company measured the lease in accordance to ASU 2016-02, “Leases” (Topic 842). Lease terms used to calculate the present value of lease payments generally do not include any options to extend, renew, or terminate the lease, as the Company does not have reasonable certainty at lease inception that these options will be exercised. The Company generally considers the economic life of its operating lease ROU assets to be comparable to the useful life of similar owned assets. The Company has elected the short-term lease exception, therefore operating lease ROU assets and liabilities do not include leases with a lease term of twelve months or less. Its leases generally do not provide a residual guarantee. The operating lease ROU asset also excludes lease incentives. Lease expense is recognized on a straight-line basis over the lease term.
As of December 31, 2023 and 2022, there were $283,847 and $383,670 million right of use (“ROU”) assets and $326,107 and $426,838 lease liabilities based on the present value of the future minimum rental payments of leases, respectively. The Company’s management believes that using an incremental borrowing rate of the minimum loan rate and the Hong Kong Dollar Best Lending Rate (“BLR”) minus 0.125% was the most indicative rate of the Company’s borrowing cost for the calculation of the present value of the lease payments; the rate used by the Company was 6.6% and 5.0% respectively.
Income Taxes
The Company recognizes deferred income tax assets or liabilities for expected future tax consequences of events recognized in the consolidated financial statements or tax returns. Under this method, deferred income tax assets and liabilities are determined based on the differences between the financial reporting and income tax bases of assets and liabilities and are measured using the income tax rates that will be in effect when the differences are expected to reverse. Valuation allowances are provided when it is more likely than not that a deferred tax asset is not realizable or recoverable in the future.
The Company determines that the tax position is more likely than not to be sustained and records the largest amount of benefit that is more likely than not to be realized when the tax position is settled. the Company recognizes interest and penalties, if any, related to uncertain tax positions in income tax expense.
Comprehensive Income
The Company presents comprehensive income in accordance with ASC Topic 220, Comprehensive Income. ASC Topic 220 states that all items that are required to be recognized under accounting standards as components of comprehensive income be reported in the consolidated financial statements. The components of comprehensive income were the net income for the years and the foreign currency translation adjustments.
NEWGENIVF LIMITED
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2023, 2022 AND 2021
(Stated in US Dollars)
NOTE 2 — SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.)
Earnings per share
The Company computes earnings per share (“EPS”) following ASC Topic 260, “Earnings per share”. Basic EPS is measured as the income or loss available to common shareholders divided by the weighted average common shares outstanding for the period. Diluted EPS presents the dilutive effect on a per-share basis from the potential conversion of convertible securities or the exercise of options and or warrants; the dilutive impacts of potentially convertible securities are calculated using the as-if method; the potentially dilutive effect of options or warranties are computed using the treasury stock method. Potentially anti-dilutive securities (i.e., those that increase income per share or decrease loss per share) are excluded from diluted EPS calculation. There were no potentially dilutive securities that were in-the-money that were outstanding during the years ended December 31, 2023, 2022 and 2021.
Related parties
The Company adopted ASC 850, Related Party Disclosures, for the identification of related parties and disclosure of related party transactions.
Commitments and contingencies
In the normal course of business, the Company is subject to contingencies, including legal proceedings and claims arising out of the business that relate to a wide range of matters, such as government investigations and tax matters. The Company recognizes its liability for such contingency if it determines it is probable that a loss has occurred and a reasonable estimate of the loss can be made. The Company may consider many factors in making these assessments including historical and the specific facts and circumstances of each matter.
Non-controlling interests
Non-controlling interests are presented as a separate component of equity on the consolidated balance sheets and net (loss) income and other comprehensive loss are attributed to controlling and non-controlling interests respectively.
Concentration of risks
Concentration of credit risk
Financial instruments that potentially expose us to concentrations of credit risk consist primarily of cash and cash equivalents and account receivable. The Company places cash and cash equivalents with financial institutions with high credit ratings and quality.
Accounts receivable primarily comprise of amounts receivable from the service customers. The Company conducts credit evaluations of customers, and generally does not require collateral or other security from its customers. The Company establishes an allowance for doubtful accounts primarily based upon the factors surrounding the credit risk of specific customers.
NEWGENIVF LIMITED
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2023, 2022 AND 2021
(Stated in US Dollars)
NOTE 2 — SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.)
Concentration of risks (cont.)
Concentration of customers
As of December 31, 2023 and 2022, two and Nil customers which individually contributed more than 10% of trade receivable, accounted for 96.3% and Nil of the Company’s trade receivable respectively.
None of the customers contributed more than 10% of revenue for years ended December 31, 2023, 2022 and 2021.
Concentration of suppliers
As of December 31, 2023 and 2022, one and four suppliers which individually contributed more than 10% of trade payable, accounted for 30.6% and 69.8% of the Company’s trade payable respectively.
For the year ended December 31, 2023, 2022 and 2021, Nil, two and two vendors which contributed more than 10% of total purchases of the Company, accounted for Nil, 55.3% and 35.6% of the Company’s total purchases respectively.
Financial instruments
The Company’s financial instruments, including cash and cash equivalents, accounts receivables, net, deposits, other receivables and deferred IPO cost, net, loan to A SPAC I, accounts payables, accrued liabilities and other payables, and due from (to) shareholders, have carrying amounts that approximate their fair values due to their short maturities. ASC Topic 820, “Fair Value Measurements and Disclosures” requires disclosing the fair value of financial instruments held by the Company. ASC Topic 825, “Financial Instruments” defines fair value and establishes a three-level valuation hierarchy for disclosures of fair value measurement that enhances disclosure requirements for fair value measures. The carrying amounts reported in the consolidated balance sheets for cash and cash equivalents, accounts and other receivables, accounts and other payables, accrued liabilities and amounts due from (to) related parties each qualify as financial instruments and are a reasonable estimate of their fair values because of the short period between the origination of such instruments and their expected realization and their current market rate of interest. The three levels of valuation hierarchy are defined as follows:
| ● | Level 1 — inputs to the valuation methodology used quoted prices for identical assets or liabilities in active markets. |
| ● | Level 2 — inputs to the valuation methodology include quoted prices for similar assets and liabilities in active markets and information that are observable for the asset or liability, either directly or indirectly, for substantially the financial instrument’s full term |
| ● | Level 3 — inputs to the valuation methodology are unobservable and significant to the fair value measurement. |
The Company analyzes all financial instruments with features of both liabilities and equity under ASC 480, “Distinguishing Liabilities from Equity” and ASC 815.
NEWGENIVF LIMITED
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2023, 2022 AND 2021
(Stated in US Dollars)
NOTE 2 — SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.)
Recent accounting pronouncements adopted
In April 2019, the FASB issued ASU 2019-04, Codification Improvements to Topic 326, Financial Instruments-Credit Losses, Topic 815, Derivatives and Hedging, and Topic 825, Financial Instruments, which amends and clarifies several provisions of Topic 326. In May 2019, the FASB issued ASU 2019-05, Financial Instruments-Credit Losses (Topic 326) Targeted Transition Relief, which amends Topic 326 to allow the fair value option to be elected for certain financial instruments upon adoption. ASU 2019-10 extended the effective date of ASU 2016-13 until December 15, 2022. This standard replaces the incurred loss methodology with an expected loss methodology that is referred to as the current expected credit loss (“CECL”) methodology. CECL requires an estimate of credit losses for the remaining estimated life of the financial asset using historical experience, current conditions, and reasonable and supportable forecasts and generally applies to financial assets measured at amortized cost, including loan receivables and held-to-maturity debt securities, and some off-balance sheet credit exposures such as unfunded commitments to extend credit. Financial assets measured at amortized cost will be presented at the net amount expected to be collected by using an allowance for expected credit losses. The Company already adopted the new standard and the Company recognizes the full impact of the new standard in these consolidated balance sheets and makes related disclosures.
Recent accounting pronouncements not yet adopted
In November 2023, the FASB issued ASU 2023-07, “Segment Reporting (Topic 280)” (“ASU 2023-07”). The amendments in ASU 2023-07 improve financial reporting by requiring disclosure of incremental segment information on an annual and interim basis for all public entities to enable investors to develop more decision useful financial analyses. Topic 280 requires a public entity to report a measure of segment profit or loss that the chief operating decision maker (CODM) uses to assess segment performance and make decisions about allocating resources. Topic 280 also requires other specified segment items and amounts, such as depreciation, amortization, and depletion expense, to be disclosed under certain circumstances. The amendments in ASU 202307 do not change or remove those disclosure requirements. The amendments in ASU 2023-07 also do not change how a public entity identifies its operating segments, aggregates those operating segments, or applies the quantitative thresholds to determine its reportable segments. The amendments in ASU 2023-07 are effective for years beginning after December 15, 2023 and interim periods within fiscal years beginning after December 15, 2024, adopted retrospectively. Management considers that the guidance does not have a significant impact on the disclosures set out in these consolidated financial statements.
In December 2023, FASB issued Accounting Standards Update (“ASU”) 2023-09, “Income Taxes (Topic 740)” (“ASU 2023-09”). The amendments in ASU 2023-09 address investor requests for more transparency about income tax information through improvements to income tax disclosures primarily related to the rate reconciliation and income taxes paid information. One of the amendments in ASU 2023-09 includes disclosure of, on an annual basis, a tabular rate reconciliation of (i) the reported income tax expense (or benefit) from continuing operations, to (ii) the product of the income (or loss) from continuing operations before income taxes and the applicable statutory federal income tax rate of the jurisdiction of domicile using specific categories, including separate disclosure for any reconciling items within certain categories that are equal to or greater than a specified quantitative threshold of 5%. ASU 2023-09 also requires disclosure of, on an annual basis, the year to date amount of income taxes paid (net of refunds received) disaggregated by federal, state, and foreign jurisdictions, including additional disaggregated information on income taxes paid (net of refunds received) to an individual jurisdiction equal to or greater than 5% of total income taxes paid (net of refunds received). The amendments in ASU2023-09 are effective for annual periods beginning after December 15, 2024, and should be applied prospectively. The Company is currently evaluating the impact of the update on the Company’s consolidated financial statements and related disclosures.
Save for elsewhere disclosed, the Company does not believe other recently issued but not yet effective accounting standards, if currently adopted, would have a material effect on the Company’s consolidated balance sheet, statement of operations and comprehensive income (loss) and statement of cash flows.
NEWGENIVF LIMITED
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2023, 2022 AND 2021
(Stated in US Dollars)
NOTE 3 — ACCOUNTS RECEIVABLE, NET
Accounts receivable, net consists of the following:
| | | December 31, | |
| | | 2023 | | | 2022 | |
| Accounts receivable | | $ | 9,393 | | | $ | 13,026 | |
| Less: allowance for expected credit loss | | | (19 | ) | | | (26 | ) |
| | | $ | 9,374 | | | $ | 13,000 | |
As of the end of each of the financial year, the aging analysis of accounts receivable, net of allowance for expected credit loss, based on the invoice date is as follows:
| | | December 31, | |
| | | 2023 | | | 2022 | |
| Within 90 days | | $ | 9,374 | | | $ | 13,000 | |
| | | $ | 9,374 | | | $ | 13,000 | |
The movement of allowances for expected credit loss is as follow:
| | | December 31, | |
| | | 2023 | | | 2022 | |
| Balance at beginning of the year | | $ | (26 | ) | | $ | (286 | ) |
| Reversal of expected credit losses | | | 7 | | | | 260 | |
| Ending balance | | $ | (19 | ) | | $ | (26 | ) |
NOTE 4 — INVENTORIES
Inventories consist of the following:
| | | December 31, | |
| | | 2023 | | | 2022 | |
| Medicines, consumables and reagents for clinical and laboratory analyses | | $ | 126,264 | | | $ | 46,910 | |
| | | $ | 126,264 | | | $ | 46,910 | |
NEWGENIVF LIMITED
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2023, 2022 AND 2021
(Stated in US Dollars)
NOTE 5 — DEPOSITS, PREPAYMENT, OTHER RECEIVABLES AND DEFERRED IPO COST, NET
Deposits, prepayment, other receivables and deferred IPO cost, net consist of the following:
| | | December 31, | |
| | | 2023 | | | 2022 | |
| | | | | | | |
| Other receivables | | $ | 15,910 | | | $ | 30,295 | |
| Deposits | | | 123,008 | | | | 40,131 | |
| Prepayment | | | 4,848 | | | | - | |
| Deferred initial public offering “IPO” cost | | | 373,677 | | | | - | |
| Less: allowance for expected credit loss | | | (14 | ) | | | (141 | ) |
| | | $ | 517,429 | | | $ | 70,285 | |
The movement of allowances for expected credit loss is as follow:
| | | December 31, | |
| | | 2023 | | | 2022 | |
| Balance at beginning of the year | | $ | (141 | ) | | $ | (115 | ) |
| Reversal of provision (Provision) | | | 127 | | | | (30 | ) |
| Effect of currency translation adjustment | | | - | | | | 4 | |
| Ending balance | | $ | (14 | ) | | $ | (141 | ) |
NEWGENIVF LIMITED
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2023, 2022 AND 2021
(Stated in US Dollars)
NOTE 6 — PLANT AND EQUIPMENT, NET
Plant and equipment, net consist of the following:
| | | December 31, | |
| | | 2023 | | | 2022 | |
| At cost: | | | | | | | | |
| Building improvement | | $ | 92,438 | | | $ | 72,519 | |
| Furniture and fixtures | | | 250,493 | | | | 246,682 | |
| Medical instruments | | | 844,809 | | | | 791,514 | |
| Motor vehicle | | | 142,936 | | | | 142,936 | |
| Office equipment | | | 150,688 | | | | 146,432 | |
| | | | 1,481,364 | | | | 1,400,083 | |
| Less: accumulated depreciation | | | (1,319,207 | ) | | | (1,277,410 | ) |
| Total | | $ | 162,157 | | | $ | 122,673 | |
Depreciation expenses for the years ended December 31, 2023 and 2022 were $31,173 and $100,533, respectively. Loss on disposal of assets for the year ended December 31, 2023 and 2022 was $Nil and $114,013, respectively, due to moving of clinic to new location in First Fertility PGS Center Limited in 2022.
No impairment loss was recorded for the years ended December 31, 2023, and 2022.
NEWGENIVF LIMITED
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2023, 2022 AND 2021
(Stated in US Dollars)
NOTE 7 — ACCRUED LIABILTIES AND OTHER PAYABLES
Accrued liabilities and other payables consist of the following:
| | | December 31, | |
| | | 2023 | | | 2022 | |
| Accrued expenses | | $ | 43,633 | | | $ | 22,345 | |
| Other tax payable | | | — | | | | 3,180 | |
| Withholding tax payable | | | 7,349 | | | | 82,240 | |
| Compensation payable (Note 1) | | | 144,015 | | | | 117,935 | |
| Other payables | | | 46,616 | | | | 64,077 | |
| | | $ | 241,613 | | | $ | 289,777 | |
| Note 1: | Compensation payable represented a claim relating to an employee of First Fertility PGS Center Limited (“FFC”). On April 23, 2023, the compensation agreement is finalized with the employee and the compensation is payable in 12 instalments within one year from 2023. |
NOTE 8 — CONTRACT LIABILITIES
Contract liabilities consist of the following:
| | | December 31, | |
| | | 2023 | | | 2022 | |
| Balance at beginning of year | | $ | 1,360,168 | | | $ | 812,158 | |
| Additions | | | 112,006 | | | | 1,360,168 | |
| Recognized to revenue during the year | | | (122,662 | ) | | | (812,158 | ) |
| Refund to customers (Note 1) | | | (1,341,575 | ) | | | - | |
| Balance at end of year | | $ | 7,937 | | | $ | 1,360,168 | |
| Note 1: | Refund of the deposits received from customer for services not rendered during 2023. China-based clients who prepaid for surrogacy and ancillary caring services requested refund of fees so such clients can appoint their own surrogate mothers in countries in which the Company does not conduct business. The Company sent the funds to accounts dictated by the clients and terminated service contract with those clients. |
NEWGENIVF LIMITED
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2023, 2022 AND 2021
(Stated in US Dollars)
NOTE 9 — LEASES
The Company has various operating leases for clinics and office spaces. The lease agreements do not specify an explicit interest rate. The Company’s management believes that the interest rate of 6.6% and 5% was the most indicative rate of the Company’s borrowing cost for the calculation of the present value of the lease payments.
As of December 31, 2023 and 2022, the right-of-use assets totaled $283,847, and $383,670, respectively.
As of December 31, 2023 and 2022, lease liabilities consist of the following:
| | | December 31, | |
| | | 2023 | | | 2022 | |
| Lease liabilities – current portion | | $ | 207,128 | | | $ | 184,651 | |
| Lease liabilities – non-current portion | | | 118,979 | | | | 242,187 | |
| Total | | $ | 326,107 | | | $ | 426,838 | |
Other lease information is as follows:
| | | December 31, | |
| | | 2023 | | | 2022 | |
| Weighted-average remaining lease term – operating leases | | | 0.92 years | | | | 1.91 years | |
| Weighted-average discount rate – operating leases | | | 5 | % | | | 5 | % |
| Short term lease cost | | $ | 114,937 | | | $ | 89,380 | |
The following is a schedule of future minimum payments under operating leases as of December 31, 2023:
| | | December 31,
2023 | |
| Not later than 1 year | | $ | 240,835 | |
| Between 1 to 2 years | | | 111,613 | |
| Between 2 to 3 years | | | 10,373 | |
| Total lease payments | | | 362,821 | |
| Less: imputed interest | | | (36,714 | ) |
| Total operating lease liabilities, net of interest | | $ | 326,107 | |
NEWGENIVF LIMITED
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2023, 2022 AND 2021
(Stated in US Dollars)
NOTE 10 — EQUITY
Ordinary shares
As at December 31, 2023, the Company is authorized to issue 5,000,000 ordinary shares. Each ordinary share is entitled to one vote. The holders of ordinary shares are also entitled to receive dividends whenever funds are legally available and when declared by the Board of Directors of the Company.
On April 3, 2024, the Company completed the business combination with A SPAC I Acquisition Corp.
The equity of the Company as of December 31, 2023 and 2022 represents 698,123 and 601,830 ordinary shares amounting to $6,981 and $6,018, respectively.
Subscription receivables
| | | December 31, | |
| | | 2023 | | | 2022 | |
| Balance at beginning of year | | $ | 319,872 | | | $ | — | |
| Issuance of shares (Note 1) | | | 2,866,856 | | | | 319,872 | |
| Settlement of subscription receivable (Note 2) | | | (219,628 | ) | | | — | |
| Total | | $ | 2,967,100 | | | $ | 319,872 | |
| Note 1: | On August 15, 2022, the Company issued and allotted additional 41,830 ordinary shares to Seazen Resources Investment Limited (“Seazen”) at the consideration of $961,538, of which other borrowings of $641,025 and $641 settlement was offset with consideration as partial settlement and $319,872 was subscription receivable due from Seazen. |
| Note 2: | On January 18, 2023, the Company received $192,308 from Seazen, reducing the subscription receivable by $192,308. On January 10, 2023, the Company issued and allotted additional 27,293 ordinary shares to Tung Donald Fan and Hok Lun Alan Lau at the consideration of $812,573. On December 4, 2023, the Company issued and allotted additional 69,000 shares to DoubleClick Services Limited at $2,054,283. Among the subscription receivable during the year, $27,320 was settled by the professional consulting service rendered during the year ended December 31, 2023. |
Additional paid-in capital
| | | December 31, | |
| | | 2023 | | | 2022 | |
| Balance at beginning of year | | $ | 1,458,941 | | | | 257,821 | |
| Directors’ remuneration (Note 1) | | | — | | | | 240,000 | |
| Issuance of shares (Note 2) | | | 2,865,893 | | | | 961,120 | |
| Total | | $ | 4,324,834 | | | | 1,458,941 | |
| Note 1: | The Company recorded remuneration to its directors, Mr. Siu, Wing Fung Alfred and Ms. Fong, Hei Yue Tina. The remuneration to Mr. Siu, Wing Fung Alfred and Ms. Fong, Hei Yue Tina was $120,000 and $120,000 for the year ended December 31, 2022, respectively. The directors considered remuneration as a capital injection rather than receiving it in cash, resulting in an $240,000 increase in paid-in capital. |
| Note 2: | On August 15, 2022, the Company issued 41,830 ordinary shares to Seazen, increasing the additional paid-in capital by $961,120. On January 10, 2023, the Company issued 27,293 ordinary shares to professional party for consulting service of 10 years, increasing the additional paid-in capital by $812,300. On December 4, 2023, the Company issued additional 69,000 shares to DoubleClick Services Limited for consulting service of 10 years, increasing the additional paid-in capital by $2,053,593. |
NOTE 11 — EMPLOYEE BENEFIT PLANS
HK SAR
The Company has a defined contribution pension scheme for its qualifying employees. The scheme assets are held under a provident fund managed by an independent fund manager. The Company and its employees are each required to make contributions to the scheme calculated at 5% of the employees’ basic salaries on monthly basis.
NEWGENIVF LIMITED
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2023, 2022 AND 2021
(Stated in US Dollars)
NOTE 11 — EMPLOYEE BENEFIT PLANS (cont.)
Thailand
The Company is obliged to make social security payments within the first 15 days of the month over which it is accrued. Special concession had been determined by the Government which saw the standard amount THB750 per month per person reduced to THB450 per month per person.
Cambodia
Every business employing one or more workers must register its business and workers with the National Social Security Fund (the “NSSF”) for the Occupational Risk Scheme (for work-related accidents and occupational diseases), the Health Care Scheme and the Pension Scheme.
Once registered, the business must pay to the NSSF:
| ● | A monthly contribution equivalent to 0.8% of each worker’s monthly average wages (between $0.40 and $2.40 per month per worker) for the Occupational Risk Scheme. |
| ● | A monthly contribution equivalent to 2.6% of a worker’s monthly average wages (between $1.30 and $7.80 per month per worker) for the Health Care Scheme. |
| ● | A monthly contribution to the compulsory Pension Scheme, which is jointly paid by the employer and the employee at the same rate of 2% (total of 4%) of the contributable wage for the first five years. The contributable wage for the Pension Scheme ranges from between KHR400,000 (approximately $100) up to KHR1,200,000 (approximately $300). |
Kyrgyzstan
The Company has a defined contribution pension scheme for its qualifying employees. The scheme assets are held under a provident fund managed by an independent fund manager. The Company and its employees are each required to make contributions to the scheme calculated at 15% and 8%, respectively of the employees’ basic salaries on monthly basis.
NOTE 12 — PROVISION FOR INCOME TAXES
Cayman Islands
NewGenIvf Limited was incorporated in the Cayman Islands and is not subject to tax on income or capital gains under current Cayman Islands law. In addition, upon payment of dividends by these entities to the shareholders, no Cayman Islands withholding tax will be imposed.
HK SAR
Under the two-tiered profits tax rates regime, Hong Kong tax residents are subject to Hong Kong Profits Tax in respect of profits arising in or derived from Hong Kong at 8.25% for the first HK$2 million of profits of the qualifying group entity, and profits above HK$2 million will be taxed at 16.5%. The profits of group entities not qualifying for the two-tiered profits tax rates regime will continue to be taxed at a flat rate of 16.5%.
Accordingly, the HK SAR profits tax is calculated at 8.25% on the first HK$2 million of the estimated assessable profits and at 16.5% on the remaining estimated assessable profits.
Thailand
The companies incorporated in Thailand are taxed on worldwide income. A company incorporated abroad is taxed on its profits arising from or in consequence of the business carried on in Thailand. The corporate income tax (CIT) rate is 20%. A foreign company not carrying on business in Thailand is subject to a final withholding tax (WHT) on certain types of assessable income (e.g. interest, dividends, royalties, rentals, and service fees) paid from or in Thailand. The rate of tax is generally 15%, except for dividends, which is 10%, while other rates may apply under the provisions of a double tax treaty (DTT).
NEWGENIVF LIMITED
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2023, 2022 AND 2021
(Stated in US Dollars)
NOTE 12 — PROVISION FOR INCOME TAXES (cont.)
Cambodia
The standard rate of corporate income tax (“CIT”) for companies and permanent establishments who are classified as medium and large taxpayers is 20%. For companies and permanent establishments who are classified as small taxpayers, the CIT rates are progressive rates from 0% to 20%. In view of the annual turnover of the company, the annual turnover ranges from KHR1 billion to KHR6 billion for service and commercial sectors, the company shall consider as the medium-sized company.
Kyrgyzstan
The company is subject to a corporate income tax on their aggregate annual income earned worldwide. Non-resident legal entities carrying out business activities through a permanent establishment in Kyrgyzstan are subject to profit tax on the income attributed to the activities of that permanent establishments.
Profit tax is calculated at a rate of 10% of aggregate annual income less allowed deductions.
Significant components of the provisions for income taxes for the year ended December 31, 2023, and 2022 were as follows:
| | | December 31, | |
| | | 2023 | | | 2022 | |
| Current tax provision Kyrgyzstan | | | — | | | | 196,116 | |
| Current tax provision Cambodia | | | — | | | | 11,323 | |
| Late penalty provision Kyrgyzstan | | | — | | | | 702 | |
| Total provision for income taxes | | $ | — | | | $ | 208,141 | |
NEWGENIVF LIMITED
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2023, 2022 AND 2021
(Stated in US Dollars)
NOTE 12 — PROVISION FOR INCOME TAXES (cont.)
| | | December 31, | |
| | | 2023 | | | 2022 | | | 2021 | |
| Income before taxes | | $ | 108,418 | | | $ | 343,988 | | | $ | 156,184 | |
| Tax expenses (credit) at the effective tax rates | | | 10,732 | | | | (124,591 | ) | | | 36,755 | |
| Tax effect on non-taxable income | | | (39,173 | ) | | | — | | | | — | |
| Tax effect on non-deductible expenses | | | — | | | | 369,101 | | | | 114,656 | |
| Tax effect on late penalty provision | | | — | | | | — | | | | 145,295 | |
| Change in valuation allowance | | | 28,441 | | | | — | | | | — | |
| Tax effect on utilization of tax losses | | | — | | | | (36,369 | ) | | | (1,990 | ) |
| Income taxes | | $ | — | | | | 208,141 | | | $ | 294,716 | |
Deferred tax asset, net
Significant components of deferred tax assets, net were as follows:
| | | December 31,
2023 | | | December 31,
2022 | |
| | | USD | | | USD | |
| Deferred tax assets: | | | | | | | | |
| – Net operating loss carry forward | | | 28,441 | | | | — | |
| Less: valuation allowance | | | (28,441 | ) | | | — | |
| Deferred tax assets, net | | | — | | | | — | |
As of December 31, 2023 and 2022, the Company had net operating loss carry forward of $164,721 and $297,207. The Company believes it is less likely than not that its operations will be able to fully utilize its deferred tax assets related to the net operating loss carry forward. As a result, the Company provided 100% allowance on deferred tax assets on net operating loss.
NOTE 13 — DISAGGREGATED REVENUES
The Company’s main business operations are to provide: (i) IVF treatment service; and (ii) surrogacy and ancillary caring services.
| | | For the year ended
December 31, | |
| Revenue from external customers | | 2023 | | | 2022 | | | 2021 | |
| IVF treatment service | | $ | 4,021,696 | | | $ | 2,819,163 | | | $ | 3,199,683 | |
| Surrogacy, ancillary caring and other services | | | 1,114,457 | | | | 3,125,027 | | | | 918,437 | |
| Total revenues | | $ | 5,136,153 | | | $ | 5,944,190 | | | $ | 4,118,120 | |
NEWGENIVF LIMITED
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2023, 2022 AND 2021
(Stated in US Dollars)
NOTE 13 — DISAGGREGATED REVENUES (cont.)
Geographical information
| | | December 31, | |
| Revenue from external customers originated from | | 2023 | | | 2022 | | | 2021 | |
| HK SAR | | $ | 34,038 | | | | — | | | $ | — | |
| Kyrgyzstan | | | 3,123,593 | | | | 5,060,973 | | | | 3,110,483 | |
| Cambodia | | | 621,619 | | | | 377,608 | | | | 313,737 | |
| Thailand | | | 1,356,903 | | | | 505,609 | | | | 693,900 | |
| Total revenues | | $ | 5,136,153 | | | | 5,944,190 | | | $ | 4,118,120 | |
The revenue information above is based on the locations where the revenue originated.
| | | December 31, | |
| Long-lived assets located at | | 2023 | | | 2022 | | | 2021 | |
| HK SAR | | $ | 584 | | | $ | — | | | | | |
| Kyrgyzstan | | | — | | | | 22,513 | | | | 20,835 | |
| Cambodia | | | 137,472 | | | | 229,085 | | | | 332,799 | |
| Thailand | | | 307,948 | | | | 254,745 | | | | 238,744 | |
| | | $ | 446,004 | | | $ | 506,343 | | | | 592,378 | |
The Company’s long-lived assets consist of plant and equipment, net and operating leases right-of-use assets, net.
NOTE 14 — RISKS
A. Credit risk
Accounts receivable
In order to minimize the credit risk, the management of the Company monitors and ensures that follow-up action is taken to recover overdue debts. The Company considers the probability of default upon initial recognition of asset and whether there has been a significant increase in credit risk on an ongoing basis throughout each reporting period. To assess whether there is a significant increase in credit risk, the Company compares the risk of a default occurring on the asset as at the reporting date with the risk of default as at the date of initial recognition. It considers available reasonable and supportive forward-looking information, such as GDP growth rate and nominal GDP per capita. Based on the impairment assessment performed by the Company, the directors consider the loss allowance for account receivables as of December 31, 2023 and 2022 is $19 and $26, respectively.
NEWGENIVF LIMITED
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2023, 2022 AND 2021
(Stated in US Dollars)
NOTE 14 — RISKS (cont.)
A. Credit risk (cont.)
Cash and cash equivalents
The credit risk on liquid funds is limited because the counterparties are banks with high credit ratings assigned by international credit-rating agencies. The Company is exposed to concentration of credit risk on liquid funds which are deposited with several banks with high credit ratings.
Deposits and other receivables, amount due from shareholders and loan to A SPAC I
The Company assessed the impairment for deposits and other receivables, due from shareholders and loan to A SPAC I individually based on internal credit rating and ageing of these debtors which, in the opinion of the directors, have no significant increase in credit risk since initial recognition. Based on the impairment assessment performed by the Company, the directors consider the loss allowance for deposits and other receivables, due from shareholders and loan to A SPAC I as of December 31, 2023 is $14, $17,818 and Nil, respectively. The loss allowance for deposits and other receivables, due from shareholders and loan to A SPAC I as of December 31, 2022 is $141, $17,059 and Nil, respectively. The loss allowance for deposits and other receivables and amount due from shareholders as of December 31, 2021 was $115 and $6,312 and Nil, respectively.
B. Interest risk
Cash flow interest rate risk
The Company is exposed to cash flow interest rate risk through the changes in interest rates related mainly to the Company’s variable-rates bank balances.
The Company currently does not have any interest rate hedging policy in relation to fair value interest rate risk and cash flow interest rate risk. The directors monitor the Company’s exposures on an ongoing basis and will consider hedging the interest rate should the need arises.
Sensitivity analysis
The sensitivity analysis below has been determined by assuming that a change in interest rates had occurred at the end of the reporting period and had been applied to the exposure to interest rates for financial instruments in existence at that date. 1% increase or decrease is used when reporting interest rate risk internally to key management personnel and represents management’s assessment of the reasonably possible change in interest rates.
If interest rates had been 1% higher or lower and all other variables were held constant, the Company’s net (loss) income for the years ended December 31, 2023, 2022 and 2021 would have increased or decreased by approximately $541, $275 and $287, respectively.
NEWGENIVF LIMITED
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2023, 2022 AND 2021
(Stated in US Dollars)
NOTE 14 — RISKS (cont.)
B. Interest risk (cont.)
Foreign currency risk
Foreign currency risk is the risk that the holding of foreign currency assets will affect the Company’s financial position as a result of a change in foreign currency exchange rates.
The Company’s monetary assets and liabilities are mainly denominated in HK$, THB, KHR and RMB which are the same as the functional currencies of the relevant group entities. Hence, in the opinion of the directors of the Company, the currency risk of US$ is considered insignificant. The Company currently does not have a foreign currency hedging policy to eliminate currency exposures. However, the directors monitor the related foreign currency exposure closely and will consider hedging significant foreign currency exposures should the need arise.
C. Economic and political risks
The Company’s operations are mainly conducted in Thailand, Cambodia and Kyrgyzstan. Accordingly, the Company’s business, financial condition, and results of operations may be influenced by changes in the political, economic, and legal environments in Thailand, Cambodia and Kyrgyzstan.
The Company’s operations in Thailand, Cambodia and Kyrgyzstan are subject to special considerations and significant risks. These include risks associated with, among others, the political, economic and legal environment and foreign currency exchange. The Company’s results may be adversely affected by changes in the political and social conditions in Thailand, Cambodia and Kyrgyzstan, and by changes in governmental policies with respect to laws and regulations, anti-inflationary measures, currency conversion, remittances abroad, and rates and methods of taxation, among other things.
D. Inflation risk
Management monitors changes in prices levels. Historically inflation has not materially impacted the Company’s consolidated financial statements; however, significant increases in the price of labor that cannot be passed to the Company’s customers could adversely impact the Company’s results of operations.
NEWGENIVF LIMITED
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2023, 2022 AND 2021
(Stated in US Dollars)
NOTE 15 — RELATED PARTY BALANCES AND TRANSACTIONS
The summary of amount due from and due to related parties as the following:
| | | | | December 31, | |
| | | Relationship | | 2023 | | | 2022 | |
| Due from shareholders consist of the following: | | | | | | | | |
| Mr. Siu Wing Fung, Alfred (“Mr. Siu”) and Ms. Fong Hei Yue, Tina (“Ms. Fong”) | | Shareholders and directors (note 1) | | $ | 354,285 | | | $ | 2,240,872 | |
| | | | | | | | | | | |
| Due to a related party consist of the following: | | | | | | | | | | |
| Harcourt Limited | | A related company (note 2) | | $ | - | | | $ | (110,773 | ) |
| (1) | Ms. Fong is the spouse of Mr. Siu. As of December 31, 2023 and 2022, the due from shareholders balance was $354,285 and $2,240,872, respectively. |
| (2) | The directors and shareholders of Harcourt Limited are Mr. Siu and Ms. Fong, Harcourt Limited therefore has the common ultimate beneficial owners with the Company. |
The balance due from shareholders consist of the following:
| | | December 31, | |
| | | 2023 | | | 2022 | |
| Due from shareholders | | $ | 372,103 | | | $ | 2,257,931 | |
| Less: allowance for expected credit loss | | | (17,818 | ) | | | (17,059 | ) |
| | | $ | 354,285 | | | $ | 2,240,872 | |
NEWGENIVF LIMITED
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2023, 2022 AND 2021
(Stated in US Dollars)
NOTE 15 — RELATED PARTY BALANCES AND TRANSACTIONS (cont.)
The movement of allowances for expected credit loss is as follow:
| | | December 31, | |
| | | 2023 | | | 2022 | |
| Balance at beginning of the year | | $ | (17,059 | ) | | $ | (6,312 | ) |
| Provision | | | (759 | ) | | | (10,747 | ) |
| Ending balance | | $ | (17,818 | ) | | $ | (17,059 | ) |
In addition to the transactions and balances detailed elsewhere in these consolidated financial statements, the Company had the following transactions with related parties:
| | | December 31, | |
| | | 2023 | | | 2022 | | | 2021 | |
| Directors’ remuneration to Mr. Siu Wing Fung, Alfred | | $ | 125,000 | | | $ | 120,000 | | | $ | 100,000 | |
| Directors’ remuneration to Ms. Fong Hei Yue, Tina | | | 125,000 | | | | 120,000 | | | | 100,000 | |
| Waiver of related party balance of Mr. Siu Wing Fung, Alfred | | | (88,151 | ) | | | — | | | | — | |
NOTE 16 — LOAN TO A SPAC I
On June 12, 2023, NewGenIvf Limited (the “Company”) and A SPAC I Acquisition Corp (“A SPAC I”) entered into a First Amendment to Merger Agreement, pursuant to which the Company agreed to provide non-interest bearing loans in an aggregate principal amount of up to $560,000 (the “Loan”) to A SPAC I to fund amounts required to further extend the period of time available for A SPAC I to consummate a business combination, and for working capital and payment of professional, administrative and operational expenses, and other purposes as mutually agreed by A SPAC I and the Company. The Loan will only become repayable upon the closing of the Acquisition Merger. As of December 31, 2023, $140,000 was outstanding under the loan. The Company completed the business combination with A SPAC I Acquisition Corp on April 3, 2024. After the combination, the balance of loan to A SPAC I was eliminated in the subsequent period.
NOTE 17 — IMPACT OF COVID-19
The COVID-19 has negatively impacted the global economy, disrupted consumer spending and global supply chains, and created significant volatility and disruption of financial markets. The Company experienced some resulting disruptions to the Company’s business operations, and the Company expected the COVID-19 pandemic could have a material adverse impact on the Company’s business and financial performance.
Due to the ongoing recession caused by the COVID-19, the Company’s business is likely to be adversely impacted. The effects of recession can also increase economic instability with vendors and customers.
NEWGENIVF LIMITED
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2023, 2022 AND 2021
(Stated in US Dollars)
NOTE 18 — CONTINGENCIES
As of December 31, 2023 and 2022, the Company was not a party to any legal or administrative proceedings.
First Fertility Bishkek LLC (“FFB”), the Company incorporated in Kyrgyzstan, did not report the current year tax to the tax authority till the reporting date since 2023. The late tax filing may lead to contingent tax penalty as of December 31, 2023. Since FFB had no profit for the year ended December 31, 2023, the tax department may not issue tax return at current tax position. The tax return is not yet filed so it is not possible to give the Company evaluation of the likelihood of the outcome or estimate the possible amount of tax penalty. The contingent tax penalty is reasonably possible and estimated at $486,706. Thus, no provision was made. Except the potential tax issue, the Company concludes that there was no contingent liability, either individually or in the aggregate, that could have resulted in an unfavorable outcome with a material adverse effect on the Company’s results of operations, consolidated financial condition, or cash flows.
NOTE 19 — segment information
The Company uses the management approach to determine reportable operating segments. The management approach considers the internal organization and reporting used by the Company’s CODM, specifically the Group’s CEO and CFO, for making decisions, allocating resources and assessing performance.
The Company does not distinguish revenues, costs and expenses between segments in its internal reporting, but instead reports costs and expenses by nature as a whole. Based on the management’s assessment, the Group determines that it has only one operating segment and therefore one reportable segment as defined by ASC 280. As such, all financial segment information required by the authoritative guidance can be found in these consolidated financial statements.
NOTE 20 — SUBSEQUENT EVENTS
The Company evaluated subsequent events and transactions that occurred after the balance sheet date up to the date that the financial statements were issued. Based upon this review, other than as described below, the Company did not identify any subsequent events that would have required adjustment or disclosure in the financial statements.
Convertible note
On February 29, 2024, A SPAC I Acquisition Corp. (“ASCA”), A SPAC I Mini Acquisition Corp. (the then name of NewGenIvf Group Limited), NewGenIvf Limited (“NewGenIvf”, the “Company”), A SPAC I Mini Sub Acquisition Corp. (the “Merger Sub”), and certain buyers named therein led by JAK Opportunities VI LLC (collectively, the “Buyers” or “JAK”) entered into a securities purchase agreement (the “Securities Purchase Agreement”), pursuant to which the NewGenIvf Group Limited agreed to issue and sell to JAK, in a private placement, an aggregate of up to $3,500,000 principal amount of convertible notes (the “Notes”), consisting of one or more tranches: (i) an initial tranche (the “Initial Tranche”) of an aggregate principal amount of Notes of up to $1,750,000 and including an original issue discount of up to aggregate $122,500, and (ii) subsequent tranches of an aggregate principal amount of Notes of up to $1,750,000 and including an original issue discount of up to aggregate $122,500.
On April 3, 2024, JAK received a certain amount of ordinary shares of the NewGenIvf Group Limited (the “Commitment Shares”), which were converted from the Company ordinary shares issued to JAK in February 2024 and equaled 295,000 ordinary shares of the NewGenIvf Group Limited, as well as an additional 100,000 ordinary shares of the NewGenIvf Group Limited, which were converted from the Company ordinary shares transferred by another shareholder of the Company to JAK in March 2024. In addition, a subsequent tranche of the Notes in the principal amount of $250,000 was issued and sold to JAK shortly after the closing of the Business Combination. As such, as of April 4, 2024, an aggregate principal amount of Notes of $2,000,000 were issued and sold to JAK.
Business combination
On April 3, 2024, the Company completed the business combination with A SPAC I Acquisition Corp. After the combination, the combined company will be named “NewGenIvf Group Limited” (“NewGenIvf Group”) and its shares and warrants are expected to begin trading on the Nasdaq Capital Market under the tickers “NIVF”, and “NIVFW”, respectively, on April 4, 2024.
NEWGENIVF GROUP LIMITED
UNAUDITED CONDENSED CONSOLIDATED BALANCE SHEETS
AS OF SEPTEMBER 30, 2024 AND 31 DECEMBER 2023
(Stated in US Dollars)
| | | 2024 | | | 2023 | |
| ASSETS | | | | | | |
| Current assets | | | | | | |
| Cash and cash equivalents | | $ | 169,661 | | | $ | 54,104 | |
| Accounts receivable, net | | | 318,859 | | | | 9,374 | |
| Inventories | | | 94,785 | | | | 126,264 | |
| Deposits, prepayment and other receivables, net | | | 394,254 | | | | 517,429 | |
| Loan to A SPAC I | | | — | | | | 140,000 | |
| Due from shareholders | | | — | | | | 354,285 | |
| Total current assets | | | 977,559 | | | | 1,201,456 | |
| | | | | | | | | |
| Non-current assets | | | | | | | | |
| Plant and equipment, net | | | 193,194 | | | | 162,157 | |
| Right-of-use assets, net | | | 293,168 | | | | 283,847 | |
| Intangible assets | | | 2,698 | | | | — | |
| Deposits, prepayment and other receivables, net | | | 6,047 | | | | — | |
| Total non-current assets | | | 495,107 | | | | 446,004 | |
| | | | | | | | | |
| TOTAL ASSETS | | $ | 1,472,666 | | | $ | 1,647,460 | |
| | | | | | | | | |
| LIABILITIES AND SHAREHOLDERS’ EQUITY | | | | | | | | |
| Current liabilities | | | | | | | | |
| Legal fee payable | | $ | 2,074,114 | | | $ | 172,626 | |
| Accrued liabilities and other payables | | | 398,208 | | | | 241,613 | |
| Contract liabilities | | | 8,400 | | | | 7,937 | |
| Due to a related party | | | 309,795 | | | | — | |
| Due to shareholders | | | — | | | | — | |
| Operating lease liabilities, current | | | 213,546 | | | | 207,128 | |
| Finance lease liabilities, current | | | — | | | | 6,446 | |
| Taxes payable | | | 486,705 | | | | 486,706 | |
| Total current liabilities | | | 3,490,768 | | | | 1,122,456 | |
| | | | | | | | | |
| Non-current liabilities | | | | | | | | |
| Operating lease liabilities, non-current | | | 122,981 | | | | 118,979 | |
| Promissory Note | | | 1,453,861 | | | | — | |
| Convertible promissory note | | | 4,300,000 | | | | — | |
| Discount on Convertible promissory note | | | (819,762 | ) | | | — | |
| Total non-current liabilities | | | 5,057,080 | | | | 118,979 | |
| | | | | | | | | |
| Total liabilities | | $ | 8,547,848 | | | $ | 1,241,435 | |
| | | | | | | | | |
| Shareholders’ equity | | | | | | | | |
| Ordinary shares, $0.01 par value, 100,000,000 and 5,000,000 shares authorized as of September 30, 2024 and December 31, 2023; 10,149,386 and 698,123 shares issued and outstanding as of September 30, 2024 and December 31, 2023, respectively | | $ | — | | | $ | 6,981 | |
| Subscription receivable | | | — | | | | (2,967,100 | ) |
| Additional paid-in capital | | | 1,415,000 | | | | 4,324,834 | |
| Accumulated deficit | | | (8,003,276 | ) | | | (461,351 | ) |
| Accumulated other comprehensive (loss) income | | | 3,370 | | | | (7,288 | ) |
| Equity attributable to the shareholders of the Company | | | (6,584,906 | ) | | | 896,076 | |
| Non-controlling interests | | | (490,276 | ) | | | (490,051 | ) |
| Total shareholders’ equity | | | (7,075,182 | ) | | | 406,025 | |
| | | | | | | | | |
| TOTAL LIABILITIES AND SHAREHOLDERS’ EQUITY | | $ | 1,472,666 | | | $ | 1,647,460 | |
The accompanying notes are an integral part of these consolidated financial statements.
NEWGENIVF GROUP LIMITED
UNAUDITED CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS AND
COMPREHENSIVE INCOME (LOSS)
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2024 AND 2023
(Stated in US Dollars)
| | | 2024 | | | 2023 | |
| Revenues | | $ | 4,159,763 | | | $ | 3,616,698 | |
| Cost of revenues | | | (2,872,004 | ) | | | (2,131,738 | ) |
| Gross profit | | | 1,287,759 | | | | 1,484,960 | |
| | | | | | | | | |
| Operating expenses | | | | | | | | |
| Selling and marketing expenses | | | (192,276 | ) | | | (11,540 | ) |
| General and administrative expenses | | | (1,166,858 | ) | | | (1,663,952 | ) |
| | | | | | | | | |
| Total operating expenses | | | (1,359,134 | ) | | | (1,675,492 | ) |
| | | | | | | | | |
| Operating income (loss) | | | (71,375 | ) | | | (190,532 | ) |
| | | | | | | | | |
| Other income (expenses), net | | | | | | | | |
| Other income, net | | | 8,404 | | | | 23,892 | |
| Interest income | | | 2,399 | | | | 396 | |
| Interest expense | | | (357,551 | ) | | | (20,922 | ) |
| Total other income (expenses), net | | | (346,748 | ) | | | 3,366 | |
| | | | | | | | | |
| Income (loss) before taxes | | | (418,123 | ) | | | (187,166 | ) |
| Provision for income taxes | | | — | | | | (83,742 | ) |
| Net income (loss) | | | (418,123 | ) | | | (270,908 | ) |
| Less: net loss attributable to non-controlling interests | | | (1,723 | ) | | | (65,476 | ) |
| Net income (loss) attributable to the shareholders of the Company | | $ | (416,400 | ) | | | (205,432 | ) |
| | | | | | | | | |
| Other comprehensive income (loss) | | | | | | | | |
| Foreign currency translation adjustment | | | 13,879 | | | | 37,270 | |
| Total comprehensive income (loss) | | | (404,244 | ) | | | (233,638 | ) |
| Less: total comprehensive loss attributable to non-controlling interests | | | 1,498 | | | | (55,938 | ) |
| Total comprehensive income attributable to the shareholders of the Company | | $ | (405,742 | ) | | | (177,700 | ) |
| | | | | | | | | |
| Earning per share – basic | | $ | (0.04 | ) | | | (0.34 | ) |
| – diluted | | $ | (0.03 | ) | | | (0.34 | ) |
| Weighted average shares outstanding – Basic | | | 10,149,386 | | | | 601,830 | |
| – Diluted | | | 14,770,914 | | | | 601,830 | |
The accompanying notes are an integral part of these consolidated financial statements.
NEWGENIVF GROUP LIMITED
UNAUDITED CONDENSED CONSOLIDATED STATEMENTS OF CHANGES IN SHAREHOLDERS’
EQUITY (DEFICIT)
FOR THE SIX MONTHS ENDED SEPTEMBER 30, 2024 and 2023
(Stated in US Dollars)
| | | Number of
shares | | | Ordinary
shares | | | Subscription
receivable | | | Additional
paid-in
capital | | | Accumulated
deficit | | | Accumulated
other
comprehensive
income/(loss) | | | Total
attributable
to the
shareholders
of the
Company | | | Non- controlling
interests | | | Total | |
| Balance, December 31, 2022 | | | 601,830 | | | $ | 6,018 | | | $ | (319,872 | ) | | $ | 1,458,941 | | | $ | (591,544 | ) | | $ | 9,570 | | | $ | 563,113 | | | $ | (462,430 | ) | | $ | 100,683 | |
| Net loss | | | — | | | | — | | | | — | | | | — | | | | (205,432 | ) | | | — | | | | (205,432 | ) | | | (65,476 | ) | | | (270,908 | ) |
| Foreign currency translation adjustment | | | — | | | | — | | | | — | | | | — | | | | — | | | | 27,732 | | | | 27,732 | | | | 9,538 | | | | 37,270 | |
| Subscription receivables | | | — | | | | — | | | | 192,308 | | | | — | | | | — | | | | — | | | | 192,308 | | | | — | | | | 192,308 | |
| Balance, September 30, 2023 | | | 601,830 | | | $ | 6,018 | | | $ | (127,564 | ) | | $ | 1,458,941 | | | $ | (796,976 | ) | | $ | 37,302 | | | $ | (577,721 | ) | | $ | (518,368 | ) | | $ | (59,353 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Balance, December 31, 2023 | | | 698,123 | | | $ | 6,981 | | | $ | (2,967,100 | ) | | $ | 4,324,834 | | | $ | (461,351 | ) | | $ | (7,288 | ) | | $ | 896,076 | | | $ | (490,051 | ) | | $ | 406,025 | |
| Net loss | | | — | | | | — | | | | — | | | | — | | | | (416,400 | ) | | | — | | | | (416,400 | ) | | | (1,723 | ) | | | (418,123 | ) |
| Foreign currency translation adjustment | | | — | | | | — | | | | — | | | | — | | | | — | | | | 13,879 | | | | 13,879 | | | | - | | | | 13,879 | |
| Business Combination | | | — | | | | (6,981 | ) | | | 2,967,100 | | | | (2,909,834 | ) | | | (7,125,525 | ) | | | (3,221 | ) | | | (7,078,461 | ) | | | 1,498 | | | | (7,076,963 | ) |
| Balance, September 30, 2024 | | | 10,149,386 | | | $ | — | | | $ | — | | | $ | 1,415,000 | | | $ | (8,003,276 | ) | | $ | 3,370 | | | $ | (6,584,906 | ) | | $ | (490,276 | ) | | $ | (7,075,182 | ) |
The accompanying notes are an integral part of these consolidated financial statements.
NEWGENIVF GROUP LIMITED
UNAUDITED CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2024 AND 2023
(Stated in US Dollars)
| | | For the Nine Months Ended
September 30, | |
| | | 2024 | | | 2023 | |
| CASH FLOWS FROM OPERATING ACTIVITIES | | | | | | |
| Net income (loss) | | $ | (418,123 | ) | | $ | (270,908 | ) |
| Adjustments to reconcile net (loss) income to net cash provided by operating activities: | | | | | | | | |
| Depreciation of plant and equipment | | | 19,300 | | | | 5,856 | |
| Amortization of right-of-use assets | | | 7,060 | | | | 56,902 | |
| Provision of expected credit loss allowance | | | — | | | | 634 | |
| Changes in operating assets and liabilities: | | | | | | | | |
| Accounts receivable | | | (309,485 | ) | | | 12,870 | |
| Inventories | | | 31,479 | | | | (17,323 | ) |
| Loan to A SPAC I | | | — | | | | (140,000 | ) |
| Deposit, prepayment and other receivables, net | | | 138,580 | | | | (8,701 | ) |
| Accrued liabilities and other payable | | | 11,741 | | | | 56,234 | |
| Legal fee payable | | | (2,007,444 | ) | | | 60 | |
| Contract liabilities | | | 463 | | | | (1,250,900 | ) |
| Operating lease liabilities | | | (89,702 | ) | | | (89,702 | ) |
| Taxes payable | | | — | | | | 87,234 | |
| Net cash provided by/(used in) operating activities | | | (2,616,131 | ) | | | (1,557,744 | ) |
| | | | | | | | | |
| CASH FLOWS FROM INVESTING ACTIVITIES | | | | | | | | |
| Purchase of plant and equipment | | | (50,337 | ) | | | (52,386 | ) |
| Purchase of intangible assets | | | (16,381 | ) | | | — | |
| Net cash used in investing activities | | | (66,718 | ) | | | (52,386 | ) |
| | | | | | | | | |
| CASH FLOWS FROM FINANCING ACTIVITIES | | | | | | | | |
| Finance lease | | | (9,317 | ) | | | (14,019 | ) |
| Amount with related parties | | | (125,394 | ) | | | 1,353,426 | |
| Subscription receivables | | | — | | | | 192,308 | |
| Convertible promissory note | | | 2,920,238 | | | | — | |
| Net cash (used in)/provided by financing activities | | | 2,785,527 | | | | 1,531,715 | |
| | | | | | | | | |
| Net (decrease)/increase in cash and cash equivalents | | | 102,678 | | | | (78,415 | ) |
| Effect of foreign currency translation on cash and cash equivalents | | | 12,879 | | | | 87,973 | |
| Cash and cash equivalents, beginning of period | | | 54,104 | | | | 27,556 | |
| Cash and cash equivalents, end of period | | $ | 169,661 | | | $ | 37,114 | |
| | | | | | | | | |
| Supplementary cash flow information: | | | | | | | | |
| Taxes paid | | $ | — | | | | — | |
| Interest paid | | $ | (357,551 | ) | | | (20,922 | ) |
| Listing fee paid | | $ | — | | | | 976,166 | |
The accompanying notes are an integral part of these consolidated financial statements.
NEWGENIVF GROUP LIMITED
NOTES TO THE UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
SEPTEMBER 30, 2024 AND 2023
(Stated in US Dollars)
NOTE 1 — ORGANIZATION AND PRINCIPAL ACTIVITIES
NewGenIvf Limited (the “Company” or the “Group”) was incorporated under the laws of the Cayman Islands on January 16, 2019 as an investment holding company.
The following is an organization chart of the Company and its subsidiaries:

As of June 30, 2024, the Company’s subsidiaries are detailed in the table as follows:
| Name | | Background | | Ownership % | | Principal activity |
| NewGenivf Limited | | ● A Cayman Islands company ● Incorporated on 16 January, 2019 | | 100% | | Investment holding |
| FFPGS (HK) Limited | | ● A Hong Kong company ● Incorporated on December 19, 2019 | | 100% | | Marketing and administrative services |
| Well Image Limited | | ● A Hong Kong company ● Incorporated on July 11, 2008 | | 100% | | Investment holding |
| Med Holdings Limited (“Med Holdings”) (Note) | | ● A Thailand company ● Incorporated on January 21, 2015 | | 49%* | | Investment holding |
| First Fertility PGS Center Limited (“FFC”) (Note) | | ● A Thailand company ● Incorporated on March 6, 2014 | | 74% | | Provision of IVF treatment |
| First Fertility Phnom Penh Limited (“FFPP”) | | ● A Cambodia company
● Incorporated on August 10, 2015 | | 100% | | Provision of IVF treatment |
NEWGENIVF GROUP LIMITED
NOTES TO THE UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
SEPTEMBER 30, 2024 AND 2023
(Stated in US Dollars)
NOTE 1 — ORGANIZATION AND PRINCIPAL ACTIVITIES (cont.)
| Name | | Background | | Ownership % | | Principal activity |
| First Fertility Bishkek LLC (“FFB”) | | ● A Kyrgyzstan company
● Incorporated on October 11, 2019 | | 100% | | Provision surrogacy and ancillary caring services |
| * | Where less than 50% of the equity of an investee is held, the Company (through its subsidiaries) holds significantly more voting rights than any other vote holder or organized company of vote holders. An assessment has been made, taking into account all the factors relevant to the relationship with the investee, to ascertain control has been established and the investee should be consolidated as a subsidiary of the Company. |
Note:
According to the Foreign Business Act (the “FBA”), the majority shareholdings of limited company incorporated in Thailand is required to be owned by Thai nationals.
With reference to the capital structure and voting rights structure of ordinary shares and preference shares (the “Share Structure”) of Med Holdings and FFC, all the preference share capital is owned by a Thai national. The ordinary shares and preference shares have the same rights and status in all respects except for the distribution of profits by way of dividends with details as follow:
| | (a) | Dividends from profits of Med Holdings and FFC shall be allocated to the holders of preference shares at a rate fixed from time to time by the board of directors prior to allocating to the holders of ordinary shares. In any event, such dividends to be allocated to the holders of preference shares shall not exceed 15% of the total amount of dividends declared from time to time; |
| | (b) | After allocation of dividends as per (a) above, the rest of the dividends shall be distributed equally amongst the holders of ordinary shares according to their shareholding ratio; |
| | (c) | The holders of preferred shares shall be entitled to dividends only in respect of the years for which the Company has declared a dividend payment, and there shall be no cumulative dividends; and |
| | (d) | Dividends allocated to the holders of preferred shares in each year shall be limited at the rate as stated in (a) only. No additional dividends shall be paid to the holders of preferred shares. |
Based upon the management’s judgement on the Shares Structure, as the Company is able to exercise majority voting power in any board meeting, the Company accounts for Med Holdings and FFC as subsidiaries on the ground that the Company is able to control Med Holdings and FFC by exercising its majority voting power in any board meetings.
NEWGENIVF GROUP LIMITED
NOTES TO THE UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
SEPTEMBER 30, 2024 AND 2023
(Stated in US Dollars)
NOTE 1 — ORGANIZATION AND PRINCIPAL ACTIVITIES (cont.)
Group reorganization
Pursuant to a group reorganization (the “group reorganization”) to rationalize the structure of the Company and its subsidiary companies (herein collectively referred to as the “Group”) in preparation for the listing of its shares, the Company becomes the holding company of the Group on February 2, 2023. As the Group were under same control of the shareholders and their entire equity interests were also ultimately held by the shareholders immediately prior to the group reorganization, the consolidated statements of income and comprehensive income, consolidated statements of changes in shareholders’ equity and consolidated statements of cash flows are prepared as if the current group structure had been in existence throughout the three-year period ended December 31, 2023, or since the respective dates of incorporation/establishment of the relevant entity, where this is a shorter period.
The unaudited condensed consolidated balance sheets as of September 30, 2024 and 2023 present the assets and liabilities of the aforementioned companies now comprising the Group which had been incorporated/established as of the relevant balance sheet date as if the current group structure had been in existence at those dates based on the same control aforementioned. The Company eliminates all significant intercompany balances and transactions in its consolidated financial statements.
The movement in the Company’s authorized share capital and the number of ordinary shares outstanding and issued in the Company are also detailed in Note 10.
NOTE 2 — SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Principles of consolidation and basis of preparation
The accompanying consolidated financial statements reflect the accounts of the Company and all of its subsidiaries in which a controlling interest is maintained. All inter-company balances and transactions have been eliminated in consolidation.
Management has prepared the accompanying consolidated financial statements and these notes in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”). The Company maintains its general ledger and journals with the accrual method accounting.
NEWGENIVF GROUP LIMITED
NOTES TO THE UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
SEPTEMBER 30, 2024 AND 2023
(Stated in US Dollars)
NOTE 2 — SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.)
Use of estimates
The preparation of the consolidated financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosures of contingent assets and liabilities as of the date of the consolidated financial statements and the reported amounts of revenues and expenses during the periods presented. Significant estimates required to be made by management include, but are not limited to, contingent tax liability for Kyrgyzstan. Actual results could differ from those estimates, and as such, differences may be material to the consolidated financial statements.
Foreign currency translation
The accompanying consolidated financial statements are presented in United States dollar (“$”), which is the reporting currency of the Company. The functional currency of the Company and its subsidiaries, FFPGS (HK) Limited and Well Image Limited, are Hong Kong dollar (“HK$”). Med Holdings and FFC use Thai baht (“THB”) as their functional currencies. First Fertility Phnom Penh Limited uses Cambodian riel (“KHR”) as its functional currency and First Fertility Bishkek LLC uses United States dollar (“USD”) as its functional currency.
Assets and liabilities denominated in currencies other than the reporting currency are translated into the reporting currency at the rates of exchange prevailing at the balance sheet date. Translation gains and losses are recognized in the consolidated statements of operations and comprehensive income as other comprehensive income or loss.
Transactions in currencies other than the reporting currency are measured and recorded in the reporting currency at the exchange rate prevailing on the transaction date. The cumulative gain or loss from foreign currency transactions is reflected in the consolidated statements of operations and comprehensive income as other income (other expenses).
The value of foreign currencies including, the HK$, THB, KHR and RMB, may fluctuate against the United States dollar. Any significant variations of the aforementioned currencies relative to the United States dollar may materially affect the Company’s financial condition in terms of reporting in USD. The following table outlines the currency exchange rates that were used in preparing the accompanying consolidated financial statements:
| | | | | | | September 30,
2024 | | | September 30,
2023 | | | December 31,
2023 | |
| Period-end | | | | $: HK$ | | | | 7.8000 | | | | 7.8000 | | | | 7.8000 | |
| Period average | | | | $: HK$ | | | | 7.8000 | | | | 7.8000 | | | | 7.8000 | |
| Period-end | | | | $: THB | | | | 36.8454 | | | | 36.4816 | | | | 34.2265 | |
| Period average | | | | $: THB | | | | 34.4021 | | | | 34.6579 | | | | 34.7867 | |
| Period-end | | | | $: KHR | | | | 4,063.3864 | | | | 4,117.2783 | | | | 4,080.0304 | |
| Period average | | | | $: KHR | | | | 4,074.3825 | | | | 4,104.8088 | | | | 4,105.4181 | |
| Period-end | | | | $: RMB | | | | 7.0812 | | | | 7.2755 | | | | 7.0971 | |
| Period average | | | | $: RMB | | | | 7.1862 | | | | 7.0556 | | | | 7.0835 | |
NEWGENIVF GROUP LIMITED
NOTES TO THE UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
SEPTEMBER 30, 2024 AND 2023
(Stated in US Dollars)
NOTE 2 — SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.)
Cash and cash equivalents
Cash and cash equivalents include cash on hand, deposits held at call with financial institutions, other short-term deposits with original maturities of three months or less that are readily convertible to known amounts of cash and which are subject to an insignificant risk of changes in value.
Deposits, prepayment, other receivables and deferred IPO cost, net
Deposits, prepayment, other receivables and deferred Initial Public Offering (“IPO”) cost, net primarily include deposits paid to suppliers, prepaid expenses, the prepaid professional fee which meets the definition of deferred IPO cost, and other deposits.
Deferred IPO costs consist of underwriting, legal, accounting and other expenses incurred through the balance sheet date that are directly related to the Initial Public Offering and that were charged to shareholders’ equity upon the completion of the Initial Public Offering.
Plant and equipment, net
Plant and equipment are stated at cost less accumulated depreciation. Depreciation is provided over their estimated useful lives, using the straight-line method. The Company typically applies a salvage value of 0%. The estimated useful lives of the plan and equipment are as follows:
| Furniture and fixtures | | 3 – 5 years |
| Leasehold improvements | | the lesser of useful life or term of lease |
| Medical instruments | | 3 – 10 years |
| Motor vehicle | | 3 – 5 years |
| Office equipment | | 3 – 5 years |
The cost and related accumulated depreciation of assets sold or otherwise retired are eliminated from the accounts, and any gain or loss are included in the Company’s results of operations. The costs of maintenance and repairs are expensed as incurred. Significant renewals and betterments that extend the useful life of an assets are capitalized.
Impairment of long-lived assets
The Company evaluates the long-lived assets for impairment whenever events or changes in circumstances indicate that the carrying amount of assets may not be recoverable. Impairment may become obsolete from a difference in the industry, introduction of new technologies, or if the Company has inadequate working capital to utilize the long-lived assets to generate adequate profits. Impairment is present if the carrying amount of an asset is less than its expected future undiscounted cash flows.
If an asset is considered impaired, a loss is recognized based on the amount by which the carrying amount exceeds the fair market value of the asset. Assets to be disposed of are reported lower the carrying amount or fair value less cost to sell.
NEWGENIVF GROUP LIMITED
NOTES TO THE UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
SEPTEMBER 30, 2024 AND 2023
(Stated in US Dollars)
NOTE 2 — SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.)
Inventories
Inventories are stated at the lower of cost and net realizable value. Costs are determined on a first-in, first-out basis. Net realizable value is based on the estimated selling prices less any estimated costs to be incurred to completion and disposal. A provision for excess and obsolete inventory will be made based primarily on forecasts of product demand. The excess balance determined by this analysis becomes the basis for excess inventory charge and the written-down value of the inventory becomes its cost. Written-down inventory would not be reversed if market conditions improve.
Other borrowings
Other borrowings are recognized initially at fair value, net of debt issuance costs incurred. Other borrowings are subsequently stated at amortized cost; any difference between the proceeds (net of debt issuance costs) and the redemption value is recognized in the consolidated statements of operations over the period of the borrowings using the effective interest method.
Ordinary shares
The Company’s ordinary shares are stated at par value of $0.01 per ordinary share. The difference between the consideration received, net of issuance cost, and the par value is recorded in additional paid-in capital. After merger, the Company’s ordinary share has no par value.
Revenue recognition
The Company adopted ASC Topic 606, Revenue from Contracts with Customers, and all subsequent ASUs that modified ASC 606 on April 1, 2017 using the full retrospective method which requires the Company to present the financial statements for all periods as if Topic 606 had been applied to all prior periods. The Company derives revenue principally from provision of In vitro fertilization (“IVF”) treatment and surrogacy and ancillary caring services. Revenue from contracts with customers is recognized using the following five steps:
| | (1) | identify its contracts with customers; |
| | (2) | identify its performance obligations under those contracts; |
| | (3) | determine the transaction prices of those contracts; |
| | (4) | allocate the transaction prices to its performance obligations in those contracts; and |
| | (5) | recognize revenue when each performance obligation under those contracts is satisfied. Revenue is recognized when promised services are transferred to the client in an amount that reflects the consideration expected in exchange for those services. |
The Company enters into service agreements with its customers that outline the rights, responsibilities, and obligations of each party. The agreements also identify the scope of services, service fees, and payment terms. Agreements are acknowledged and signed by both parties. All the contracts have commercial substance, and it is probable that the Company will collect considerations from its customers for service component.
NEWGENIVF GROUP LIMITED
NOTES TO THE UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
SEPTEMBER 30, 2024 AND 2023
(Stated in US Dollars)
NOTE 2 — SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.)
Revenue recognition (cont.)
The Company derives its revenues from two sources: (1) revenue from IVF treatment, and (2) revenue from surrogacy and ancillary caring services.
Revenue from IVF treatment
In vitro fertilization (“IVF”) treatment is an assisted reproductive technique where eggs and sperm are collected and fertilized in laboratory to become embryo. Fertilized embryo is then implanted to the customer or a surrogate mother. IVF treatment involves the performance of a series of medical treatment and procedures that are not separately distinct and only brings benefits to customer when embryo is successfully implanted, therefore revenue from IVF treatment is recognized at a point in time when it is completed in clinic. The completion of this treatment is evidenced by a written IVF report indicating successful embryo implantation. The Company collects payment from customer in advance for IVF treatment. The amount of revenue recognized from contract liabilities to the Company’s result of operations can be found in Note 8 below.
Revenue from surrogacy and ancillary caring services
The Company provides surrogacy and ancillary caring services solely in Kyrgyzstan. Embryo from blood parents is implanted to surrogate mother contracted by the Company. During pregnancy period, the Company provides ancillary caring services including regular body check and provision of vitamins, supplements and medicines to surrogate mothers. The key performance obligation is identified as a single performance obligation where a baby is born, therefore revenue from surrogacy and ancillary caring services is recognized at a point in time when surrogate mother gives birth. The Company collects approximately 40% of contract sum upfront, and remaining contract sum is collected in installments across pregnancy period of surrogate mother. The amount of revenue recognized from contract liabilities to the Company’s result of operations can be found in Note 8 below.
Contract related assets and liabilities are classified as current assets and current liabilities. Significant balance sheet accounts related to the revenue cycle are as follows:
Account receivables, net
Accounts receivable, net are stated at the original amount less an allowance for expected credit loss on such receivables. The allowance for expected credit loss is estimated based upon the Company’s assessment of various factors including historical experience, the age of the accounts receivable balances, current general economic conditions, future expectations and customer specific quantitative and qualitative factors that may affect the Company’s customers’ ability to pay. An allowance is also made when there is objective evidence for the Company to reasonably estimate the amount of probable loss.
NEWGENIVF GROUP LIMITED
NOTES TO THE UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
SEPTEMBER 30, 2024 AND 2023
(Stated in US Dollars)
NOTE 2 — SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.)
Revenue recognition (cont.)
Contract liabilities
Contract liabilities represent considerations received from customers in advance of satisfying the Company’s performance obligations under the contract. These amounts are expected to be earned within 12 months and are classified as current liabilities.
Expected credit loss
ASU No. 2016-13, Financial Instruments — Credit Losses (Topic 326): Measurement of Credit Losses on Financial Instruments requires entities to use a current lifetime expected credit loss methodology to measure impairments of certain financial assets. Using this methodology will result in earlier recognition of losses than under the current incurred loss approach, which requires waiting to recognize a loss until it is probable of having been incurred. There are other provisions within the standard that affect how impairments of other financial assets may be recorded and presented, and that expand disclosures. Expected credit losses are probability-weighted estimates of credit losses. Credit losses are measured at the present value of all cash shortfalls (i.e., the difference between the cash flows due to the entity in accordance with the contract and the cash flows that the Company expects to receive). ECLs are discounted at the effective interest rate of the financial asset.
Retirement benefits
Retirement benefits in the form of mandatory government-sponsored defined contribution plans are charged to either expense as incurred or allocated to wages as part of cost of revenues.
Segment information
Operating segments are identified as components of an enterprise about which separate discrete financial information is available for evaluation by the chief operating decision maker (the “CODM”), or decision making group, in making decisions on how to allocate resources and assess performance. The Company operates and manages in one operating segment. The Company defines its CODM as Mr. Siu Wing Fund Alfred, the Company’s Chief Executive Officer. Since the Company operates in one operating segment, all required financial segment information can be found in the consolidated financial statements. The long-lived assets and revenue from external customers as of September 30, 2024 and 2023 by geographical area are presented in Note 13.
NEWGENIVF GROUP LIMITED
NOTES TO THE UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
SEPTEMBER 30, 2024 AND 2023
(Stated in US Dollars)
NOTE 2 — SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.)
Leases
The Company measured the lease in accordance to ASU 2016-02, “Leases” (Topic 842). Lease terms used to calculate the present value of lease payments generally do not include any options to extend, renew, or terminate the lease, as the Company does not have reasonable certainty at lease inception that these options will be exercised. The Company generally considers the economic life of its operating lease ROU assets to be comparable to the useful life of similar owned assets. The Company has elected the short-term lease exception, therefore operating lease ROU assets and liabilities do not include leases with a lease term of twelve months or less. Its leases generally do not provide a residual guarantee. The operating lease ROU asset also excludes lease incentives. Lease expense is recognized on a straight-line basis over the lease term.
As of September 30, 2024 and December 31, 2023, there were $293,168 and $ 283,847 right of use (“ROU”) assets and $336,527 and $326,107 lease liabilities based on the present value of the future minimum rental payments of leases, respectively. The Company’s management believes that using an incremental borrowing rate of the minimum loan rate and the Hong Kong Dollar Best Lending Rate (“BLR”) minus 0.125% was the most indicative rate of the Company’s borrowing cost for the calculation of the present value of the lease payments; the rate used by the Company was 6.6% and 5.0% respectively.
Income Taxes
The Company recognizes deferred income tax assets or liabilities for expected future tax consequences of events recognized in the consolidated financial statements or tax returns. Under this method, deferred income tax assets and liabilities are determined based on the differences between the financial reporting and income tax bases of assets and liabilities and are measured using the income tax rates that will be in effect when the differences are expected to reverse. Valuation allowances are provided when it is more likely than not that a deferred tax asset is not realizable or recoverable in the future.
The Company determines that the tax position is more likely than not to be sustained and records the largest amount of benefit that is more likely than not to be realized when the tax position is settled. the Company recognizes interest and penalties, if any, related to uncertain tax positions in income tax expense.
Comprehensive Income
The Company presents comprehensive income in accordance with ASC Topic 220, Comprehensive Income. ASC Topic 220 states that all items that are required to be recognized under accounting standards as components of comprehensive income be reported in the consolidated financial statements. The components of comprehensive income were the net income for the years and the foreign currency translation adjustments.
NEWGENIVF GROUP LIMITED
NOTES TO THE UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
SEPTEMBER 30, 2024 AND 2023
(Stated in US Dollars)
NOTE 2 — SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.)
Earnings per share
The Company computes earnings per share (“EPS”) following ASC Topic 260, “Earnings per share”. Basic EPS is measured as the income or loss available to common shareholders divided by the weighted average common shares outstanding for the period. Diluted EPS presents the dilutive effect on a per-share basis from the potential conversion of convertible securities or the exercise of options and or warrants; the dilutive impacts of potentially convertible securities are calculated using the as-if method; the potentially dilutive effect of options or warranties are computed using the treasury stock method. Potentially anti-dilutive securities (i.e., those that increase income per share or decrease loss per share) are excluded from diluted EPS calculation. There were no potentially dilutive securities that were in-the-money that were outstanding during the nine months ended September 30, 2024 and 2023.
Related parties
The Company adopted ASC 850, Related Party Disclosures, for the identification of related parties and disclosure of related party transactions.
Commitments and contingencies
In the normal course of business, the Company is subject to contingencies, including legal proceedings and claims arising out of the business that relate to a wide range of matters, such as government investigations and tax matters. The Company recognizes its liability for such contingency if it determines it is probable that a loss has occurred and a reasonable estimate of the loss can be made. The Company may consider many factors in making these assessments including historical and the specific facts and circumstances of each matter.
Non-controlling interests
Non-controlling interests are presented as a separate component of equity on the consolidated balance sheets and net (loss) income and other comprehensive loss are attributed to controlling and non-controlling interests respectively.
Concentration of risks
Concentration of credit risk
Financial instruments that potentially expose us to concentrations of credit risk consist primarily of cash and cash equivalents and account receivable. The Company places cash and cash equivalents with financial institutions with high credit ratings and quality.
Accounts receivable primarily comprise of amounts receivable from the service customers. The Company conducts credit evaluations of customers, and generally does not require collateral or other security from its customers. The Company establishes an allowance for doubtful accounts primarily based upon the factors surrounding the credit risk of specific customers.
NEWGENIVF GROUP LIMITED
NOTES TO THE UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
SEPTEMBER 30, 2024 AND 2023
(Stated in US Dollars)
NOTE 2 — SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.)
Concentration of risks (cont.)
Concentration of customers
As of September 30, 2024 and December 31, 2023, one and two customers which individually contributed more than 10% of trade receivable, accounted for 15% and 96.3% of the Company’s trade receivable respectively.
None of the customers contributed more than 10% of revenue for nine months ended September 30, 2024 and 2023.
Concentration of suppliers
As of September 30, 2024 and December 31, 2023, one and four suppliers which individually contributed more than 10% of trade payable, accounted for 13.6% and 30.6% of the Company’s trade payable respectively.
For the nine months ended September 30, 2024 and 2023, one and one vendors which contributed more than 10% of total purchases of the Company, accounted for 15.3% and 21.5% of the Company’s total purchases respectively.
Financial instruments
The Company’s financial instruments, including cash and cash equivalents, accounts receivables, net, deposits, other receivables and deferred IPO cost, net, loan to A SPAC I, accounts payables, accrued liabilities and other payables, and due from (to) shareholders, have carrying amounts that approximate their fair values due to their short maturities. ASC Topic 820, “Fair Value Measurements and Disclosures” requires disclosing the fair value of financial instruments held by the Company. ASC Topic 825, “Financial Instruments” defines fair value and establishes a three-level valuation hierarchy for disclosures of fair value measurement that enhances disclosure requirements for fair value measures. The carrying amounts reported in the consolidated balance sheets for cash and cash equivalents, accounts and other receivables, accounts and other payables, accrued liabilities and amounts due from (to) related parties each qualify as financial instruments and are a reasonable estimate of their fair values because of the short period between the origination of such instruments and their expected realization and their current market rate of interest. The three levels of valuation hierarchy are defined as follows:
| | ● | Level 1 — inputs to the valuation methodology used quoted prices for identical assets or liabilities in active markets. |
| | ● | Level 2 — inputs to the valuation methodology include quoted prices for similar assets and liabilities in active markets and information that are observable for the asset or liability, either directly or indirectly, for substantially the financial instrument’s full term |
| | ● | Level 3 — inputs to the valuation methodology are unobservable and significant to the fair value measurement. |
The Company analyzes all financial instruments with features of both liabilities and equity under ASC 480, “Distinguishing Liabilities from Equity” and ASC 815.
NEWGENIVF GROUP LIMITED
NOTES TO THE UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
SEPTEMBER 30, 2024 AND 2023
(Stated in US Dollars)
NOTE 2 — SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.)
Recent accounting pronouncements adopted
In April 2019, the FASB issued ASU 2019-04, Codification Improvements to Topic 326, Financial Instruments-Credit Losses, Topic 815, Derivatives and Hedging, and Topic 825, Financial Instruments, which amends and clarifies several provisions of Topic 326. In May 2019, the FASB issued ASU 2019-05, Financial Instruments-Credit Losses (Topic 326) Targeted Transition Relief, which amends Topic 326 to allow the fair value option to be elected for certain financial instruments upon adoption. ASU 2019-10 extended the effective date of ASU 2016-13 until December 15, 2022. This standard replaces the incurred loss methodology with an expected loss methodology that is referred to as the current expected credit loss (“CECL”) methodology. CECL requires an estimate of credit losses for the remaining estimated life of the financial asset using historical experience, current conditions, and reasonable and supportable forecasts and generally applies to financial assets measured at amortized cost, including loan receivables and held-to-maturity debt securities, and some off-balance sheet credit exposures such as unfunded commitments to extend credit. Financial assets measured at amortized cost will be presented at the net amount expected to be collected by using an allowance for expected credit losses. The Company already adopted the new standard and the Company recognizes the full impact of the new standard in these consolidated balance sheets and makes related disclosures.
Recent accounting pronouncements not yet adopted
In November 2023, the FASB issued ASU 2023-07, “Segment Reporting (Topic 280)” (“ASU 2023-07”). The amendments in ASU 2023-07 improve financial reporting by requiring disclosure of incremental segment information on an annual and interim basis for all public entities to enable investors to develop more decision useful financial analyses. Topic 280 requires a public entity to report a measure of segment profit or loss that the chief operating decision maker (CODM) uses to assess segment performance and make decisions about allocating resources. Topic 280 also requires other specified segment items and amounts, such as depreciation, amortization, and depletion expense, to be disclosed under certain circumstances. The amendments in ASU 202307 do not change or remove those disclosure requirements. The amendments in ASU 2023-07 also do not change how a public entity identifies its operating segments, aggregates those operating segments, or applies the quantitative thresholds to determine its reportable segments. The amendments in ASU 2023-07 are effective for years beginning after December 15, 2023 and interim periods within fiscal years beginning after December 15, 2024, adopted retrospectively. Management considers that the guidance does not have a significant impact on the disclosures set out in these consolidated financial statements.
In December 2023, FASB issued Accounting Standards Update (“ASU”) 2023-09, “Income Taxes (Topic 740)” (“ASU 2023-09”). The amendments in ASU 2023-09 address investor requests for more transparency about income tax information through improvements to income tax disclosures primarily related to the rate reconciliation and income taxes paid information. One of the amendments in ASU 2023-09 includes disclosure of, on an annual basis, a tabular rate reconciliation of (i) the reported income tax expense (or benefit) from continuing operations, to (ii) the product of the income (or loss) from continuing operations before income taxes and the applicable statutory federal income tax rate of the jurisdiction of domicile using specific categories, including separate disclosure for any reconciling items within certain categories that are equal to or greater than a specified quantitative threshold of 5%. ASU 2023-09 also requires disclosure of, on an annual basis, the year to date amount of income taxes paid (net of refunds received) disaggregated by federal, state, and foreign jurisdictions, including additional disaggregated information on income taxes paid (net of refunds received) to an individual jurisdiction equal to or greater than 5% of total income taxes paid (net of refunds received). The amendments in ASU2023-09 are effective for annual periods beginning after December 15, 2024, and should be applied prospectively. The Company is currently evaluating the impact of the update on the Company’s consolidated financial statements and related disclosures.
Save for elsewhere disclosed, the Company does not believe other recently issued but not yet effective accounting standards, if currently adopted, would have a material effect on the Company’s consolidated balance sheet, statement of operations and comprehensive income (loss) and statement of cash flows.
NEWGENIVF GROUP LIMITED
NOTES TO THE UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
SEPTEMBER 30, 2024 AND 2023
(Stated in US Dollars)
NOTE 3 — ACCOUNTS RECEIVABLE, NET
Accounts receivable, net consists of the following:
| | | September 30, | | | December 31, | |
| | | 2024 | | | 2023 | |
| Accounts receivable | | $ | 318,841 | | | $ | 9,393 | |
| Less: allowance for expected credit loss | | | (18 | ) | | | (19 | ) |
| | | $ | 318,859 | | | $ | 9,374 | |
As of the end of each of the financial year, the aging analysis of accounts receivable, net of allowance for expected credit loss, based on the invoice date is as follows:
| | | September 30, | | | December 31, | |
| | | 2024 | | | 2023 | |
| Within 90 days | | $ | 318,841 | | | $ | 9,374 | |
| | | $ | 318,841 | | | $ | 9,374 | |
The movement of allowances for expected credit loss is as follow:
| | | September 30, | | | December 31, | |
| | | 2024 | | | 2023 | |
| Balance at beginning of the year | | $ | (19 | ) | | $ | (26 | ) |
| Reversal (provision) of provision | | | 1 | | | | 7 | |
| Effect of currency translation adjustment | | | — | | | | — | |
| Ending balance | | $ | (18 | ) | | $ | (19 | ) |
NOTE 4 — INVENTORIES
Inventories consist of the following:
| | | September 30, | | | December 31, | |
| | | 2024 | | | 2023 | |
| Medicines, consumables and reagents for clinical and laboratory analyses | | $ | 94,785 | | | $ | 126,264 | |
| | | $ | 94,785 | | | $ | 126,264 | |
NEWGENIVF GROUP LIMITED
NOTES TO THE UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
SEPTEMBER 30, 2024 AND 2023
(Stated in US Dollars)
NOTE 5 — DEPOSITS, PREPAYMENT, OTHER RECEIVABLES AND DEFERRED IPO COST, NET
Deposits, prepayment, other receivables and deferred IPO cost, net consist of the following:
| | | September 30, | | | December 31, | |
| | | 2024 | | | 2023 | |
| | | | | | | |
| Other receivables | | $ | 35,253 | | | $ | 15,910 | |
| Deposits | | | 144,079 | | | | 123,008 | |
| Prepayment | | | 214,937 | | | | 4,848 | |
| Deferred initial public offering “IPO” cost | | | — | | | | 373,677 | |
| Less: allowance for expected credit loss | | | (15 | ) | | | (14 | ) |
| | | $ | 394,254 | | | $ | 517,429 | |
The movement of allowances for expected credit loss is as follow:
| | | September 30, | | | December 31, | |
| | | 2024 | | | 2023 | |
| Balance at beginning of the year | | $ | (14 | ) | | $ | (141 | ) |
| Reversal of provision (Provision) | | | (1 | ) | | | 127 | |
| Effect of currency translation adjustment | | | — | | | | — | |
| Ending balance | | $ | (15 | ) | | $ | (14 | ) |
NEWGENIVF GROUP LIMITED
NOTES TO THE UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
SEPTEMBER 30, 2024 AND 2023
(Stated in US Dollars)
NOTE 6 — PLANT AND EQUIPMENT, NET
Plant and equipment, net consist of the following:
| | | September 30, | | | December 31, | |
| | | 2024 | | | 2023 | |
| At cost: | | | | | | |
| Building improvement | | $ | 96,020 | | | $ | 92,438 | |
| Furniture and fixtures | | | 260,199 | | | | 250,493 | |
| Medical instruments | | | 877,542 | | | | 844,809 | |
| Motor vehicle | | | 148,473 | | | | 142,936 | |
| Office equipment | | | 156,527 | | | | 150,688 | |
| | | | 1,538,761 | | | | 1,481,364 | |
| Less: accumulated depreciation | | | (1,345,567 | ) | | | (1,319,207 | ) |
| Total | | $ | 193,194 | | | $ | 162,157 | |
Depreciation expenses for the nine months ended September 30, 2024 and 2023 were $26,360 and $19,461, respectively. No loss on disposal of assets for the nine months ended September 30, 2024 and 2023.
No impairment loss was recorded for the nine months ended September 30, 2024, and 2023.
NEWGENIVF GROUP LIMITED
NOTES TO THE UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
SEPTEMBER 30, 2024 AND 2023
(Stated in US Dollars)
NOTE 7 — ACCRUED LIABILTIES AND OTHER PAYABLES
Accrued liabilities and other payables consist of the following:
| | | September 30, | | | December 31, | |
| | | 2024 | | | 2023 | |
| Accrued expenses | | $ | 250,039 | | | $ | 43,633 | |
| Withholding tax payable | | | 7,378 | | | | 7,349 | |
| Compensation payable (Note 1) | | | 19,322 | | | | 144,015 | |
| Other payables | | | 121,469 | | | | 46,616 | |
| | | $ | 398,208 | | | $ | 241,613 | |
| | Note 1: | Compensation payable represented a claim relating to an employee of First Fertility PGS Center Limited (“FFC”). On April 23, 2023, the compensation agreement is finalized with the employee and the compensation is payable in 12 instalments within one year from 2023. |
NOTE 8 — CONTRACT LIABILITIES
Contract liabilities consist of the following:
| | | September 30, | | | December 31, | |
| | | 2024 | | | 2023 | |
| Balance at beginning of period | | $ | 7,937 | | | $ | 1,360,168 | |
| Additions | | | 37,592 | | | | 112,006 | |
| Recognized to revenue during the period | | | (37,129 | ) | | | (122,662 | ) |
| Refund to customers (Note 1) | | | — | | | | (1,341,575 | ) |
| Balance at end of period | | $ | 8,400 | | | $ | 7,937 | |
| Note 1: | Refund of the deposits received from customer for services not rendered during 2023. China-based clients who prepaid for surrogacy and ancillary caring services requested refund of fees so such clients can appoint their own surrogate mothers in countries in which the Company does not conduct business. The Company sent the funds to accounts dictated by the clients and terminated service contract with those clients. |
NEWGENIVF GROUP LIMITED
NOTES TO THE UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
SEPTEMBER 30, 2024 AND 2023
(Stated in US Dollars)
NOTE 9 — LEASES
The Company has various operating leases for clinics and office spaces. The lease agreements do not specify an explicit interest rate. The Company’s management believes that the interest rate of 6.6% and 5% was the most indicative rate of the Company’s borrowing cost for the calculation of the present value of the lease payments.
As of September 30, 2024 and December 31, 2023, the right-of-use assets totaled $293,168 and $283,847, respectively.
As of September 30, 2024 and December 31,2023, lease liabilities consist of the following:
| | | September 30, | | | December 31, | |
| | | 2024 | | | 2023 | |
| Lease liabilities – current portion | | $ | 213,546 | | | $ | 207,128 | |
| Lease liabilities – non-current portion | | | 122,981 | | | | 118,979 | |
| Total | | $ | 336,527 | | | $ | 326,107 | |
Other lease information is as follows:
| | | September 30, | | | December 31, | |
| | | 2024 | | | 2023 | |
| Weighted-average remaining lease term – operating leases | | | 0.67 years | | | | 0.92 years | |
| Weighted-average discount rate – operating leases | | | 5 | % | | | 5 | % |
| Short term lease cost | | $ | 100,009 | | | $ | 114,937 | |
The following is a schedule of future minimum payments under operating leases as of September 30, 2024:
| | | September 30,
2024 | |
| Not later than 1 year | | $ | 214,375 | |
| Between 1 to 2 years | | | 84,910 | |
| Between 2 to 3 years | | | 37,600 | |
| Total lease payments | | | 336,885 | |
| Less: imputed interest | | | (358 | ) |
| Total operating lease liabilities, net of interest | | $ | 336,527 | |
NEWGENIVF GROUP LIMITED
NOTES TO THE UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
SEPTEMBER 30, 2024 AND 2023
(Stated in US Dollars)
NOTE 10 — EQUITY
Ordinary shares
As at September 30, 2024, the Company is authorized to issue 201,200,000 ordinary shares with no par value. Each ordinary share is entitled to one vote. The holders of ordinary shares are also entitled to receive dividends whenever funds are legally available and when declared by the Board of Directors of the Company.
On April 3, 2024, the Company completed the business combination with A SPAC I Acquisition Corp.
The equity of the Company as of September 30, 2024 and December 31, 2023 represents 10,149,386 and 698,123 ordinary shares amounting to $0 and $6,981, respectively.
Subscription receivables
| | | September 30, | | | December 31, | |
| | | 2024 | | | 2023 | |
| Balance at beginning of year | | $ | 2,967,100 | | | $ | 319,872 | |
| Issuance of shares (Note 1) | | | — | | | | 2,866,856 | |
| Settlement of subscription receivable (Note 2) | | | — | | | | (219,628 | ) |
| Business combination | | | (2,967,100 | ) | | | — | |
| Total | | $ | — | | | $ | 2,967,100 | |
| Note 1: | On August 15, 2022, the Company issued and allotted additional 41,830 ordinary shares to Seazen Resources Investment Limited (“Seazen”) at the consideration of $961,538, of which other borrowings of $641,025 and $641 settlement was offset with consideration as partial settlement and $319,872 was subscription receivable due from Seazen. |
| Note 2: | On January 18, 2023, the Company received $192,308 from Seazen, reducing the subscription receivable by $192,308. On January 10, 2023, the Company issued and allotted additional 27,293 ordinary shares to Tung Donald Fan and Hok Lun Alan Lau at the consideration of $812,573. On December 4, 2023, the Company issued and allotted additional 69,000 shares to DoubleClick Services Limited at $2,054,283. Among the subscription receivable during the year, $27,320 was settled by the professional consulting service rendered during the year ended December 31, 2023. |
Additional paid-in capital
| | | September 30, | | | December 31, | |
| | | 2024 | | | 2023 | |
| Balance at beginning of period | | $ | 4,324,834 | | | $ | 1,458,941 | |
| Issuance of shares (Note 1) | | | — | | | | 2,865,893 | |
| Business combination | | | (2,909,834 | ) | | | — | |
| Total | | $ | 1,415,000 | | | $ | 4,324,834 | |
| Note 1: | On August 15, 2022, the Company issued 41,830 ordinary shares to Seazen, increasing the additional paid-in capital by $961,120. On January 10, 2023, the Company issued 27,293 ordinary shares to professional party for consulting service of 10 years, increasing the additional paid-in capital by $812,300. On December 4, 2023, the Company issued additional 69,000 shares to DoubleClick Services Limited for consulting service of 10 years, increasing the additional paid-in capital by $2,053,593. |
NOTE 11 — EMPLOYEE BENEFIT PLANS
HK SAR
The Company has a defined contribution pension scheme for its qualifying employees. The scheme assets are held under a provident fund managed by an independent fund manager. The Company and its employees are each required to make contributions to the scheme calculated at 5% of the employees’ basic salaries on monthly basis.
NEWGENIVF GROUP LIMITED
NOTES TO THE UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
SEPTEMBER 30, 2024 AND 2023
(Stated in US Dollars)
NOTE 11 — EMPLOYEE BENEFIT PLANS (cont.)
Thailand
The Company is obliged to make social security payments within the first 15 days of the month over which it is accrued. Special concession had been determined by the Government which saw the standard amount THB750 per month per person reduced to THB450 per month per person.
Cambodia
Every business employing one or more workers must register its business and workers with the National Social Security Fund (the “NSSF”) for the Occupational Risk Scheme (for work-related accidents and occupational diseases), the Health Care Scheme and the Pension Scheme.
Once registered, the business must pay to the NSSF:
| | ● | A monthly contribution equivalent to 0.8% of each worker’s monthly average wages (between $0.40 and $2.40 per month per worker) for the Occupational Risk Scheme. |
| | ● | A monthly contribution equivalent to 2.6% of a worker’s monthly average wages (between $1.30 and $7.80 per month per worker) for the Health Care Scheme. |
| | ● | A monthly contribution to the compulsory Pension Scheme, which is jointly paid by the employer and the employee at the same rate of 2% (total of 4%) of the contributable wage for the first five years. The contributable wage for the Pension Scheme ranges from between KHR400,000 (approximately $100) up to KHR1,200,000 (approximately $300). |
Kyrgyzstan
The Company has a defined contribution pension scheme for its qualifying employees. The scheme assets are held under a provident fund managed by an independent fund manager. The Company and its employees are each required to make contributions to the scheme calculated at 15% and 8%, respectively of the employees’ basic salaries on monthly basis.
NOTE 12 — PROVISION FOR INCOME TAXES
Cayman Islands
NewGenIvf Limited was incorporated in the Cayman Islands and is not subject to tax on income or capital gains under current Cayman Islands law. In addition, upon payment of dividends by these entities to the shareholders, no Cayman Islands withholding tax will be imposed.
HK SAR
Under the two-tiered profits tax rates regime, Hong Kong tax residents are subject to Hong Kong Profits Tax in respect of profits arising in or derived from Hong Kong at 8.25% for the first HK$2 million of profits of the qualifying group entity, and profits above HK$2 million will be taxed at 16.5%. The profits of group entities not qualifying for the two-tiered profits tax rates regime will continue to be taxed at a flat rate of 16.5%.
Accordingly, the HK SAR profits tax is calculated at 8.25% on the first HK$2 million of the estimated assessable profits and at 16.5% on the remaining estimated assessable profits.
Thailand
The companies incorporated in Thailand are taxed on worldwide income. A company incorporated abroad is taxed on its profits arising from or in consequence of the business carried on in Thailand. The corporate income tax (CIT) rate is 20%. A foreign company not carrying on business in Thailand is subject to a final withholding tax (WHT) on certain types of assessable income (e.g. interest, dividends, royalties, rentals, and service fees) paid from or in Thailand. The rate of tax is generally 15%, except for dividends, which is 10%, while other rates may apply under the provisions of a double tax treaty (DTT).
NEWGENIVF GROUP LIMITED
NOTES TO THE UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
SEPTEMBER 30, 2024 AND 2023
(Stated in US Dollars)
NOTE 12 — PROVISION FOR INCOME TAXES (cont.)
Cambodia
The standard rate of corporate income tax (“CIT”) for companies and permanent establishments who are classified as medium and large taxpayers is 20%. For companies and permanent establishments who are classified as small taxpayers, the CIT rates are progressive rates from 0% to 20%. In view of the annual turnover of the company, the annual turnover ranges from KHR1 billion to KHR6 billion for service and commercial sectors, the company shall consider as the medium-sized company.
Kyrgyzstan
The company is subject to a corporate income tax on their aggregate annual income earned worldwide. Non-resident legal entities carrying out business activities through a permanent establishment in Kyrgyzstan are subject to profit tax on the income attributed to the activities of that permanent establishments.
Profit tax is calculated at a rate of 10% of aggregate annual income less allowed deductions.
Significant components of the provisions for income taxes for the six months ended September 30, 2024, and 2023 were as follows:
| | | September 30, | |
| | | 2024 | | | 2023 | |
| Current tax provision Kyrgyzstan | | $ | — | | | $ | 83,742 | |
| Current tax provision Cambodia | | | — | | | | — | |
| Late penalty provision Kyrgyzstan | | | — | | | | — | |
| Total provision for income taxes | | $ | — | | | $ | 83,742 | |
NEWGENIVF GROUP LIMITED
NOTES TO THE UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
SEPTEMBER 30, 2024 AND 2023
(Stated in US Dollars)
NOTE 12 — PROVISION FOR INCOME TAXES (cont.)
| | | September 30, | |
| | | 2024 | | | 2023 | |
| Income/(loss) before taxes | | $ | (418,123 | ) | | $ | (187,166 | ) |
| Tax expenses/(credit) at the effective tax rates | | | (170,443 | ) | | | (116,672 | ) |
| Tax effect on non-taxable income | | | (761 | ) | | | — | |
| Tax effect on non-deductible expenses | | | 119,130 | | | | 204,226 | |
| Tax effect on utilization of tax losses | | | 52,074 | | | | (3,812 | ) |
| Income taxes | | $ | — | | | $ | 83,742 | |
Deferred tax asset, net
Significant components of deferred tax assets, net were as follows:
| | | September 30,
2024 | | | December 31,
2023 | |
| | | USD | | | USD | |
| Deferred tax assets: | | | | | | |
| – Net operating loss carry forward | | | 28,441 | | | | 28,441 | |
| Less: valuation allowance | | | (28,441 | ) | | | (28,441 | ) |
| Deferred tax assets, net | | | — | | | | — | |
As of September 30, 2024 and December 31, 2023, the Company had net operating loss carry forward of $164,721 and $164,721. The Company believes it is less likely than not that its operations will be able to fully utilize its deferred tax assets related to the net operating loss carry forward. As a result, the Company provided 100% allowance on deferred tax assets on net operating loss.
NOTE 13 — DISAGGREGATED REVENUES
The Company’s main business operations are to provide: (i) IVF treatment service; and (ii) surrogacy and ancillary caring services.
| | | Nine months ended
September 30, | |
| Revenue from external customers | | 2024 | | | 2023 | |
| IVF treatment service | | $ | 3,637,250 | | | $ | 3,162,400 | |
| Surrogacy, ancillary caring and other services | | | 522,513 | | | | 454,298 | |
| Total revenues | | $ | 4,159,763 | | | $ | 3,616,698 | |
NEWGENIVF GROUP LIMITED
NOTES TO THE UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
SEPTEMBER 30, 2024 AND 2023
(Stated in US Dollars)
NOTE 13 — DISAGGREGATED REVENUES (cont.)
Geographical information
| | | Nine months ended
September 30, | |
| Revenue from external customers originated from | | 2024 | | | 2023 | |
| HK SAR | | $ | — | | | $ | — | |
| Kyrgyzstan | | | 2,066,992 | | | | 2,184,214 | |
| Cambodia | | | 461,232 | | | | 424,130 | |
| Thailand | | | 1,631,539 | | | | 1,008,354 | |
| Total revenues | | $ | 4,159,763 | | | $ | 3,616,698 | |
The revenue information above is based on the locations where the revenue originated.
| | | September 30, | | | December 31, | |
| Long-lived assets located at | | 2024 | | | 2023 | |
| HK SAR | | $ | 260 | | | $ | 584 | |
| Cambodia | | | 132,414 | | | | 137,472 | |
| Thailand | | | 362,433 | | | | 307,948 | |
| | | $ | 495,107 | | | $ | 446,004 | |
The Company’s long-lived assets consist of plant and equipment, net and operating leases right-of-use assets, net.
NOTE 14 — RISKS
A. Credit risk
Accounts receivable
In order to minimize the credit risk, the management of the Company monitors and ensures that follow-up action is taken to recover overdue debts. The Company considers the probability of default upon initial recognition of asset and whether there has been a significant increase in credit risk on an ongoing basis throughout each reporting period. To assess whether there is a significant increase in credit risk, the Company compares the risk of a default occurring on the asset as at the reporting date with the risk of default as at the date of initial recognition. It considers available reasonable and supportive forward-looking information, such as GDP growth rate and nominal GDP per capita. Based on the impairment assessment performed by the Company, the directors consider the loss allowance for account receivables as of September 30, 2024 and December 31, 2023 is $Nil and $19, respectively.
NEWGENIVF GROUP LIMITED
NOTES TO THE UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
SEPTEMBER 30, 2024 AND 2023
(Stated in US Dollars)
NOTE 14 — RISKS (cont.)
A. Credit risk (cont.)
Cash and cash equivalents
The credit risk on liquid funds is limited because the counterparties are banks with high credit ratings assigned by international credit-rating agencies. The Company is exposed to concentration of credit risk on liquid funds which are deposited with several banks with high credit ratings.
Deposits and other receivables, amount due from shareholders and loan to A SPAC I
The Company assessed the impairment for deposits and other receivables, due from shareholders and loan to A SPAC I individually based on internal credit rating and ageing of these debtors which, in the opinion of the directors, have no significant increase in credit risk since initial recognition. Based on the impairment assessment performed by the Company, the directors consider the loss allowance for deposits and other receivables, due from shareholders and loan to A SPAC I as of September 30, 2024 is $14, $Nil and $ Nil, respectively. The loss allowance for deposits and other receivables, due from shareholders and loan to A SPAC I as of December 31, 2023 is $14, $17,818 and Nil, respectively.
B. Interest risk
Cash flow interest rate risk
The Company is exposed to cash flow interest rate risk through the changes in interest rates related mainly to the Company’s variable-rates bank balances.
The Company currently does not have any interest rate hedging policy in relation to fair value interest rate risk and cash flow interest rate risk. The directors monitor the Company’s exposures on an ongoing basis and will consider hedging the interest rate should the need arises.
Sensitivity analysis
The sensitivity analysis below has been determined by assuming that a change in interest rates had occurred at the end of the reporting period and had been applied to the exposure to interest rates for financial instruments in existence at that date. 1% increase or decrease is used when reporting interest rate risk internally to key management personnel and represents management’s assessment of the reasonably possible change in interest rates.
If interest rates had been 1% higher or lower and all other variables were held constant, the Company’s net (loss) income for the nine months ended September 30, 2024 and 2023 would have increased or decreased by approximately $1,202 and $773, respectively.
NEWGENIVF GROUP LIMITED
NOTES TO THE UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
SEPTEMBER 30, 2024 AND 2023
(Stated in US Dollars)
NOTE 14 — RISKS (cont.)
B. Interest risk (cont.)
Foreign currency risk
Foreign currency risk is the risk that the holding of foreign currency assets will affect the Company’s financial position as a result of a change in foreign currency exchange rates.
The Company’s monetary assets and liabilities are mainly denominated in HK$, THB, KHR and RMB which are the same as the functional currencies of the relevant group entities. Hence, in the opinion of the directors of the Company, the currency risk of US$ is considered insignificant. The Company currently does not have a foreign currency hedging policy to eliminate currency exposures. However, the directors monitor the related foreign currency exposure closely and will consider hedging significant foreign currency exposures should the need arise.
C. Economic and political risks
The Company’s operations are mainly conducted in Thailand, Cambodia and Kyrgyzstan. Accordingly, the Company’s business, financial condition, and results of operations may be influenced by changes in the political, economic, and legal environments in Thailand, Cambodia and Kyrgyzstan.
The Company’s operations in Thailand, Cambodia and Kyrgyzstan are subject to special considerations and significant risks. These include risks associated with, among others, the political, economic and legal environment and foreign currency exchange. The Company’s results may be adversely affected by changes in the political and social conditions in Thailand, Cambodia and Kyrgyzstan, and by changes in governmental policies with respect to laws and regulations, anti-inflationary measures, currency conversion, remittances abroad, and rates and methods of taxation, among other things.
D. Inflation risk
Management monitors changes in prices levels. Historically inflation has not materially impacted the Company’s consolidated financial statements; however, significant increases in the price of labor that cannot be passed to the Company’s customers could adversely impact the Company’s results of operations.
NEWGENIVF GROUP LIMITED
NOTES TO THE UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
SEPTEMBER 30, 2024 AND 2023
(Stated in US Dollars)
NOTE 15 — RELATED PARTY BALANCES AND TRANSACTIONS
The summary of amount due from and due to related parties as the following:
| | | | | September 30, | | | December 31, | |
| | | Relationship | | 2024 | | | 2023 | |
| Due from shareholders consist of the following: | | | | | | | | | | |
| Mr. Siu Wing Fung, Alfred (“Mr. Siu”) and Ms. Fong Hei Yue, Tina (“Ms. Fong”) | | Shareholders and directors (note 1) | | $ | — | | | $ | 354,285 | |
| | | | | | | | | | | |
| Due to a related party consist of the following: | | | | | | | | | | |
| Mr. Siu Wing Fung, Alfred (“Mr. Siu”) and Ms. Fong Hei Yue, Tina (“Ms. Fong”) | | Shareholders and directors (note 1) | | $ | (221,203 | ) | | $ | — | |
The balance due from shareholders consist of the following:
| | | September 30, | | | December 31, | |
| | | 2024 | | | 2023 | |
| Due (to) from shareholders | | $ | (221,203 | ) | | $ | 372,103 | |
| Less: allowance for expected credit loss | | | — | | | | (17,818 | ) |
| | | $ | (221,203 | ) | | $ | 354,285 | |
NEWGENIVF GROUP LIMITED
NOTES TO THE UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
SEPTEMBER 30, 2024 AND 2023
(Stated in US Dollars)
NOTE 15 — RELATED PARTY BALANCES AND TRANSACTIONS (cont.)
The movement of allowances for expected credit loss is as follow:
| | | September 30, | | | December 31, | |
| | | 2024 | | | 2023 | |
| Balance at beginning of the period | | $ | (17,818 | ) | | $ | (17,059 | ) |
| Provision | | | — | | | | (759 | ) |
| Revised | | | 17,818 | | | | — | |
| Ending balance | | $ | — | | | $ | (17,818 | ) |
In addition to the transactions and balances detailed elsewhere in these consolidated financial statements, the Company had the following transactions with related parties:
| | | September 30, | |
| | | 2024 | | | 2023 | |
| Directors’ remuneration to Mr. Siu Wing Fung, Alfred | | $ | 93,750 | | | $ | 90,000 | |
| Directors’ remuneration to Ms. Fong Hei Yue, Tina | | | 93,750 | | | | 90,000 | |
NEWGENIVF GROUP LIMITED
NOTES TO THE UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
SEPTEMBER 30, 2024 AND 2023
(Stated in US Dollars)
NOTE 16 — CONTINGENCIES
As of September 30, 2024 and 2023, the Company was not a party to any legal or administrative proceedings.
First Fertility Bishkek LLC (“FFB”), the Company incorporated in Kyrgyzstan, did not report the current year tax to the tax authority till the reporting date since 2023. The late tax filing may lead to contingent tax penalty as of September 30, 2024. The tax return is not yet filed so it is not possible to give the Company evaluation of the likelihood of the outcome or estimate the possible amount of tax penalty. The contingent tax penalty is reasonably possible and estimated at $486,706. Thus, no futher provision was made. Except the potential tax issue, the Company concludes that there was no contingent liability, either individually or in the aggregate, that could have resulted in an unfavorable outcome with a material adverse effect on the Company’s results of operations, consolidated financial condition, or cash flows.
NOTE 17 — segment information
The Company uses the management approach to determine reportable operating segments. The management approach considers the internal organization and reporting used by the Company’s CODM, specifically the Group’s CEO and CFO, for making decisions, allocating resources and assessing performance.
The Company does not distinguish revenues, costs and expenses between segments in its internal reporting, but instead reports costs and expenses by nature as a whole. Based on the management’s assessment, the Group determines that it has only one operating segment and therefore one reportable segment as defined by ASC 280. As such, all financial segment information required by the authoritative guidance can be found in these consolidated financial statements.
NOTE 18 — SUBSEQUENT EVENTS
In accordance with ASC Topic 855, “Subsequent Events”, which establishes general standards of accounting for and disclosure of events that occur after the balance sheet date but before consolidated financial statements are issued, the Company evaluated all events or transactions that occurred after September 30, 2024, up through the date the Company.
Subsequent to the end of September 2024, $1,300,000 of convertible promissory note was converted into ordinary share by note holder in November 2024, thereby increasing our shareholder equity by $1,300,000.











