
Virtual KOL Investor Event Series Part II: �Review FG-3246 Development Program in Metastatic Castration-Resistant Prostate Cancer � �Hosted by FibroGen Inc. Wednesday, June 26, 2024

This presentation contains “forward-looking” statements that involve substantial risks and uncertainties. All statements other than statements of historical facts contained in this presentation, including statements regarding our future financial condition, business strategy, and plans, and objectives of management for future operations, are forward looking statements. These forward-looking statements can generally be identified by terminology such as “believe,” “will,” “may,” “estimate,” “continue,” “anticipate,” “contemplate,” “intend,” “target,” “project,” “should,” “plan,” “expect,” “predict,” “could,” “or potentially,” or by the negative of these terms or other similar expressions. Forward-looking statements appear in a number of places throughout this presentation and include statements regarding our intentions, beliefs, projections, outlook, analyses, or current expectations concerning, among other things, ongoing and planned development and clinical trials, the potential safety, efficacy, reimbursement, convenience, or clinical and pharmaco-economic benefits of our product candidates, and the potential markets for any of our product candidates. Forward-looking statements involve known and unknown risks, uncertainties, assumptions, and other factors that may cause our actual results, performance, or achievements to be materially different from any future results, performance, or achievements expressed or implied by the forward-looking statements, including the other risks and uncertainties that are described in the Risk Factors section of our most recent annual report on Form 10-K or quarterly report on Form 10-Q filed with the Securities and Exchange Commission. Forward-looking statements represent our management’s beliefs and assumptions only as of the date of this presentation. Except as required by law, we assume no obligation to update these forward-looking statements publicly, or to update the reasons why actual results could differ materially from those anticipated in the forward-looking statements, even if new information becomes available in the future. Forward-Looking Statements

FibroGen Strategic Pillars and Investment Highlights Pamrevlumab readouts for pancreatic cancer, targeting a significant unmet medical need and representing a multi-billion-dollar revenue opportunity: ADC=antibody drug conjugate; CIA=chemotherapy-induced anemia; IND=investigational new drug; mAb=monoclonal antibody; mCPRC=metastatic castration-resistant prostate cancer. Pamrevlumab Pivotal Clinical Trial Readouts $214.7M in cash, cash equivalents, investments, and accounts receivable as of March 31, 2024. Expected to fund operating plans into 2026. Growing Roxadustat Revenue and Cash Flow Strong Balance Sheet Growing revenue and cash flow stream from roxadustat; approved in > 40 countries and commercialized by AstraZeneca and Astellas. sNDA accepted in China for chemotherapy induced anemia, approval decision expected in 2H 2024. FibroGen regains rights to roxadustat in AZ territories (excluding China and South Korea): creating potential partnership opportunities in indications such as anemia in patients with LR-MDS. Early-Stage Oncology Pipeline FG-3246 (CD46-targeting ADC) for mCRPC: Data from multiple Phase 1 studies in 2024. Initiation of Phase 2 monotherapy dose optimization study in 2H 2024. FG-3165 (Galectin-9 targeting mAb) for solid tumors: IND clearance in 2Q 2024. FG-3175 (CCR8 targeting mAb) for solid tumors: IND in 2025. Precision PromiseSM Phase 2/3 topline expected mid-2024 LAPIS Phase 3 topline expected 3Q 2024

Rahul Aggarwal, MD Associate Director for Clinical Sciences, UCSF Helen Diller Family Comprehensive Cancer Center �Professor of Medicine UCSF Division of Hematology/Oncology FG-3246 is an investigational drug and not approved for marketing by any regulatory authority. Review FG-3246 Development Program in Metastatic Castration-Resistant Prostate Cancer

Dr. Rahul Aggarwal Prostate Cancer Deep Dive Rahul Aggarwal, MD

Prostate Cancer Facts 3.4 million men live with prostate cancer in the US It is the second most common cancer type after breast cancer. ~13% of men will be diagnosed with prostate cancer at some point during their lifetime While most men diagnosed with prostate cancer can still live long lives, there are ~ 65K drug treatable cases in the US annually, where cancer has spread (metastasized) and become castrate resistant (mCRPC) 5-year survival in mCRPC is ~30%4,5 Prostate Cancer is the Most Common Cancer in Men and Has a High Unmet Need for Treatment Options That Extend Survival in Late-Stage Disease Therapies that extend survival in patients who are ineligible or progressed on ARSI and/or chemo Therapies with novel MOAs for patients with advanced mCRPC who progressed on available treatment options Identification of predictive molecular markers in conjunction with novel therapies to inform patient selection Optimal combination and sequencing of therapies Highest Unmet Needs in mCRPC 1. SEER, 2024. https://seer.cancer.gov/statfacts/html/prost.html. 2. DRG, 2024. 3. Bernal A, et al. Pharmaceuticals (Basel). 2024;17(3):351. 4. American Cancer Society 2024. 5. NIH 2024.

Prostate Cancer Diagnosis and Progression Overview Source: DRG, 2024 Newly Diagnosed Low & Intermediate Risk (Hormone Sensitive) Newly Diagnosed High Risk & N1M0 (Hormone Sensitive) Newly Diagnosed Metastatic (Hormone Sensitive) Biochemical Recurrent Cases Biochemically Recurrent (Hormone Sensitive) Rising PSA (biochemical progression) following primary treatment & remain hormone sensitive Rising PSA despite continued hormonal treatment Castrate-resistant at the time of initial biochemical recurrence Metastatic (Hormone Sensitive) 1L Metastatic Castrate Resistant 2L Metastatic Castrate Resistant 3L+ Metastatic Castrate Resistant ~32K Drug Treatable US ~21K Drug Treatable US ~12K Drug Treatable US Diagnosed Incident Cases Metastatic Recurrent Cases Non-Metastatic Castrate-Resistant

Prostate Cancer Diagnosis and Progression Overview Source: DRG, 2024 Newly Diagnosed Low & Intermediate Risk (Hormone Sensitive) Newly Diagnosed High Risk & N1M0 (Hormone Sensitive) Newly Diagnosed Metastatic (Hormone Sensitive) Biochemical Recurrent Cases Biochemically Recurrent (Hormone Sensitive) Rising PSA (biochemical progression) following primary treatment & remain hormone sensitive Rising PSA despite continued hormonal treatment Castrate-resistant at the time of initial biochemical recurrence Metastatic (Hormone Sensitive) 1L Metastatic Castrate Resistant 2L Metastatic Castrate Resistant 3L+ Metastatic Castrate Resistant ~32K Drug Treatable US ~21K Drug Treatable US ~12K Drug Treatable US Diagnosed Incident Cases Metastatic Recurrent Cases Non-Metastatic Castrate-Resistant Potential placement of FG-3246 within treatment paradigm: For treatment of CD46high patients in 1L or 2L mCRPC as monotherapy or in combination For 1L mCRPC patients who progressed on ARSI in mHSPC or nmCRPC For 2L mCRPC patients who progressed on no more than 1 ARSI and have not received chemo in mCRPC FG-3246 is an investigational drug and not approved for marketing by any regulatory authority.

Dr. Rahul Aggarwal mCRPC Treatment Landscape
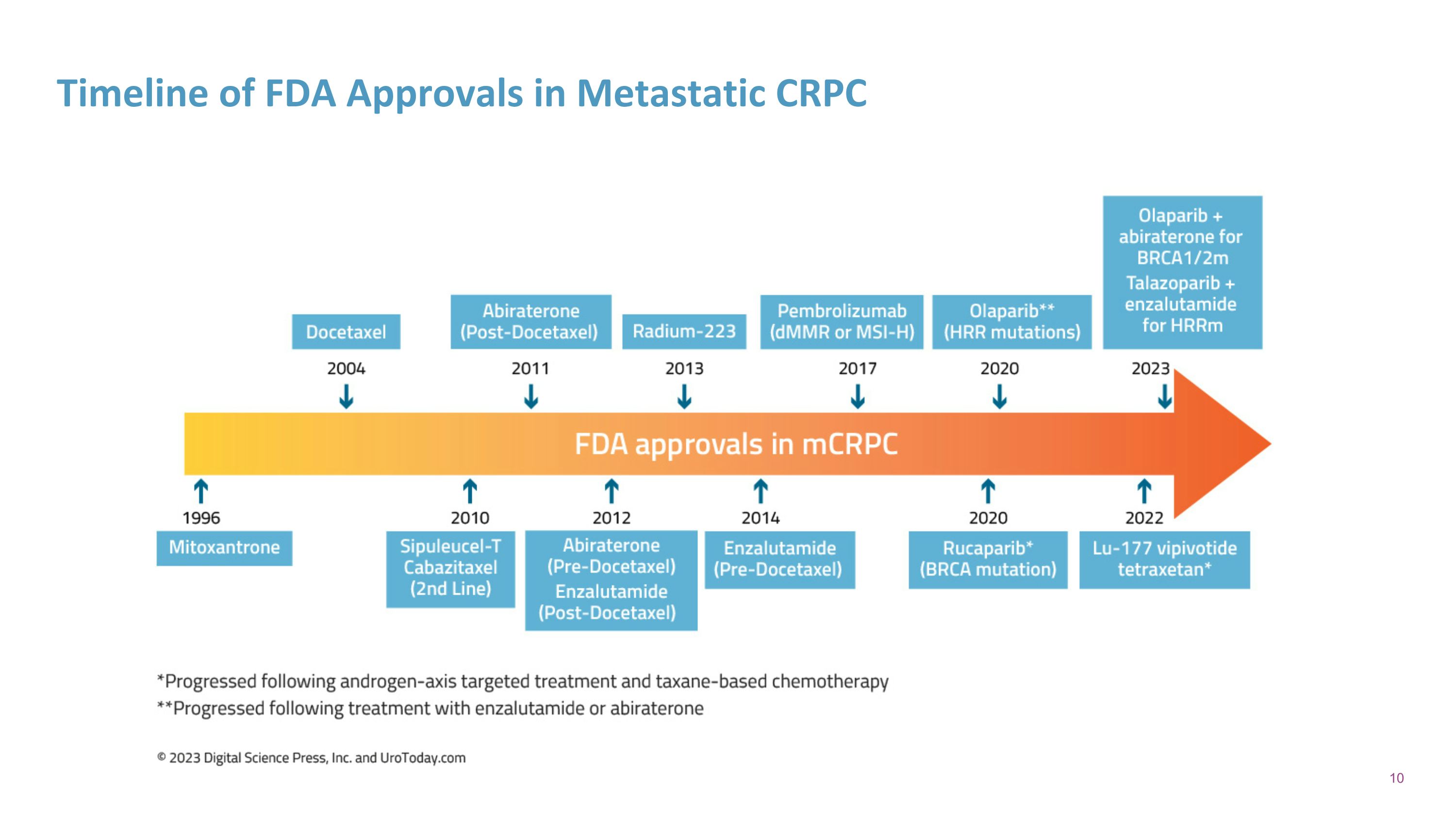
Timeline of FDA Approvals in Metastatic CRPC

mCRPC Treatment Algorithm based on NCCN Guidelines Version 3.2024 Color Key Abbreviations: AR-Tx = AR-targeted therapy AA = abiraterone ENZA = enzalutamide APA = apalutamide DARO = darolutamide D = docetaxel CABAZ = cabazitaxel AVPC = aggressive variant PCa NEPC = neuroendocrine PCa Lu-PSMA = 177Lu-PSMA-617 Plat = Platinum *MSI-H/dMMR Algorithm based on NCCN Guidelines Version 3.2024 Note: ADT continued with all combinations Ra-223 Plat NEPC BSC AA or ENZA D D Plat Sip-T AA or ENZA AA or ENZA CABAZ CABAZ or D rechallenge AA or ENZA Lu-PSMA CABAZ or D AA or ENZA D PARPi Pembro Prior AR-Tx only Prior D only Prior AR-Tx + D No prior AR-Tx or D No visc. mets Unselected Bone only MSI-H*, TMB-H BRCA/HRRm AVPC PSMA+ Ra-223 Sip-T PARPi Pembro Plat Ra-223 PARPi Pembro Plat Ra-223 PARPi Pembro Immunotherapy Chemotherapy AR-targeted therapy Targeted therapy Radiotherapy

Evolving Treatment Landscape in Post-AR, Chemo-Naïve mCRPC Non-Targeted Efficacy Benchmarks (US, 2024): ARSI Switch rPFS: 5.5m-6.5m, Chemo rPFS: 8.0m-8.5m Targeted Efficacy Benchmarks (US, 2024): Post ARSI/ Pre Chemo rPFS: 9.5m-12m Rucaparib* vs. AA/ENZA or D �(TRITON3) in BRCA mut Months 177Lu-PSMA-617* vs. AA/ENZA (PSMAfore) in ITT *Trial met primary endpoint, FDA approval pending **Included patients with visceral disease or measurable extrapelvic adenopathy. While we cannot make direct comparisons to other trials due to differences in study design, analysis methods, population sizes, controls and phases of development, we are encouraged there are opportunities for new treatments 177LuPNT2002* vs. AA/ENZA (SPLASH) Cabozantinib + atezo* vs AA/ENZA (CONTACT-02)** NA NA Active Control

Intermediate Endpoints in Metastatic CRPC Radiographic Progression-Free Survival PSA50 and PSA90 response rates Objective response rate The only surrogate endpoint that has been accepted for regulatory approval BICR and use of standard criteria (PCWG3 + modified RECIST 1.1) Useful for gauging preliminary anti-tumor activity May not be as applicable for all agents (e.g. radium-223) Useful for gauging preliminary anti-tumor activity Requires measurable disease by RECIST 1.1 (only ~ 30-40% of mCRPC)

Recently reported results for Investigational treatments in development for mCRPC Drug Name/Company Target/MOA Population PSA50 ORR (%) rPFS (median) or duration of tx Safety ARX517 Ambrx (now JNJ) ADC (amberstatin) targeting PSMA Metastatic CRPC, no biomarker selection, ≥ 2 prior systemic tx 52% (dose levels ≥ 2.0 mg/kg) 50% (2/4 patients) Not reported AE discontinuation rate 3% Grade 3 TRAEs 9.2% AMG 509 Amgen BiTE targeting STEAP1 Metastatic CRPC, no biomarker selection, at least one ARSI, 1-2 prior taxanes 59% (at higher dose levels) 41% (at higher dose levels) Not reported Cytokine release, myalgia/arthalgia, fatigue, anemia DS-7300 Daiichi Sankyo ADC (deruxtecan) targeting B7-H3 Metastatic CRPC, no biomarker selection, median 6 prior lines of therapy 21% (4/19 pts) 25% (15/59 evaluable pts) 4.8 months (3.9 – 5.9) Grade ≥ 3 TRAEs include anemia, neutropenia, diarrhea, nausea/vomiting JANX007 Janux BiTE targeting PSMA Metastatic CRPC, no biomarker selection 83% (starting step ≥ 0.2 mg, 5/6 patients) Not reported Not reported Cytokine release, other safety data no yet reported While we cannot make direct comparisons to other trials due to differences in study design, analysis methods, population sizes, controls and phases of development, we are encouraged there are opportunities for new treatments.

Dr. Rahul Aggarwal FG-3246 Product Characteristics and Mechanism of Action FG-3246 is an investigational drug and not approved for marketing by any regulatory authority.

FG-3246 Therapeutic Novel antibody-drug conjugate (ADC) Targeting antibody: YS5FL is a fully-human IgG1 monoclonal �antibody to tumor-selective epitope of CD46 Payload: MMAE - Potent anti-microtubule agent CD46 Transmembrane protein negatively regulates the complement system Upregulated during tumorigenesis Binds both C3b + C4b Helps tumors evade complement-dependent cytotoxicity (CDC) Overexpressed in prostate cancer, colorectal cancer, and other solid tumors vs. normal tissue PET46 Biomarker Utilizes same targeting antibody as FG-3246 89Zr biomarker demonstrated specific uptake in CD46 positive tumors FG-3246 – Potential First-in-Class ADC for the Treatment of mCRPC Mouse Human FG-3246 is an investigational drug and not approved for marketing by any regulatory authority.

Dr. Rahul Aggarwal FG-3246 Clinical Data Overview FG-3246 is an investigational drug and not approved for marketing by any regulatory authority.
Dr. Rahul Aggarwal FOR46-001 Phase I monotherapy study of FG-3246 administered Q3W in patients with metastatic castration resistant prostate cancer (mCRPC) Study sponsored and conducted by FORTIS NCT03575819 FG-3246 is an investigational drug and not approved for marketing by any regulatory authority.

Study Overview Phase 1 Study Design Primary Endpoints: Evaluate the safety and tolerability of FG-3246 in mCRPC patients, Determine the MTD and/or recommended Phase 2 dose in mCRPC patients Secondary Endpoints: Characterize the PK of FG-3246, YS5FL, and MMAE, Efficacy including rPFS, PSA50, and objective response rate Exploratory Endpoint: Evaluate potential relationships between CD46 expression and measures of antitumor activity Phase 1 monotherapy Phase 1, first-in-human, dose-escalation with expansion study of FG-3246 in patients with mCRPC Dose escalation, n = 33 Dose expansion, n = 23 Adenocarcinoma, n=18 Neuroendocrine, n=5 Initial accelerated titration for FG-3246 doses < 1.0 mg/kg (expanded to 3 if ≥ 1 Grade 2 treatment-related AE or a DLT), transitioning to standard 3 + 3 escalation FG-3246 starting dose level 0.1 mg/kg every 21 days, administered IV on Day 1 of each 21-day cycle Primary prophylaxis with G-CSF not mandated; secondary prophylaxis required for Grade ≥ 3 neutropenia Study Endpoints FG-3246 is an investigational drug and not approved for marketing by any regulatory authority.

Study Overview Eligibility Criteria Progressive metastatic castration resistant prostate cancer (CRPC) by PCWG3 criteria Prior treatment with at least one androgen signaling inhibitor (e.g., abiraterone, enzalutamide) No prior taxane for the treatment of metastatic CRPC Prior taxane for castration-sensitive disease allowed Dose expansion only Availability of CRPC tissue from newly acquired or archival tumor sample No histologic evidence of small cell neuroendocrine prostate cancer CD46 expression by IHC not required for eligibility Assessed retrospectively using a commercially-available monoclonal Ab �(clone 3F1, different epitope from YS5FL) when archival CRPC tissue available NCT0357819 Phase 1 monotherapy
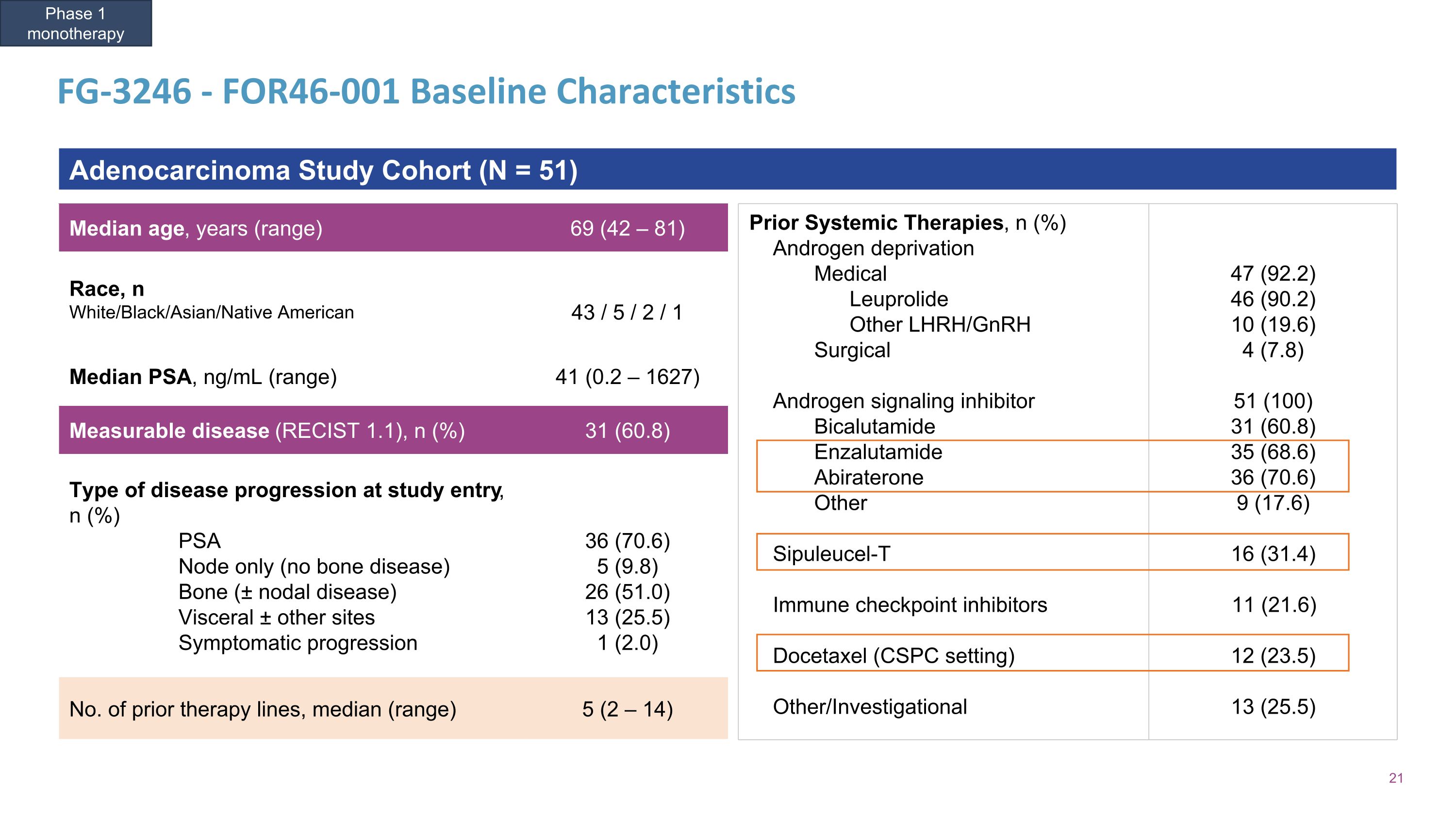
FG-3246 - FOR46-001 Baseline Characteristics Median age, years (range) 69 (42 – 81) Race, n White/Black/Asian/Native American 43 / 5 / 2 / 1 Median PSA, ng/mL (range) 41 (0.2 – 1627) Measurable disease (RECIST 1.1), n (%) 31 (60.8) Type of disease progression at study entry, n (%) PSA Node only (no bone disease) Bone (± nodal disease) Visceral ± other sites Symptomatic progression 36 (70.6) 5 (9.8) 26 (51.0) 13 (25.5) 1 (2.0) No. of prior therapy lines, median (range) 5 (2 – 14) Prior Systemic Therapies, n (%) Androgen deprivation Medical Leuprolide Other LHRH/GnRH Surgical Androgen signaling inhibitor Bicalutamide Enzalutamide Abiraterone Other Sipuleucel-T Immune checkpoint inhibitors Docetaxel (CSPC setting) Other/Investigational 47 (92.2) 46 (90.2) 10 (19.6) 4 (7.8) 51 (100) 31 (60.8) 35 (68.6) 36 (70.6) 9 (17.6) 16 (31.4) 11 (21.6) 12 (23.5) 13 (25.5) Adenocarcinoma Study Cohort (N = 51) Phase 1 monotherapy

Safe and generally well tolerated Adverse events consistent with other MMAE based antibody-drug conjugate (ADC) therapies Peripheral Neuropathy All Grades – 34.1%, Grade 3 or higher – 2.3% Neutropenia All Grades – 45.5%, Grade 3 or higher – 36.4% Infusion-Related Reactions All Grades - 47.7%, Grade 3 or higher – 2.3% No ocular toxicities 2.7 mg/kg ajbw declared as the MTD in the study; number and severity of AEs were dose-exposure related Dose optimization in the phase 2 trial will encompass 2 dose levels 2.4 mg/kg ajbw and 1.8 mg/kg ajbw FG-3246 Phase 1 Safety Summary Phase 1 monotherapy

All Grades by Patient (≥ 10%) All Grades N (%) ≥ Grade 3�N (%) Fatigue 25 (56.8) 3 (6.8) Weight decreased 23 (52.3) 1 (2.3) Infusion related reaction 21 (47.7) 1 (2.3) Nausea 20 (45.5) 0 Neutropenia 20 (45.5) 16 (36.4) Constipation 19 (43.2) 0 Decreased appetite 16 (36.4) 1 (2.3) Diarrhoea 16 (36.4) 0 Neutrophil count decreased 16 (36.4) 13 (29.5) White blood cell count decreased 16 (36.4) 12 (27.3) Neuropathy peripheral 15 (34.1) 1 (2.3) Anaemia 14 (31.8) 3 (6.8) Arthralgia 14 (31.8) 0 Alopecia 13 (29.5) 0 Hypoalbuminaemia 11 (25.0) 1 (2.3) Vomiting 11 (25.0) 0 Alanine aminotransferase increased 10 (22.7) 0 Aspartate aminotransferase increased 10 (22.7) 0 Back pain 10 (22.7) 1 (2.3) Lymphocyte count decreased 10 (22.7) 3 (6.8) All Grades by Patient (≥ 10%) All Grades N (%) ≥ Grade 3�N (%) Blood alkaline phosphatase increased 9 (20.5) 1 (2.3) Oedema peripheral 9 (20.5) 0 Abdominal pain 8 (18.2) 0 Blood creatinine increased 8 (18.2) 0 Dyspnoea 8 (18.2) 0 Hypocalcaemia 8 (18.2) 2 (4.5) Hypokalaemia 8 (18.2) 1 (2.3) Hypophosphotaemia 8 (18.2) 0 Pain in extremity 8 (18.2) 1 (2.3) Headache 7 (15.9) 0 Hyponatraemia 7 (15.9) 3 (6.8) Peripheral sensory neuropathy 7 (15.9) 0 Pyrexia 7 (15.9) 0 Blood lactate dehydrogenase increased 6 (13.6) 0 Hypomagnesaemia 6 (13.6) 0 Lymphopenia 6 (13.6) 1 (2.3) Tachycardia 6 (13.6) 0 Fall 5 (11.4) 0 Insomnia 5 (11.4) 0 Phase 1 monotherapy FG-3246 Safety Profile Consistent with Other MMAE-ADCs�Selected Cohorts - Safety (N=44) Number and severity of AEs were dose-exposure related; No new safety signals; All AEs were managed by institutional standard of care. Sources: Table 14.3.1.3.7 Summary of Grade ≥ 3 TEAE by Preferred Term Decreasing Frequency Table 14.3.1.3.9 Summary of All Grade TEAE by Preferred Term Decreasing Frequency Selected Cohorts: Dose escalation cohorts-level > 1.2 mg/kg, combined with cohort 1 (adenocarcinoma) of the Expansion cohort

8.7 Month rPFS - Potentially Clinically Meaningful Efficacy Phase 1 monotherapy Median rPFS (months) Selected Cohorts (N=40): 8.7 months �(n=27: 26 PD; & 1 Death Event) Selected Cohorts: Dose escalation cohorts-level > 1.2 mg/kg, combined with cohort 1 (adenocarcinoma) of the Expansion cohort
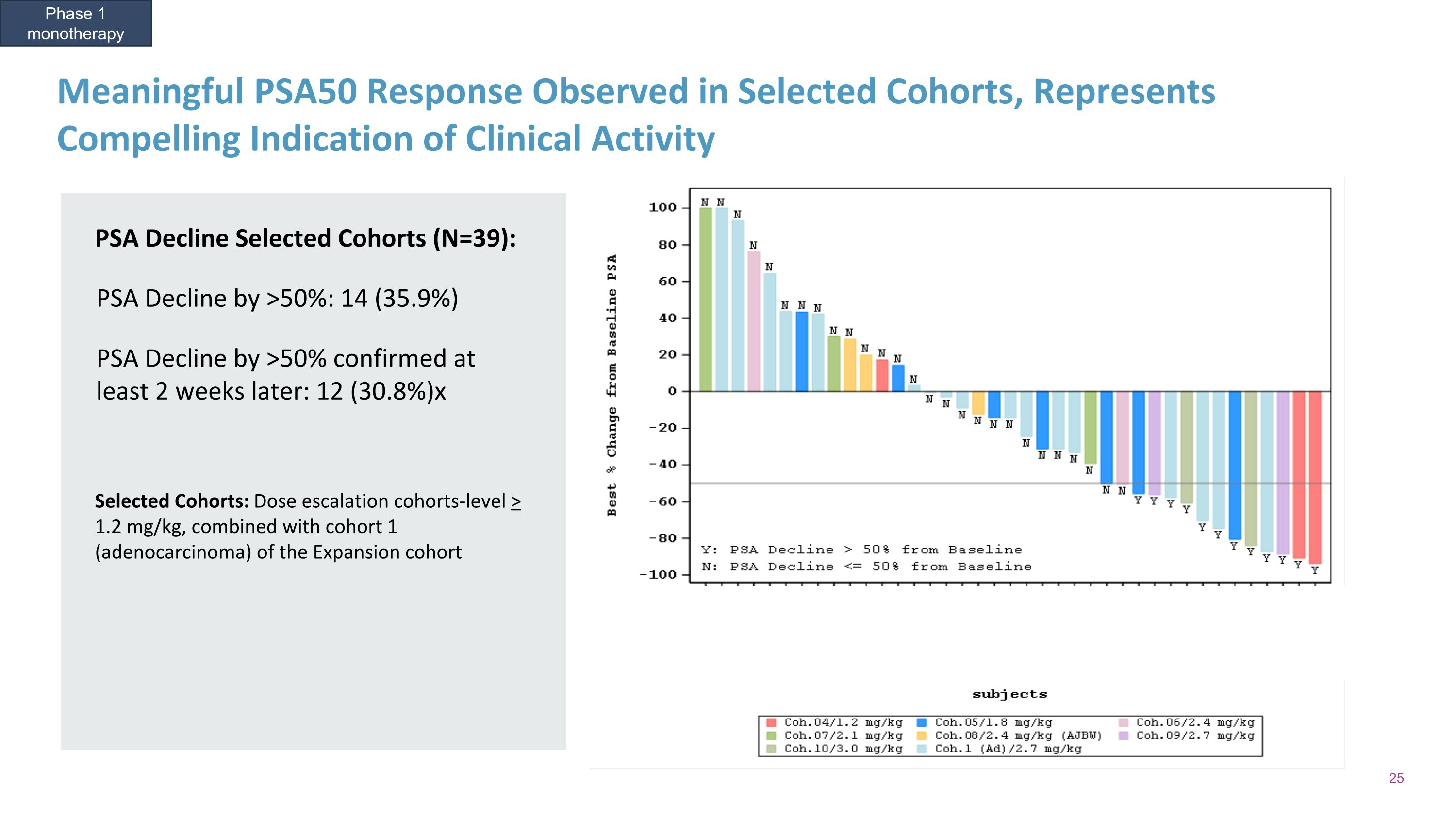
Meaningful PSA50 Response Observed in Selected Cohorts, Represents Compelling Indication of Clinical Activity Phase 1 monotherapy PSA Decline Selected Cohorts (N=39): PSA Decline by >50%: 14 (35.9%) PSA Decline by >50% confirmed at least 2 weeks later: 12 (30.8%)x Selected Cohorts: Dose escalation cohorts-level > 1.2 mg/kg, combined with cohort 1 (adenocarcinoma) of the Expansion cohort

Selected Cohorts (N=25) Confirmed ORR 5 (20%) Complete Response 0 Partial Response 5 (20%) Stable Disease 15 (60%) Prog. Dis 4 (16%) Indeterminate 1 (4%) Confirmed Objective Response Rate RECIST Evaluable Set (Selected Cohorts) Selected Cohorts: Dose escalation cohorts-level > 1.2 mg/kg, combined with cohort 1 (adenocarcinoma) of the Expansion cohort Phase 1 monotherapy

Duration of Response (DOR) in Selected Cohorts Median Tumor DOR (months) Selected Cohorts (N=25): 7.5 (N=5; 5 with ORR) Selected Cohorts: Dose escalation cohorts-level > 1.2 mg/kg, combined with cohort 1 (adenocarcinoma) of the Expansion cohort Phase 1 monotherapy

Single agent activity in heavily pretreated mCRPC (median 5 prior lines of therapy) 8.7 months rPFS Objective Response Rate (ORR) 20% Duration Of Response (DoR) 7.5 months PSA50 = 36% Safe and well tolerated to date Doses selected for Phase 2/3 registrational trial: 1.8 and 2.4 mg/kg 2.4 mg/kg and/or 1.8 mg/kg with secondary G-CSF prophylaxis to provide best risk/benefit profile Dose Optimization in the phase 2 component of the registration trial FG-3246 Phase 1 Monotherapy Summary Phase 1 monotherapy FG-3246 is an investigational drug and not approved for marketing by any regulatory authority.

Dr. Rahul Aggarwal Investigator Sponsored Study by Dr Rahul Aggarwal FG-3246 + Enza Phase 1b ASCO 2024 Interim Data Phase 1b Dose Escalation Study of FG-3246, a Novel Antibody-Drug Conjugate Targeting a Tumor Specific Epitope of CD46, in Combination with Enzalutamide (Enza) in Patients with mCRPC NCT05011188 FG-3246 is an investigational drug and not approved for marketing by any regulatory authority.

ARSIs Up-regulate CD46 in mCRPC and Sensitizes to FG-3246 Tx Provides rationale for combination therapy Su et al., JCI Insight 2018 LNCaP-C4-2B Du145 CD46 ADC Ctr ADC Ctr ADC + ASI ASI + CD46 ADC shows enhanced killing (EC50 on LNCaP-C4-2B: 13 pM vs. 226 pM)

Phase 1b Primary Endpoint Maximum tolerated dose (MTD) and recommended phase 2 dose (RP2D) of FG-3246 in combination w/ enzalutamide Secondary Endpoints PSA50 Response Rate Objective Response Rate by RECIST 1.1 criteria Median radiographic progression-free survival (rPFS) Overall Survival Frequency and severity of adverse events by CTCAE version 5.0 Exploratory Endpoints: Evaluating the association between… Tumor characteristics & AR transcriptional signature score with clinical outcomes. CD46 expression by immunohistochemistry with clinical outcomes Uptake on 89Zr-DFO-YS5 PET with clinical outcomes: 1-3 mCi 89Zr-DFO-YS5 was administered, and PET/CT imaging was performed 5-7 days post-injection on a Siemens Biograph Vision PET/CT scanner Study Design: Primary, Secondary and Exploratory Endpoints Eligible patients must have progressive mCRPC per PCWG3 criteria, at least 1 prior androgen-signaling inhibitor (ASI); no prior taxane for CRPC and an ECOG performance status ≤1. Phase 1b combination Presented at ASCO June 2024
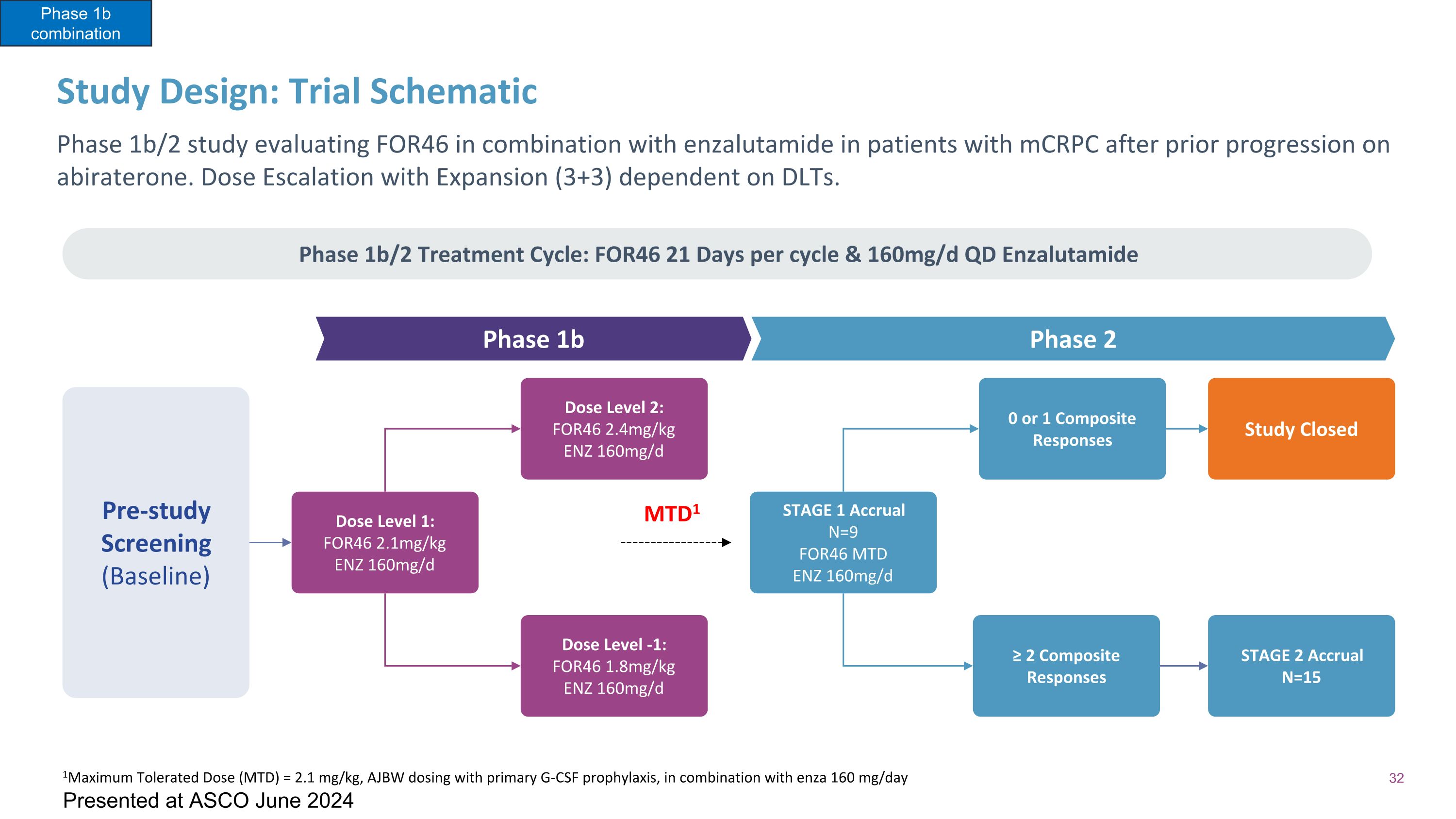
Study Design: Trial Schematic Phase 1b/2 study evaluating FOR46 in combination with enzalutamide in patients with mCRPC after prior progression on abiraterone. Dose Escalation with Expansion (3+3) dependent on DLTs. Phase 1b/2 Treatment Cycle: FOR46 21 Days per cycle & 160mg/d QD Enzalutamide Phase 1b Phase 2 Pre-study Screening (Baseline) Dose Level 1: FOR46 2.1mg/kg ENZ 160mg/d Dose Level 2: FOR46 2.4mg/kg ENZ 160mg/d Dose Level -1: FOR46 1.8mg/kg ENZ 160mg/d STAGE 1 Accrual N=9 FOR46 MTD ENZ 160mg/d 0 or 1 Composite Responses ≥ 2 Composite Responses Study Closed STAGE 2 Accrual N=15 MTD1 1Maximum Tolerated Dose (MTD) = 2.1 mg/kg, AJBW dosing with primary G-CSF prophylaxis, in combination with enza 160 mg/day Phase 1b combination Presented at ASCO June 2024

Treatment-related Adverse Events Treatment-related AEs that lead to treatment discontinuation included increased LFTs (transaminases) (n=1), Neuropathy, Grade 2 (n=3), and Hyponatremia (n =1) Phase 1b combination Presented at ASCO June 2024

Clinical Outcomes: DoT, rPFS Median time on treatment for patients in all dose levels (N = 17) was 6 months (range: 1 – 18) with 3 patients still on treatment. The preliminary estimate of median radiographic progression-free survival was 10.2 months. Phase 1b combination Duration of Treatment Radiographic Progression-free Survival Presented at ASCO June 2024

Clinical Outcomes: PSA The majority of patients (12/17 evaluable, 71%) experienced a decline in PSA levels post-treatment 5 of the 12 patients (42%) that experienced PSA decline had previous progression on enzalutamide There were two patients who experienced ≥ 50% decline from baseline in PSA Phase 1b combination PSA Change from Baseline Presented at ASCO June 2024

Zr-89 labeled YS5 Demonstrates Tumor Specific Uptake Phase 1b combination Axial PET/CT fusion images obtained 5 days following administration of 89Zr-DFO-YS5 in a 72 year old man with history of prostate adenocarcinoma, with prior treatments of Lupron, abiraterone, and enzalutamide, and a PSA of 53.5. PET/CT revealed multiple small lymph node metastases in the pelvis, retroperitoneum, and supraclavicular regions, and bone metastases in the pelvis. Presented at ASCO June 2024

The MTD and recommended phase 2 dose of FOR46 was established as 2.1 mg/kg adjusted body weight with primary G-CSF prophylaxis in combination with enzalutamide 160 mg daily Preliminary anti-tumor activity was observed with PSA declines in 12/17 (71%) of evaluable pts, and a preliminary estimate of median rPFS of 10.2 months Accrual is ongoing in Phase 2 with mandatory [89Zr]-YS5 PET imaging performed during screening to determine its potential utility in patient selection Phase 1b Conclusions: FG-3246 + Enzalutamide Phase 1b combination FG-3246 is an investigational drug and not approved for marketing by any regulatory authority. Presented at ASCO June 2024

Opportunity �mCRPC and Lifecycle Management Deyaa Adib, MD FibroGen Chief Medical Officer

FG-3246 Presents a Unique Opportunity in mCRPC Novel Mechanism of Action and potential First-in-Class Opportunity ADC – antibody against novel target tethered to a validated chemotherapy payload Binds a unique epitope on CD46 present on cancer cells, including prostate/colorectal, but absent in most normal tissues Phase 1 Monotherapy Efficacy Results Adenocarcinoma selected cohorts receiving ≥ 1.2 mg/kg: Median rPFS of 8.7 months PSA decline by >50%: 36% ORR: 20% Consistent Safety Profile Adverse events consistent with those observed with other MMAE-based ADC therapies 1 3 4 Potential Opportunity in Multiple Cancer Types Multiple lines of mCRPC Colorectal and other solid tumors 5 Investigating PET Biomarker Diagnostic CD46 biomarker diagnostic, PET46, in development for screening, patient selection and enrichment 2

Development of a CD46 Biomarker is an Integral Part of the Development Strategy Likely that patient selection biomarker is required to achieve clinically differentiated profile in prostate cancer, based on early clinical data and highly competitive mCRPC market Estimate that 50%-70% of mCRPC patients will be CD46high PSMA PET biomarker have demonstrated positive impact on patient outcomes PET-based biomarker currently considered superior to CD46 IHC in prostate cancer due to higher accuracy, applicability to patients with bone-only disease who are not amenable for IHC testing (~50% of advanced mCRPC) Exploratory Phase 2 trial required to assess utility of PET46 and CD46 IHC for patient selection and to select best patient selection strategy prior to Phase 3 trial

Planning for Seamless Phase 2/3 RCT > 2L Pre-Chemo mCRPC Randomized, dose optimization, biomarker-driven, adaptive phase 2/3 design: FG-3246 vs. ARSI switch* Ph3 in CD46high patients: FG-3246 vs. ARSI switch* Open label-adaptive design Primary EP: rPFS Secondary EP: OS Biomarker strategy: PET46 vs. IHC Mandatory CD46 PET & IHC (archival tissue) at screening FG-3246 Dose Optimization Primary EP: ORR, DOR Secondary EP: rPFS, PSA50, PSA90 Exploratory: Association between CD46 and rPFS, ORR and PSA50 1:1 Randomization Dose Level 1 (N=25) 2.4 mg/kg AJBW Dose Level 2 (N=25): 1.8 mg/kg AJBW Decision on optimal dose & biomarker Plus: signals for rPFS, PSA50, ORR & DOR FG 3246 monotherapy n= ~200 ARSI Switch* n= ~200 *Pending upon consultation with FDA summer 2024 Phase 2 Phase 3 1:1

Treatment Option Landscape for Pre-chemo mCRPC and Target FG3246 Product Profile FG-3246 Target Product Profile ARSI switch Chemo PSMA targeted radioligands Patient population CD46-high selected Unselected Unselected PSMA-high selected rPFS (months) >10m 5.6m-6.4m 8.3m 9.5 - 12.0m OS (months) 28.6m ~20m (not yet mature) 19.0m ~19m (not yet mature) Safety & Tolerability: Adverse Events In line with other MMAE containing ADCs Comparable to FG-3246 FG-3246 compares favorably FG-3246 compares favorably Dosing & Administration IV every 3 weeks PO twice daily IV IV, every 6 weeks for up to 6 doses Patient Cost per Course of Therapy NA ~$186K (2024) (Xtandi) ~$62k (2024) (6.2k x 10 cycles, branded) ~273k (2024) REFERENCES Internal Estimates PSMAfore, SPLASH KEYNOTE-921 PSMAfore, SPLASH While we cannot make direct comparisons to other trials due to differences in study design, analysis methods, population sizes, controls and phases of development, we are encouraged there are opportunities for new treatments

Pursue accelerated registrational path to pre-chemo mCRPC Establish FG-3246 in pre-chemo mCRPC in CD46high patients (monotherapy & combination therapy) Establish PET46 biomarker as standard of care diagnostic Expand footprint in prostate cancer by moving into earlier stages of disease Launch in other solid tumor type (e.g., R/R mCRC) Explore novel combinations and additional solid tumors FG-3246 & PET46 Biomarker - Vision and Strategy Horizon 1: Establish Horizon 2: Expand Horizon 3: Explore

Q&A Session

Thank You












































